Abstract
Objective
Early and accurate staging of ovarian cancer is paramount to disease survival. Conventional imaging including FDG PET/CT are limited in the evaluation of small metastatic lesions. 18F-Fluciclovine has minimal urine and bowel excretion allowing optimal visualization of the abdomen and pelvis. This study examines 18F-fluciclovine uptake in known primary and recurrent ovarian cancer.
Methods
Seven patients with a confirmed diagnosis of epithelial ovarian cancer underwent 18F-fluciclovine PET/CT imaging. Forty-one (41) lesions were identified with 18F-fluciclovine and confirmed to be true positive (n = 41). We aim to explore if 18F-fluciclovine uptake in ovarian lesions were greater than background uptake of bone marrow, blood pool, and bladder. Quantification analysis was performed to determine max and mean standard uptake values (SUVmax and SUVmean) of known and suspected lesions compared to SUVmean uptake of background structures.
Results
18F-Fluciclovine demonstrated 100% sensitivity (41/41) for uptake in known ovarian lesions. The average SUVmax (±SD) uptake of known ovarian lesions was 5.9 (±2.6) and 5.1 (±2.0) on early and delayed images, respectively. The average tumor SUVmax to SUVmean of background (±SD) (T:B) ratios on early and delay were 1.9 (±0.8), 2.1 (±0.9) for marrow; 3.8 (±1.8), 3.4 (±1.5) for aorta; and 8.4 (±4.3), 1.5 (±1.7) for bladder, respectively.
Conclusion
18F-Fluciclovine uptake in malignant ovarian lesions was above background levels suggesting its feasibility in the imaging of ovarian cancer. Due to increasing tracer washout via the urinary bladder over time, early imaging at 4 min post injection is favorable.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ovarian cancer ranks fifth in cancer deaths among women, accounting for more deaths than any other cancer of the female reproductive system [1]. Accurate evaluation of neoplastic involvement is vital for optimal treatment selection for any cancer which often utilizes imaging. Ovarian cancer initial staging and restaging according to the V5.2022 NCCN guidelines is predominantly performed with the use of conventional imaging (CI), such as ultrasound (US), computed tomography (CT), and magnetic resonance imaging (MRI). Although CT is predominantly used for staging, it may have low sensitivities in the detection of small peritoneal metastatic lesions and often may underestimate patients with diffuse disease who may benefit from neoadjuvant chemotherapy [2]. Final staging of ovarian cancer is still performed by surgery, known as debulking/staging laparotomy [3,4,5,6]. An FDG PET/CT is primarily reserved as a problem-solving tool for indeterminate lesions on CI when it may alter management [3]. Although many publications suggest that FDG may be superior to CI, it is yet to be incorporated into the guidelines as an early staging/restaging modality [7,8,9,10]. The limited use of FDG PET/CT for initial and restaging may be due to false-positive tracer uptake in benign inflammatory structures (uterine fibroids and reactive lymph nodes), benign ovary lesions, and physiological (uterus, bladder, and loops of bowel) processes, which may lower tumor to background ratios. False negatives are associated with low cellular mucosal lesions with minimal FDG uptake [7]. As clinical practice is progressing into precision medicine, it is important to improve the diagnostic performance of imaging in GYN cancer. Hence, it is essential to evaluate the use of alternative molecular pathways within ovarian lesions with the use of amino acid analogs.
Amino acids are the building blocks for protein and are essential for cell metabolism and proliferation. Amino acids enter the cell via amino acid transporters (AAT), which are often upregulated in various cancer cells [11]. In prostate cancer cells, large neutral amino acid transporters (systems L: LAT1, LAT3, and LAT4) and alanine-serine-cysteine transporters (systems ASC: ASCT1, ASCT2) are overexpressed [12,13,14]. In ovarian cancer, LAT1 is highly expressed, and its overexpression is an independent factor for predicting poor overall survival [15, 16]. Although ASCT2 is an important glutamine transporter that is often overexpressed in many types of malignancies, its role in epithelial ovarian cancer is unclear [17].
A literature review using amino acid tracers in GYN malignancies is limited. A single study with naturally occurring amino acid 11C-methionine demonstrated promising results in ovarian cancers with 7/7 (100%) tracer uptake in malignant lesions. The study concluded that ovarian cancers could be effectively imaged with 11C-methionine PET scans [18]. Although imaging with 11C-methionine was promising, the use of naturally occurring labeled amino acids is not optimal for imaging because of increased metabolites in nontarget organs.
18F-Fluciclovine is a synthetic amino acid, and unlike 11C-methionine, it will not be metabolized. Therefore, 18F-fluciclovine has great potential to demonstrate better target to background ratios in malignant ovarian lesions. 18F-Fluciclovine PET tracer was approved in May 2016 by the Food and Drug Administration (FDA) for prostate cancer imaging. Unlike the glucose analog FDG, 18F-fluciclovine has minimal to no early renal excretion and bladder accumulation [19,20,21] (Fig. 1). 18F-Fluciclovine has proven highly effective in identifying indolent prostate cancer recurrence in small pelvic lymph nodes and demonstrated high performance in detecting local recurrence within the prostate bed [19, 21,22,23]. 18F-Fluciclovine is not a prostate-specific radiotracer and has been investigated in several other malignancies, including brain, breast, and lung, with promising results [24]. Consequently, we believe 18F-fluciclovine may have an application in the imaging of GYN cancers. To our knowledge, this is the first research evaluating 18F-fluciclovine uptake in ovarian cancer.
Normal physiological distribution of the PET radiotracers. Left: 18F-FDG-PET images demonstrating intense urine exertion to the urinary bladder and normal variant uptake within the loop of bowel. Right: 18F-fluciclovine PET image demonstrating minimal urinary excretion in early (4 min) images. Intense tracer uptake is seen in the liver and pancreas. Increasing uptake over time is noted within the skeletal muscles (images obtained from thighs to skull base)
In this study, we aimed to assess the uptake of 18F-fluciclovine in known ovarian lesions over time (primary aim) in a similar method used to evaluate prostate cancer. Similarly, we aimed to define the optimal imaging time with the highest tumor to background ratios (secondary aim).
Methods
This prospective observational trial was aimed to evaluate the uptake of 18F-fluciclovine in PET/CT imaging of GYN cancers. The study included three arms: ovarian, cervical, and endometrial cancers. Each arm was analyzed separately to explore 18F-fluciclovine uptake in GYN malignancies. The ovarian arm will serve as the scope of this paper.
Patient Selection
Seven patients with a median age (range) of 61 (42–70) and a confirmed diagnosis of epithelial ovarian cancer (1/7 primary, 6/7 recurrence) were evaluated in this study (Table 1). Inclusive criteria included patients with a confirmed diagnosis of ovarian cancer (biopsy-proven and/or scheduled for subsequent surgery). Patients with systemic chemotherapy in the past 3 months were excluded.
Image Protocol
The patients underwent PET/CT imaging on a Philips Ingenuity 64 Slice TOF PET/CT scanner. Each patient was positioned supine on scanner table with arms above head, if possible. A CT scan from the mid thighs to skull base (80–120 mA; 120 kVp) was acquired followed by an injection of 10 mCi (±20%) of 18F-flucicolvine diluted in up to 10 mL of normal saline. Injection of the 18F-fluciclovine was administered via a venous catheter in the right arm to avoid the appearance of residual tracer uptake in the venous system, which could interfere with the visualization of the left supraclavicular lymph node (Virchow’s node). 18F-Fluciclovine PET images were acquired at 3 min per bed with 6 bed positions for a total of 18 min per scan. Imaging was done from distal thighs to skull base at dual-time points: early imaging (4–22 min) and delayed imaging (24–42 min).
Image Interpretation
Image quantification, qualification, and interpretation were completed by a single, board-certified nuclear medicine physician with more than 10 years of experience evaluating 18F-flucicolvine PET/CT scans. Images were reconstructed with iterative technique and interpreted on Hermes (Hermes Medical Solutions, Stockholm, Sweden) fused software. An exploratory analysis of SUVmax and SUVmean of malignant, benign, and background regions of interest (ROI) was performed on the early and delayed images. Suspicious lesions were defined as PET positive if uptake was equal to or higher than that of the bone marrow. For lesions smaller than 1 cm, positivity threshold was lowered due to partial volume artifact and lesions were defined as positive when uptake was significantly above blood pool and approaching marrow. This approach to image interpretation is consistent with 18F-fluciclovine reading guidelines and prior studies [25, 26].
Time-Activity Curves and Quantitation Analysis
Lesion ROIs were visually defined by the interpreter. Background ROI structures included the liver (3D ROI contoured on segments 6/7), bone marrow (ROI contoured to include all L3 vertebral body), blood pool (2D ROI within the aortic lumen, just above the bifurcation), and urinary bladder (ROI contoured to include all bladder lumen). The lesions ROI were contoured to include the entire soft tissue. SUVmax and SUVmean values were plotted into time activity curves including early and delayed time points to examine the characteristics of uptake in known malignant ovarian lesions in relation to benign background (T:B ratios) and tracer urinary excretion over time.
Truth Verifications
The primary method of truth verification of positive lesions identified by 18F-fluciclovine PET/CT was histology. Patients with history of histologically confirmed epithelial lesions with biochemical recurrence (elevated CA-125) and abnormal CT or MRI for metastatic disease (M1) did not require additional biopsies, and verification was defined based on clinical imaging presentation and original biopsy. For lymph node (N) and primary lesions (T) verification, histology confirmation was used. All patients were followed for 6 months after 18F-fluciclovine images. Any post-imaging biopsies performed as per standard practice contributed to the aim of the study.
Results
PET Findings
18F-Fluciclovine PET/CT images in 7 women (1 primary, 6 recurrence) demonstrated 100% sensitivity. Overall, 46 lesions were defined as malignant. Of the 46 suspicious lesions evaluated in the ovarian cancer patients, 41 had truth verification and were defined as true positive. A total of 5 lesions (suspected lymph nodes) could not be verified due to lack of biopsy. Among the 41 verified positive lesions, 2 lesions were within the ovaries and 39 were metastatic lesions. Of the 39 metastatic lesions, 16 were abdominal implants, 3 were liver lesions, 6 were lung nodules, 8 were bone lesions, and 6 were metastatic lymph nodes.
Time Activity Curve of 18F-Fluciclovine Uptake in Malignant Lesions in Relation to Background Structures and Urine Excretion
Uptake over time for the 41 verified positive malignant lesions, background liver, marrow, and blood pool demonstrated a down sloping time activity curve from early (4 min) to delay (42 min) images. Despite the down sloping curve, uptake within the malignant lesions was persistently higher compared to marrow background. The tumor average SUVmax (±SD) uptake was 5.9 (±2.6) and 5.1 (±2.0) on early and delayed images, respectively (Fig. 2). The average background SUVmean (±SD) on early and delay images were 8.9 (±2.6), 8.7 (±2.7) for liver; 3.2 (±0.7), 2.5 (±0.5) for marrow; and 1.7 (±0.4), 1.5 (±0.4) for aorta. Uptake within the bladder increased significantly over time and approached tumor uptake on delay images with SUVmean (±SD) of 0.7 (±0.3) on early imaged and 5.2 (±3.6) on delay.
Time-activity graph: ovarian cancer SUVmax tumor compared to SUVmean background at early and delayed time points demonstrating significantly higher tumor uptake than background for aorta and marrow. There was only slight decrease in tumor uptake in the delayed images. Background uptake in the bladder increased significantly from early to delay images. In fact, on delayed imaging, bladder uptake was greater than tumor uptake
Time Activity Curve of Tumor SUVmax to Background SUVmean Ratio
The average tumor SUVmax to background SUVmean; T:B (±SD) ratios on early and delay images were 1.9 (±0.8), 2.1 (±0.9) for marrow and 3.8 (±1.8), 3.4 (±1.5) for aorta, respectively. Tumor to bladder uptake ratio decreased over time due to increased tracer washout via the bladder on delay images. T:B were 8.4 (±4.3) on early images and 1.5 (±1.7) on delay (Fig. 3).
Time-activity graph: ovarian cancer SUVmax tumor to SUVmean background ratio at early and delayed time points demonstrating a steady uptake ratio for both aorta and marrow. There was a significant decrease from early tumor to bladder ratio when compared to the delayed images indicating overlapping SUV values for tumor and bladder
Discussion
Ovarian cancer initial staging and restaging according to the V5.2022 NCCN guidelines is predominantly performed with the use of CI. An FDG PET/CT is primarily reserved as a problem-solving tool for indeterminate lesions on CI when it may alter management [1]. Although FDG PET literature review suggests it has a higher diagnostic performance compared to CI, its clinical benefit as first-line modality was not yet achieved and is likely attributed to high false positives [6, 7]. As clinical practice is progressing into precision medicine with the recent FDA approval of new cancer specific PET tracers [27,28,29], it is important to improve the accuracy of ovarian cancer staging and restaging by evaluating the use of molecular imaging targeting different biological pathways in this malignancy.
18F-Fluciclovine is a synthetic amino acid analog that has been widely used in patients with recurrent prostate cancer. 18F-Fluciclovine is primarily transported into the cell by amino acid transposers which are upregulated in deferent malignancies. LAT1 transport is upregulated in ovarian cancer [16].
In this study, we set out to evaluate the uptake of 18F-fluciclovine by ovarian epithelial cancer in patients with histologically proven initial or recurrent disease. We aimed to evaluate the uptake in malignant lesions and to compare it to adjacent background tissue, in a similar methodology used to investigate the use of 18F-fluciclovine in prostate cancer [30]. We hypothesized that uptake on early and delay images (4–42 min post injection) will be higher than bone marrow and blood pool. Delay tracer secretion by the urine was evaluated to optimize imaging time for future cohorts.
In this cohort of 41 verified malignant lesions of ovarian cancer, we found 100% sensitivity. Our results are similar to C11-methione naturally occurring amino acid PET tracer which reported 100% sensitivity (7/7) in patients with ovarian cancer [18].
18F-Fluciclovine uptake to the cell via the amino acid transporters is likely a 1:1 ratio; for every amino acid that goes into the cell, one amino acid comes out. Therefore, prior data on 18F-fluciclovine dosimetry in prostate cancer reported on a down sloping time activity uptake from 0 to 60 min post injections [20]. Our cohort also demonstrated similar results with a downsloping curve. Similar to prostate cancer lesions, uptake within malignant ovarian lesions was persistently higher than that of bone marrow or blood pool at early and delay images (Figs. 2, 3) [19, 30].
18F-Fluciclovine normal excretion is mainly via the urine and usually occurs at later imaging time. To optimize image quality and mitigate overlapping of delayed bladder tracer washout with pelvic lesions tracer uptake, it is recommended to obtain the PET images at 4 min post injection and avoid emptying the bladder prior to the exam [21, 25, 26]. In the USA, the use of Foley catheter is not routinely performed for PET imaging and was not evaluated as a method in this cohort. In this study, we found a pattern of increasing delayed urine tracer washout with overlapping uptake to that of the tumor (24–42 min). The average of tumor to bladder ratio on early 4 min post injection images was 8.1 and decreased to 1.5 at 24–42 min images. As such, optimal imaging time will likely be similar to that of imaging in prostate cancer (Figs. 4 and 5) [25].
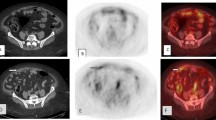
A 42-year-old female presented with suspicious ascites. A CT scan demonstrated abnormal pelvic lesions suspicious for ovarian cancer and revealed a pleural effusion. Biopsy confirmed a poorly differentiated ovarian cancer. B, C Subsequent 18F-fluciclovine PET/CT demonstrated intense uptake within the known pelvic masses as well as pulmonary lesions (red arrows). Whole body early time point images (B) demonstrate minimal bladder activity in comparison to delayed time point images (C) with markedly increased tracer activity in the bladder (blue arrow). This could potentially obscure pelvic lesions. Transaxial images demonstrate the abnormal pelvic findings (D, G) as well two abnormal foci of 18F-fluciclovine uptake in the chest compatible with pulmonary metastases (E, F, H, I). The pulmonary lesions were not detected on the staging CT due to overlying pleural effusion; however, they would demonstrate tracer uptake even in the presence of one
18F-Fluciclovine delay time point images at approximately 24–42 min post injections are not commonly used in clinical practice. However, delay imaging was reported to have a value in increasing specificity of positive lymph node in patients with biochemical recurrence prostate cancer due to more rapid washout in benign lesions compare to true malignant lesions [19, 22]. This cohort set out to look at uptake within known positive lesions only and the specificity of positive lesions was not evaluated. In this cohort, 5/46 lesions with suspicious uptake were lymph nodes with no histological verifications and therefore excluded from this analysis.
Overall, our findings are promising, as this is among the first cohorts to our knowledge to support the potential use of a new molecular tracer such as 18F-fluciclovine to evaluate patients whom standard of care imaging protocols are limited. A recent exploratory study examines the potential use of 68Ga-fibroblast activating protein (FAPI)-PET/CT in gynecological malignancies. The study presented promising results, with average SUVmax of 9.3 [31], where 18F-fluciclovine in our study demonstrated average SUVmax of 8.9. The benefit of FAP over 18F-fluciclovine is the low liver uptake for the evaluation of hepatic metastatic lesions.
The main limitation of this study is the small cohort and heterogeneity of its population. Our cohort included only 1/7 patient with initial staging. Hence, the majority of the proven malignant lesions were metastatic recurrence and only one patient had adnexal masses. As most patients with recurrent ovarian cancer underwent hysterectomy, this cohort could not evaluate the uptake of 18F-fluciclovine in benign fibroid lesions or pre-menopausal uterine uptake [31]. As well in this analysis we did not evaluate discrepancies of true positive lesions on 18F-fluciclovine PET/CT with available CI, therefore the additional value of 18F-fulicilovine over CI will need to be determined in a dedicated cohort. Despite its limitations, the presented data is promising, with high tumor to background ratios. We believe that this exploratory study should be examined in a large clinical trial to evaluate its potential clinical use in patients with ovarian cancer. This preliminary data can assist in the future design of a larger cohort to evaluate the use of 18F-fluciclovine in patients with ovarian cancer.
Conclusions
18F-Fluciclovine uptake in malignant ovarian lesions was above background levels suggesting its feasibility in the imaging of ovarian cancer. Due to increasing bladder activity over time, early imaging at 4 min post injection is favorable.
References
American Cancer Society (n.d.) Key statistics for ovarian cancer. https://www.cancer.org/cancer/ovarian-cancer/about/key-statistics.html. Accessed 12 Jan 2023
Hu TWY, Nie D, Gou JH, Li ZY (2018) Predictive significance of preoperative CT findings for suboptimal cytoreduction in advanced ovarian cancer: a meta-analysis. Cancer Manag Res 10:2019–2030
National Comprehensive Cancer Network (n.d.) NCCN guidelines. Ovarian cancer https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf. Accessed 12 Jan 2023
Forstner R, Hricak H, Occhipinti KA, Powell CB, Frankel SD, Stern JL (1995) Ovarian cancer: staging with CT and MR imaging. Radiology 197:619–626
Nougaret S, Addley HC, Colombo PE et al (2012) Ovarian carcinomatosis: how the radiologist can help plan the surgical approach. Radiographics 32:1775–1800 discussion 1800-1773
Forstner R, Meissnitzer M, Cunha TM (2016) Update on imaging of ovarian cancer. Curr Radiol Rep 4:31
Khiewvan B, Torigian DA, Emamzadehfard S et al (2017) An update on the role of PET/CT and PET/MRI in ovarian cancer. European journal of nuclear medicine and molecular imaging 44:1079–1091
Feng Z, Liu S, Ju X et al (2021) Diagnostic accuracy of (18)F-FDG PET/CT scan for peritoneal metastases in advanced ovarian cancer. Quant Imaging Med Surg 11:3392–3398
Rusu G, Achimaș-Cadariu P, Piciu A, Căinap SS, Căinap C, Piciu D (2021) A comparative study between 18F-FDG PET/CT and conventional imaging in the evaluation of progressive disease and recurrence in ovarian carcinoma. Healthcare (Basel) 9(6):666
Forstner R (2021) Imaging of ovarian cancer: from early detection to post-treatment relapse. EMJ Radiol:21–00086
Ganapathy V, Thangaraju M, Prasad PD (2009) Nutrient transporters in cancer: relevance to Warburg hypothesis and beyond. Pharmacol Ther 121:29–40
Washburn LC, Sun TT, Byrd B, Hayes RL, Butler TA (1979) 1-Aminocyclobutane[11C]carboxylic acid, a potential tumor-seeking agent. J Nucl Med 20:1055–1061
Okudaira H, Oka S, Ono M et al (2014) Accumulation of trans-1-amino-3-[(18)F]fluorocyclobutanecarboxylic acid in prostate cancer due to androgen-induced expression of amino acid transporters. Mol Imaging Biol 16:756–764
Fuchs BC, Bode BP (2005) Amino acid transporters ASCT2 and LAT1 in cancer: partners in crime? Semin Cancer Biol 15:254–266
Kaira K, Nakamura K, Hirakawa T et al (2015) Prognostic significance of L-type amino acid transporter 1 (LAT1) expression in patients with ovarian tumors. Am J Transl Res 7:1161–1171
Sato K, Miyamoto M, Takano M, Furuya K, Tsuda H (2019) Significant relationship between the LAT1 expression pattern and chemoresistance in ovarian clear cell carcinoma. Virchows Archiv 474:701–710
Guo H, Xu Y, Wang F et al (2018) Clinical associations between ASCT2 and p-mTOR in the pathogenesis and prognosis of epithelial ovarian cancer. Oncol Rep 40:3725–3733
Lapela M, Leskinen-Kallio S, Varpula M et al (1995) Metabolic imaging of ovarian tumors with carbon-11-methionine: a PET study. J Nucl Med 36:2196–2200
Schuster DM, Nieh PT, Jani AB et al (2014) Anti-3-[(18)F]FACBC positron emission tomography-computerized tomography and (111)In-capromab pendetide single photon emission computerized tomography-computerized tomography for recurrent prostate carcinoma: results of a prospective clinical trial. J Urol 191:1446–1453
Nye JA, Schuster DM, Yu W, Camp VM, Goodman MM, Votaw JR (2007) Biodistribution and radiation dosimetry of the synthetic nonmetabolized amino acid analogue anti-18F-FACBC in humans. J Nucl Med 48:1017–1020
Lovrec P, Schuster DM, Wagner RH, Gabriel M, Savir-Baruch B (2020) Characterizing and mitigating bladder radioactivity on (18)F-fluciclovine PET/CT. J Nucl Med Technol 48:24–29
Andriole GL, Kostakoglu L, Chau A et al (2018) The impact of positron emission tomography with (18)F-fluciclovine on the management of patients with biochemical recurrence of prostate cancer: results from the LOCATE trial. J Urol. 201(2):322–331
Robertson MS, Sakellis CG, Hyun H, Jacene HA (2020) Extraprostatic uptake of (18)F-fluciclovine: differentiation of nonprostatic neoplasms from metastatic prostate cancer. AJR Am J Roentgenol 214:641–648
Savir-Baruch B, Zanoni L, Schuster DM (2018) Imaging of prostate cancer using fluciclovine. Urol Clin North Am 45:489–502
Savir-Baruch B, Banks KP, McConathy JE et al (2018) ACR-ACNM practice parameter for the performance of fluorine-18 fluciclovine-PET/CT for recurrent prostate cancer. Clin Nucl Med 43:909–917
Tade FI, Sajdak RA, Gabriel M, Wagner RH, Savir-Baruch B (2019) Best practices for (18)F-fluciclovine PET/CT imaging of recurrent prostate cancer: a guide for technologists. J Nucl Med Technol 47:282–287
PYLARIFY® (piflufolastat F 18) injection, for intravenous use initial U.S. approval: 2021 https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214793s000lbl.pdf. Accessed 12 Jan 2023
CERIANNA™ (fluoroestradiol F 18) injection, for intravenous use initial U.S. approval: 2020 https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212155s000lbl.pdf. Accessed 12 Jan 2023
NETSPOT (kit for the preparation of gallium Ga 68 dotatate injection), for intravenous use initial U.S. approval: 2016. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208547s000lbl.pdf. Accessed 12 Jan 2023
Schuster D, Votaw J, Nieh P et al (2007) Initial experience with the radiotracer anti-1-amino-3-F-18-fluorocyclobutane-1-carboxylic acid with PET/CT in prostate carcinoma. J Nucl Med 48:56–63
Dendl K, Koerber SA, Finck R et al (2021) (68)Ga-FAPI-PET/CT in patients with various gynecological malignancies. Eur J Nucl Med Mol Imaging 48:4089–4100
Acknowledgements
This study is in memory of Adi Ran-Cohen, MD (1980–2017) OB/GYN resident physician and mother who bravely fought ovarian cancer.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The senior author and principal investigator, Bital Savir-Baruch, MD, has received research grants from Blue Earth Diagnostics (BED). She has also served as a consultant for Blue Earth Diagnostics (BED), General Electric, and Curium. No other potential conflicts of interest relevant to this article exist.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Buehner, T.M., Liotta, M., Potkul, R.K. et al. Initial Experience with the Radiotracer 18F-Fluciclovine PET/CT in Ovarian Cancer. Mol Imaging Biol 26, 45–52 (2024). https://doi.org/10.1007/s11307-023-01807-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-023-01807-8