Abstract
Pupose
Obstructive sleep apnea (OSA) is associated with various metabolic disorders, and oxidative stress was suggested to play an important role. In the present study, we aimed to investigate serum adiponectin and oxidative stress markers, especially protein carbonyls, and to evaluate the correlation between these parameters and lipid, insulin and fasting glucose concentrations in OSA patients and controls.
Method
Blood was drawn from healthy male volunteers following full-night polysomnographic evaluation. Subjects were classified as controls (n = 24), mild OSA group (n = 9) and moderate-severe OSA group (n = 17) according to their apnea–hypopnea indices (AHIs). Serum lipids, fasting glucose, adiponectin, malondialdehyde (MDA), protein carbonyl concentrations, and paraoxonase activities were measured in all subjects.
Results
Results of this study indicated that serum adiponectin concentrations were significantly decreased and MDA and protein carbonyl concentrations were significantly elevated in OSA patients compared to the controls. Protein carbonyl and MDA concentrations were significantly and positively correlated with AHI, while a significant negative correlation was found between adiponectin concentrations and AHI. Adiponectin levels were negatively correlated with MDA levels.
Conclusion
Results of this study, which is the first human study investigating and describing serum protein carbonyl concentrations in OSA patients, reveal that OSA causes increments in oxidative damage and decreases adiponectin levels. The recurrent hypoxia-reoxygenation attacks in OSA patients may activate oxidative stress, elevating sympathetic activity and leading to low levels of adiponectin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) syndrome is characterized by repetitive episodes of complete or partial upper airway obstruction that occur during sleep. The former results in cessation of breathing (apnea); the latter results in hypoventilation (hypopnea). Intermittent hypoxia and reoxygenation due to repetitive apnea attacks are blamed for free radical generation and oxidative stress. Several studies have shown a direct [1–3] or an indirect OSA involvement [4, 5] in the generation of reactive oxygen species. It has been suggested that in OSA, intermittant hypoxia-related free radicals and systemic inflammation might be associated with the progression of several pathophysiological features such as atherosclerosis, cardio- and cerebrovascular, as well as neurodegenerative diseases [5, 6].
OSA is associated with metabolic abnormalities, including insulin resistance [7–9] and dyslipidemia [10–12], as well. Obesity is suspected in the development of OSA in many cases. However, it is not yet entirely clear if it is apnea or obesity which results in the metabolic abnormalities, such as, insulin resistance and dislipidemia, seen in some OSA cases [11, 13, 14]. Recent reports have indicated a positive relationship between OSA syndrome and insulin resistance, independent of obesity [7, 15, 16]. It is postulated that the cerebral activation and increased sympathetic output related to sleep-disordered breathing might provide a stress stimulus that triggers the production of inflammatory cytokines such as TNF-α and develops insulin resistance [7, 15].
Adiponectin is one of a number of adipocytokines produced from adipocytes. Its antiinflammatory and insulin-sensitizing properties are well described. Serum adiponectin levels are decreased in obese patients as well as in type 2 diabetics and OSA patients [17–19]. Although insulin resistance and obesity are strongly associated, it is demonstrated that insulin resistance in OSA is not only caused by obesity but is also an independent feature of OSA [20]. Some authors suggest decreased adiponectin levels in OSA patients as an important determinant of insulin sensitivity [21], while some investigators report an association with reduced levels of adiponectin independently of insulin resistance [19].
The role and association between adiponectin, oxidative stress, and OSA draws attention to the complex interactions taking part in obesity, insulin resistance, and sleep-disordered breathing. Since studies about the prevalence of OSA indicate that OSA affects 24% and 9% of middle-aged men and women, respectively [22] and the prevelence of OSA may be increasing because of recent obesity trends [23], studies are needed to better define various metabolic processes taking place in OSA disorders.
Relying on the proposed effect of OSA-related oxidative stress on expression of inflammatory cytokines [24] and the inhibitory effect of TNF-α on adiponectin synthesis and secretion [25], we suggested a causal relationship between OSA related oxidative stress and adiponectin levels and resulting metabolic effects. We therefore aimed to investigate the serum levels of oxidative stress indicators [malondialdehyde (MDA), protein carbonyl content (PCC) and paraoxonase (PON) activity], adiponectin, lipids, fasting insulin, and glucose concentrations and to evaluate the association between these parameters in OSA patients and control subjects.
Methodology
Subjects
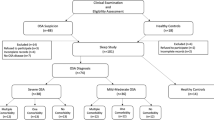
Fifty consequtive male subjects who were found to have OSA (n = 26) or classified as simple snorers (controls) (n = 24) during sleep studies at the sleep laboratory were included in this study. Exclusion criteria were use of any medications, personal or family history of psychiatric disorders, history of alcohol and drug abuse, and any other significant medical illnesses such as cancer, cardiovascular, cerebrovascular, pulmonary, or neuromuscular disease. A questionnaire that inquired about the presence of any history of snoring, witnessed apnea, excessive daytime sleepiness, and Epworth sleepiness scale was completed for each patient in the presence of the bed partner. Demographic information (age, gender, smoking habits) and anthropometric measurements [height, weight, body mass index (BMI; weight/height squared)] were obtained on presentation to the sleep center. All subjects gave informed consent, and the protocol was approved by the Ethics Committee of the Uludağ University Hospital.
Sleep study
Full polysomnography was performed in all patients (Compumedics P-series Sleep System; Compumedics Sleep; Melbourne, Australia). All participants reported to the sleep laboratory at approximately 8:30 p.m., and polysomnography was initiated at approximately 10:30 p.m. Polysomnographic recordings included two EEG channels (C3/A2 and O2/A1), two electrooculogram channels, one submental electromyogram channel, and one ECG channel. Ventilatory monitoring included recording of oronasal airflow (with an oronasal thermistor), hemoglobin oxygen saturation by pulse oximetry (oxygen saturation measured via a finger oximeter), respiratory movement (with an inductive plethysmography) including chest and abdomen, and body position. Sleep staging was performed according to the standard criteria of Rechtschaffen and Kales. To assess ventilation during sleep, nasal airflow was analyzed carefully. Apnea was defined as episodes lasting at least 10 s with airflow cessation. Hypopnea was defined as episodes lasting at least 10 s with reductions of thermistor signal amplitude by at least 50% and associated fall of at least 3% in oxygen saturation or an arousal. The sum of time spent in apnea and hypopnea was divided by the total sleep time to obtain by the apnea–hypopnea index (AHI). Subjects with AHI ≥5 were considered to have OSA. Subjects with AHI <5 were included in the control group.
Blood sampling and biochemical assays
Single blood samples were drawn between 8:00 and 9:00 a.m. after the sleep study. Blood samples were centrifuged within 30 min at 4°C at 3,000 g for 10 min to obtain serum samples. Total cholesterol, triglycerides, high-density lipoprotein (HDL)-cholesterol, and glucose were measured enzymatically on the same day, using kits obtained from Abbott Laboratories (USA). Plasma low-density lipoprotein (LDL)-cholesterol was determined from the values of total cholesterol and HDL-cholesterol using the Friedwald's formula. Sera that were appropriately separated were stored at −80°C until they were analyzed for insulin, adiponectin, MDA levels, PCC, and PON activity measurements. Fasting insulin was determined by electrochemiluminescense on Elecys 2000, using kits from Roche Diagnostics (USA). Adiponectin was measured by radioimmunoassay using LINCO Research Human Adiponectin RIA kits. Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as insulin (mU/l) × (glucose [mg/dl] ×0.055)/22.5 [26] to estimate insulin resistance. Serum MDA levels, measured by using HPLC as described by Young et al. [27], and serum PCCs, measured by the spectrophotometric method described by Reznick et al. [28], were used to evaluate oxidative stress. PON activity was determined according to the spectrophotometric method by Eckerson et al. [29].
Statistical analysis
Statistical analysis was performed using statistical software (SPSS for Windows, version 13.0; SPSS; Chicago, IL). After assessing for approximate normal distribution, all continuous variables were summarized in terms of means (standard error). The difference between the groups was compared using one-way analysis of variance and post hoc LSD tests or Kruskal–Wallis and Mann–Whitney U tests, where appropriate. Pearson correlation analysis was performed to test the relationship between the parameters. Confounding effect of smoking on various parameters was tested by using general linear models. p value of <0.05 was considered statistically significant.
Results
In this study, we compared 26 OSA patients (AHI ≥5, range 5.2–80.0) with 24 non-apneic controls (AHI <5, range 0.2–4.5). OSA patients are further divided into two groups as mild OSA (AHI =5–15, range 5.2–12.9) (n = 9) and moderate-severe OSA (AHI >15, range 24.4–80.0) (n = 17) patients. According to the classic OSA classification, only two of the participants were classified as moderate OSA patients (AHI =24.4 and 25.1) in the study. Therefore, these subjects were evaluated with the severe OSA patients, and accordingly, the group was named as moderate-severe OSA patients. The demographics of the 50 patients are presented in Table 1.
The ages, body mass indices, waist circumferences, hip circumferences, waist/hip ratios, smoking, and drinking habits of the subjects were not different between the three groups.
The lipid profile, fasting glucose, insulin, adiponectin concentrations, MDA levels, PCCs, and PON activities of the three groups are compared in Table 2. There was no significant difference in serum lipids, glucose, and insulin concentrations between the three groups. HOMA-IR, the expression of insulin resistance, was not statistically different between the three groups either. Serum adiponectin concentration of OSA patients in both mild and moderate-severe OSA groups was significantly lower compared to those of controls (p < 0.05). While serum MDA and PCC were not different between the mild OSA group and control subjects, these oxidative stress indicators were significantly higher in moderate-severe OSA patients compared to the controls (p < 0.05). Serum PON activity did not show any significant difference between the OSA patients and controls.
The Pearson’s correlation coefficient of AHI with serum MDA was found to be 0.51 (p < 0.001), with PCC 0.39 (p < 0.01), and with adiponectin −0.34 (p < 0.05). The association between MDA, PCC, adiponectin, and AHI is shown in Fig. 1.
Although adiponectin concentrations showed significant negative correlation with MDA concentrations (r = −0.32, p < 0.05), significant correlations were not observed between adiponectin and PCC or PON activity. Correlations between the parameteres of oxidative stress, anthropometric, and blood data are presented in Table 3.
Discussion
Research over the past several years indicate that OSA is an oxidative stress disorder. Although a few studies have reported negative results, most have shown oxidative stress to be associated with OSA [30–32]. Studies demonstrating that oxidative stress ameliorates after successful treatment with nasal continuous positive airway pressure made it evident that augmented formation of reactive oxygen species and the resultant oxidative stress are prominent features of OSA [5, 33, 34]. Increased oxidative stess in OSA patients has been studied by evaluating various parameters such as oxidative damage markers or antioxidant defence mechanisms in serum [5, 35]. To our knowledge, oxidative damage to serum proteins in OSA patients is investigated in only one study so far [36]. In their study, Sonka et al. have studied advanced oxidation protein products, which they found elevated in OSA patients; however, the difference from the controls was not statistically significant. The current study is the first attempt to assess both lipid and protein oxidation markers in the same cohort. In this study, we demonstrate, for the first time, that OSA causes oxidative damage to serum proteins. Our data reveal that patients with moderate-severe OSA (characterized by elevated AHI) have evidence for greater oxidative stress (both serum lipid peroxidation and serum protein oxidation) than healthy controls. The increases in oxidative stress markers being significant in merely the moderate-severe OSA patients suggest an association between the oxidant damage and the severity of OSA. The significant positive correlations of AHI with serum MDA and serum PCCs supports this premise. Smoking is well known to cause oxidative stress and related effects [37]. Since the percentage of smokers were higher in OSA patients than in controls, we tested if smoking was a confounding factor in this study population by using general linear models and found that smoking was not a confounder in MDA, PCC, PON, and adiponectin results.
According to our results, PON activity is not affected by the consequences of OSA . Lavie [38] studied PON activity in controls and OSA patients with and without cardiovascular disease (CVD). They found that PON activity was different only between controls and OSA patients with CVD. In other words, participants (either control or OSA) without CVD had comparable PON activities. They stated that a cardiovascular condition was significantly associated with down-regulation of PON activity. The controls and OSA patients in the present study had no history of CVD. Therefore, the unchanged PON activity is in accordance with the results Lavie et al. observed in their controls and OSA patients without CVD. This finding supports the thought that PON activity alterations are related to the precence of CVD in OSA patients.
Adiponectin, an adipocyte-derived hormone, has been found to have potential antidiabetic, anti-atherosclerotic, and anti-inflammatory properties [39]. Although its association with obesity and CVDs is well documented, its role in OSA remains controversial. Also, the interaction between adiponectin and oxidative stress is not entirely clear yet. It is suggested that the sequence of events in OSA—breathing cessation, nocturnal hypoxia, continuous brief arousals, and sleep fragmentation—activates oxidative stress [40] and elevates sympathetic activity, promoting the expression of inflammatory cytokines like TNF-α and IL-I [24] . Increased TNF-α has an inhibitory effect on adiponectin synthesis and secretion, causing decreases in the serum levels of this cytokine [25, 41]. In the current study, we tried to study an association between oxidative stress and adiponectin in OSA patients and found that adiponectin levels were significantly lower in both mild and moderate-severe OSA groups compared to those of healthy controls. Also, we found a significant negative correlation between adiponectin and MDA concentrations (r = −0.32; p < 0.05), supporting the proposed relationship between OSA, oxidative stress, and adiponectin levels. However, similar associations were not detected between adiponectin and the two other oxidative stress markers, PON and PCC, we tested in this study. Since PON activity was not altered in OSA patients of this study, any statistical association was not expected between PON activity and adiponectin concentrations. PCC, on the other hand, was significantly elevated in moderate-severe OSA patients and its concentration was significantly correlated to AHI. The failure to demonstrate a significant association between adiponectin and PCC may be because of the relatively small sample size. Therefore, to investigate the relationship of this oxidative stress marker with adiponectin in larger OSA groups in future studies would provide further data for beter discussion.
Low adiponectin concentrations are associated with insulin resistance and obesity [20]. However, in the present study, estimation of insulin resistance using HOMA-IR method in OSA patients was not significantly different from the controls despite the significant decrements in adiponectin concentrations. Makino et al. found significantly higher fasting glucose and fasting insulin levels in their severe OSA patients and accordingly calculated significantly greater insulin resistance for this group [21]. In their study, which was carried out on a wide population of OSA patients, the subjects had various systemic and metabolic diseases and the prevelance of the clinical conditions as well as BMI tended to be greater in conjunction with the severity of OSA. On the other hand, they reported significant negative association between adiponectin and insulin resistance, although plasma adiponectin levels were not different between the OSA groups and were not correlated to AHI. An important strength of the present study is that only normotensive and otherwise healthy male patients were included. Therefore, the subjects of the present study had no known metabolic or chronic diseases, and fasting serum glucose as well as insulin concentrations of OSA patients were not different from those of controls. This may explain the unaltered insulin resistance in OSA patients. The relationship between insulin resistance and other metabolic variates is one of complex interactive regulation. The missing association between adiponectin levels and insulin resistance in the present study may be because the adiponectin concentrations were decreased due to the consequences of OSA related oxidative stress, while insulin resistance was not still affected.
Dyslipidemia is also a prevalent finding among patients with sleep apnea. Increased total serum cholesterol and triglycerides levels and decreased HDLs independent of age and body mass index [10, 11]. The lipid profile was not changed in OSA patients compared to controls in the present study. This inconsistance with the previous reports is probably because the patient profiles of the before-mentioned investigations included CVD patients, whereas the subjects in our study were otherwise healthy OSA patients.
In summary, the findings of our study emphasize the impact of sleep-disordered breathing on oxidative damage and provides evidence for the literature that serum proteins are oxidatively damaged in OSA. Markers of oxidative stress being elevated and adiponectin levels being reduced in OSA patients, despite the unchanged lipid profiles and insulin resistance between OSA patients and controls, raise the question if the changes in oxidative stress markers and adiponectin levels could represent early signs for the subsequent development of metabolic disorders. We suggest that otherwise healthy OSA patients are exposed to oxidative stress and develop lower adiponectin levels which may later cause cardiometabolic consequences, affecting markers such as visceral adiposity, insulin resistance, and lipid profile. This needs to be investigated in future studies by periodically following the changes in oxidative stress markers, adiponectin, visceral adiposity, lipid profiles and insulin resistance in otherwise healthy OSA patients for a better discussion on this context.
This study has a number of limitations; the study population and the number of parameters tested were restricted because of financial limitations. Therefore, small sample sizes had to be used in statistical analyses. Also, to determine inflammatory cytokine levels and/or parameters of sympathetic activity, evidence should be provided to better discuss their pathophysiological importance in OSA.
References
Carpagnano GE, Kharitonov SA, Resta O, Foschino-Barbaro MP, Gramiccioni E, Barnes PJ (2003) 8-Isoprostane, a marker of oxidative stress, is increased in exhaled breath condensate of patients with obstructive sleep apnea after night is reduced by continious positive air way pressure therapy. Chest 124:1386–1392
Christou K, Markoulis N, Moulas AN, Pastaka C, Gourgoulianis KI (2003) Reactive oxygen metabolites (ROMs) as an index of oxidative stress in obstructive sleep apnea patients. Sleep Breath 7:105–110
Saarelainen S, Lehtimaki T, Jaak-Kola O, Poussa T, Nikkila M, Solakivi T, Nieminen MM (1996) Autoantibodies against oxidised low-density lipoprotein in patients with obstructive sleep apneoea. Clin Chem Lab Med 37:517–520
Jordan W, Berger C, Cohrs S, Rodenbeck A, Mayer G, Niedmann PD, vanAhsen N, Rüther E, Kornhuber J, Bleich S (2004) CPAP-therapy efficiently lowers serum homocysteine in obstructive sleep apnea syndrome. J Neural Transm 111:683–689
Lavie L (2003) Obstructive sleep apnoea syndrome—an oxidative stress disorder. Sleep Med Rev 7:35–51
Banno K, Kryger MH (2007) Sleep apnea: clinical investigations in humans. Sleep Med 8:400–426
Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS (2002) Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med 165:670–676
Punjabi NM, Sorkin JD, Katzel LI, Goldberg AP, Schwartz AR, Smith PL (2002) Sleepdisordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir Crit Care Med 165:677–682
Meslier N, Gagnadoux F, Giraud P, Person C, Ouksel H, Urban T, Racineux J-L (2003) Impaired glucose-insulin metabolism in males with obstructive sleep apnoea syndrome. Eur Respir J 22:156–160
Newman AB, Nieto FJ, Guidry U, Lind BK, Redline S, Shahar E, Pickering TG, Quan SF (2001) Sleep Heart Health Study Research Group. Relation of sleepdisordered breathing to cardiovascular disease risk factors: the Sleep Heart Health Study. Am J Epidemiol 154:50–59
McArdle N, Hillman D, Beilin L, Watts G (2007) Metabolic risk factors for vascular disease in obstructive sleep apnea: a matched controlled study. Am J Respir Crit Care Med 175:190–195
Tan KC, Chowa W-S, Lama JC, Lama B, Wong W-K, Tamb S, Ipl MS (2006) HDL dysfunction in obstructive sleep apnea. Atherosclerosis 184:377–382
Sharma SK, Kumpawat S, Goel A, Banga A, Ramakrishnan L, Chaturvedi P (2007) Obesity and not obstructive sleep apnea is responsible for metabolic abnormalities in a cohort with sleep disordered breathing. Sleep Medicine 8:12–17
Ciftci TU, Kokturk O, Bukan N, Bilgihan A (2005) Leptin and ghrelin levels in patients with obstructive sleep apnea syndrome. Respiration 72:395–401
Punjabi NM, Sorkin JD, Katzel LI, Goldberg AP, Schwartz AR, Smith PL (2002) Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir Crit Care Med 165:677–682
Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE (2004) Sleep Heart Health Study Investigators. Sleepdisordered breathing, glucose intolerance, and insulin resistance: the sleep heart health Study. Am J Epidemiol 160:521–530
Lihn AS, Pedersen SB, Richelsen B (2005) Adiponectin: action, regulation and association to insulin sensitivity. Obes Rev 6:13–21
Fantuzzi G (2005) Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol 115:911–919
Masserini B, Morpurgo PS, Donadio F, Baldessari C, Bossi R, Beck-Peccoz P, Orsi E (2006) Reduced levels of adiponectin in sleep apnea syndrome. J Endocrinol Invest 29:700–705
Harsch IA, Schahin SP, Radespiel-Tröger M, Weintz O, Jahreiss H, Fuchs FS, Wiest GH, Hahn EG, Lohmann T, Konturek PC, Ficker JH (2004) Continious positive airway pressure tratment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am J Respir Crit Care Med 169:156–162
Makino S, Handa H, Suzukawa K, Fujiwara M, Nakamura M, Muraoka S, Takasago I, Tanaka Y, Hashimato K, Sugimoto T (2006) Obstructive sleep apnoea syndrome, plasma adiponectin levels, and insulin resistance. Clin Endocrinol 64:12–19
Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S (1993) The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 328:1230–1235
Banno K, Walld R, Kryger MH (2005) Increasing obesity trends in patients with sleep breathing disorders referred to a sleep disorders centre. J Clin Sleep Med 1:364–366
Vgontzas AN, Papanicolaou DA, Bixler EO, Kales A, Tyson K, Chrousos GP (1997) Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity. J Clin Endocrinol Metab 82:1313–1316
Soares AF, Guichardant M, Cozzone D, Bernoud-Hubac N, Bouzaïdi-Tiali N, Lagarde M, Géloën A (2005) Effects of oxidative stress on adiponectin secretion and lactate production in 3T3-L1 adipocytes. Free Radic Biol Med 38:882–889
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
Young IS, Trimble ER (1991) Measurement of malondialdehyde in plasma by high performance liquid chromatography with fluorimetric detection. Ann Clin Biochem 28:504–508
Reznick AZ, Packer L (1994) Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol 233:357–363
Eckerson HW, Wyte CM, La Du BN (1983) The human serum paraoxonase/arylesterase polymorphism. Am J Hum Genet 35:1126–1138
Lavie L, Vishnevsky A, Lavie P (2004) Evidence for lipid peroxidation in obstructive sleep apnea. Sleep 27:123–128
Barcelo A, Miralles C, Barbe F, Vila M, Pons S, Agusti AG (2000) Abnormal lipid peroxidation in patients with sleep apnoea. Eur Respir J 16:644–647
Itzhaki S, Dorchin H, Clark G, Lavie L, Lavie P, Pillar G (2007) The effects of 1-year treatment with a herbst mandibular advancement splint on obstructive sleep apnea, oxidative stress, and endothelial function. Chest 131:740–749
Lavie L (2005) Sleep-disordered breathing and cerebrovascular disease: a mechanistic approach. Neurol Clin 23:1059–1075
Suzuki YJ, Jain V, Park AM, Day RM (2006) Oxidative stress and oxidant signaling in obstructive sleep apnea and associated cardiovascular diseases. Free Radic Biol Med 40:1683–1692
Arnardottir ES, Mackiewicz M, Gislason T, Teff KL, Pack AI (2009) Molecular sigantures of obstructive sleep apnea in adults: a riview and perspective. Sleep 32:447–470
Sonka K, Fialová L, Volná J, Jiroutek P, Vávrová J, Kemlink D, Pretl M, Kalousová M (2008) Advanced oxidation protein products in obstructive sleep apnea. Prague Med Rep 109:159–165
Tanriverdi H, Evrengul H, Kuru O, Tanriverdi S, Seleci D, Enli Y, Kaftan HA, Kilic M (2006) Cigarette smoking induced oxidative stress may impair endothelial function and coronary blood flow in angiographically normal coronary arteries. Circ J 70(5):593–599
Lavie P (2004) Evidence for lipid peroxidation in obstructive sleep apnea. Sleep 27(1):123–8
Trujillo ME, Scherer PE (2005) Adiponectin—journey from an adipocyte secretory protein to biomarker of the metabolic syndrome. J Intern Med 257:167–175
Buckley TM, Schatzberg AF (2005) On the interactions of the hypothalamic–pituitary–adrenal (HPA) axis and sleep: normal HPA axis activity and circadian rythm, exemplary sleep disorders. J Clin Endocrinol Metab 90:3106–3114
Bruun JM, Lihn AS, Verdich C, Pedersen SB, Toubro S, Astrup A, Richelsen B (2003) Regulation of adiponectin by adipose tissue-derived cytokines: in vivo and in vitro investigations in humans. Am J Physiol Endocrinol Metab 285:527–533
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vatansever, E., Surmen-Gur, E., Ursavas, A. et al. Obstructive sleep apnea causes oxidative damage to plasma lipids and proteins and decreases adiponectin levels. Sleep Breath 15, 275–282 (2011). https://doi.org/10.1007/s11325-010-0378-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-010-0378-8