Abstract
Purpose
The purpose of this study was to evaluate the safety, dosimetry, and apparent receptor occupancy (RO) of [64Cu]DOTA-patritumab, a radiolabeled monoclonal antibody directed against HER3/ERBB3 in subjects with advanced solid tumors.
Procedures
Dosimetry subjects (n = 5) received [64Cu]DOTA-patritumab and underwent positron emission tomography (PET)/X-ray computed tomography (CT) at 3, 24, and 48 h. Evaluable RO subjects (n = 3 out of 6) received [64Cu]DOTA-patritumab at day 1 and day 8 (after 9.0 mg/kg patritumab) followed by PET/CT at 24 h post-injection. Endpoints included safety, tumor uptake, and efficacy.
Results
The tumor SUVmax (± SD) was 5.6 ± 4.5, 3.3 ± 1.7, and 3.0 ± 1.1 at 3, 24, and 48 h in dosimetry subjects. The effective dose and critical organ dose (liver) averaged 0.044 ± 0.008 mSv/MBq and 0.46 ± 0.086 mGy/MBq, respectively. In RO subjects, tumor-to-blood ratio decreased from 1.00 ± 0.32 at baseline to 0.57 ± 0.17 after stable patritumab, corresponding to a RO of 42.1 ± 3.
Conclusions
[64Cu]DOTA-patritumab was safe. These limited results suggest that this PET-based method can be used to determine tumor-apparent RO.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human epidermal growth factor receptor 3 (HER3) is expressed in a variety of solid tumors of epithelial origin (e.g., non-small cell lung, breast, colon, and ovarian cancers) and is a novel target for cancer therapy [1, 2]. Patritumab (U3-1287, AMG 888), a first-in-class fully human anti-HER3 monoclonal antibody (immunoglobulin G, subclass 1), binds the extracellular domain of HER3 and promotes receptor internalization, leading to the inhibition of basal and ligand-induced HER3 activation and downstream signaling [3, 4]. In tumor cell models, patritumab reduced cellular migration, proliferation, and anchorage-independent growth [3, 4]. In phase 1 studies, patritumab (up to 20 mg/kg) produced mild adverse events (fatigue, diarrhea, nausea, decreased appetite, dysgeusia), stable disease as the best response in 50.9 % of the subjects, and no dose-limiting toxicities (DLTs) [5].
We previously reported the results of a microPET study with [64Cu]DOTA-patritumab in athymic nu/nu mice with xenograft tumors (BxPC3, a human pancreatic adenocarcinoma). We designed a study to evaluate the tumor uptake of the radiolabeled conjugate, as well as apparent tumor HER3-receptor occupancy [6]. In animals given 0.5 μg of [64Cu]DOTA-patritumab, microPET imaging showed an intense xenograft tumor uptake of the tracer at 24 and 48 h following injection. In addition, after co-injection of 800 μg of patritumab, the tracer uptake in the tumor was reduced by more than 50 % at 24 h.
Based on the microPET findings, this phase 1 study was performed to determine whether a positron emission tomography (PET) with [64Cu]DOTA-patritumab could predict HER3-receptor occupancy for a given serum concentration of patritumab in subjects with advanced solid tumors. The safety and antitumor efficacy of [64Cu]DOTA-patritumab were also assessed.
Materials and Methods
Study Design
This study (ClinicalTrials.gov NCT01479023) was performed under an investigator-sponsored IND (114334). The study was approved by the Washington University Institutional Review Board and Radioactive Drug Research Committee prior to patient enrollment. Written informed consent was obtained for each participant. This open-label study evaluated subjects with refractory, advanced solid tumors, who were expected to have HER3 expression (Supplemental Table 1). The study consisted of two parts: (1) an imaging phase that consisted of two cohorts (dosimetry subjects and receptor occupancy subjects); and (2) a patritumab monotherapy phase.
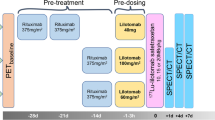
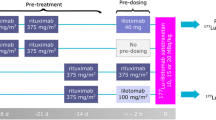
In part 1 of the study, dosimetry subjects were administered [64Cu]DOTA-patritumab (8.8–15 mCi, ≤0.2 mg DOTA-patritumab/injection) on day 1, followed by whole-body PET/CT at 3, 24, and 48 h (± 3 h) after tracer injection to evaluate dosimetry of [64Cu]DOTA-patritumab and assess tumor uptake by PET/CT (Supplemental Figure 1). Receptor occupancy (RO) subjects were administered [64Cu]DOTA-patritumab (8.1–15 mCi, ≤0.2 mg DOTA-patritumab/injection) at two separate times (days 1 and 8), followed by whole-body PET/CT at 24 h (± 3 h) after each injection. On day 1, only the [64Cu]DOTA-patritumab was administered. On day 8, each patient who had a detectable tumor uptake of [64Cu]DOTA-patritumab in the baseline study and received one infusion of an unlabeled patritumab (9.0 mg/kg) given over approximately 60 min. This administration was followed approximately 3 h later by an injection over approximately 10 min of [64Cu]DOTA-patritumab to quantify the tumor uptake of [64Cu]DOTA-patritumab in order to assess apparent HER3-receptor occupancy for a given plasma concentration of the unlabeled patritumab (Supplemental Figure 1).
In part 2, all dosimetry subjects (starting on day 8) and RO subjects with detectable tumors (starting on day 29) could receive the unlabeled patritumab monotherapy (18.0 mg/kg loading dose, followed by 9.0 mg/kg every 3 weeks) until disease progression, unacceptable toxicity, withdrawal of consent, or death.
[64Cu]DOTA-Patritumab Preparation and Dosing
Cu-64 (half-life = 12.7 h, β+ = 17 %, β− = 40 %) was produced at the Washington University cyclotron facility. [7] [64Cu]DOTA-patritumab was prepared in compliance with the Good Manufacturing Practices by previously published methods in the Biologic Therapy Core Facility of the Siteman Cancer Center at Washington University [7, 8]. The [64Cu]DOTA-patritumab was produced using aseptic techniques with radiochemical purity (required to be ≥95 %) determined by radioactive thin-layer chromatography (Bioscan) [8]. The final product contained 10–30 mCi of [64Cu]DOTA-patritumab with specific activity ranging from 100–400 μCi/μg.
The human radiation dose estimates for [64Cu]DOTA-patritumab were extrapolated from animal biodistribution data in non-tumor-bearing nu/nu female mice [6]. The estimated effective dose (ED) was 0.035 and 0.046 mSv/MBq (0.13 and 0.17 rem/mCi) for men and women, respectively. The critical organ dose was 0.21 mGy/MBq (0.76 rad/mCi) to the liver. Based on the expected 555 MBq (15 mCi) administered activity of [64Cu]DOTA-patritumab, EDs were projected to range from 19.5 to 25.5 mSv (1.95 to 2.55 rem/mCi) in men and women, respectively. A 296 MBq (8 mCi) dosage was chosen as a lower limit expected to provide an acceptable image quality.
Imaging
PET/CT was performed with the subjects in supine position with a Siemens Biograph 40 TruePoint TrueV PET/CT scanner. A spiral low-dose CT scan (120 kVp, 50 effective mAs, pitch of 1) used for attenuation correction and localization of PET findings was obtained from the top of the skull through the upper thighs. Emission images were reconstructed with the same iterative algorithm parameters throughout the study. The emission images were corrected for scatter and attenuation.
Human Dosimetry
Dosimetry cohort PET/CT images were inspected to identify organs with activity exceeding that of the background. Volumes of interest (VOIs) were drawn on the identified organs to obtain the mean radioactivity. The area under the curve (AUC) was computed as the trapezoidal sum of the observed data plus pure physical decay for the tail after the end of the scanned data. The initial organ activity (t=0) was assumed to be identical to the organ activity measured at 3 h and 6.2 % of the blood activity was assigned to the heart left ventricle content. The red marrow residence time was derived from the blood residence time by the method of Wessels, et al. [9]. The AUC to infinity was computed, multiplied by the scaled organ weight, and normalized to the injected activity to yield the organ residence time in hours. The standard organ weights were scaled, using the patient’s weight, to the standard human male body weight used in Organ Level Internal Dose Assessment/Exponential Modeling anthropomorphic adult male model [10]. Individual patient remainder of the body residence times were calculated by the maximal residence time for 64Cu (12.7 hr/ln (2)) minus the measured residence times to the lungs, kidney, spleen, liver and derived residence time to the red marrow and heart content. The resulting residence times for each patient were entered into OLINDA/EXM software to compute absorbed doses.
Image Evaluation and Determination of Apparent Receptor Occupancy
The tumor uptake of [64Cu]DOTA-patritumab at disease sites was qualitatively evaluated by a single experienced nuclear radiologist to be either detectable or non-detectable if the tumor uptake was or was not greater than that within the surrounding tissues/organs. Subjects whose tumors demonstrated a detectable uptake were considered evaluable for the RO cohort, received the unlabeled patritumab, and received a second PET/CT scan on day 9. In addition, the images were evaluated semi-quantitatively by measurement of the tumor maximum standardized uptake value (SUVmax) and the tumor-to-blood ratio (T/B). VOIs were manually drawn around the entire tumor (up to five lesions) to obtain SUVmax. This analysis was performed at all imaging time points in the dosimetry cohort and at 24 h for the RO cohort. Blood pool and liver activity was assessed as in the dosimetry subjects.
The quantitative measures of the tumor tracer uptake at 24 h were correlated with tumor HER3 expression by immunohistochemistry. In previously obtained archived diagnostic tissue samples, HER3 expression was measured by a validated immunohistochemistry (IHC) method at Mosaic Laboratories (Lake Forest, CA). HER3 (mouse clone 5A12) IHC staining in tumor cells was evaluated by a pathologist and reactivity was reported using a semiquantative H-score. The H-score was calculated as the percentage of cells at different staining intensities 0 (unstained), 1+ (weak staining), 2+ (moderate staining), and 3+ (strong staining) determined by visual assessment, with the H-score calculated using the formula 1 × (% 1 + cells) + 2 × (% 2 + cells) + 3 × (% 3 + cells) [11].
Apparent HER3-receptor occupancy was determined based on a simple competitive binding assay concept in which tumor uptake in RO participants was compared on the PET/CT obtained at baseline and that obtained after administration of the unlabeled patritumab. The ratio of tumor activity to blood pool activity was calculated for each lesion and was denoted as the volume of distribution (VT). The baseline VT was marked as VT D− (VT without dose D of an unlabeled drug) The VT in the presence of dose D of the unlabeled patritumab was noted as VT D+. At a given dose, the apparent receptor occupancy (ROapp) was calculated: ROapp = (VT D − − VT D +)/VT D −.
Pharmacokinetics
Blood samples were obtained in the dosimetry cohort for part 1 on days 1 (pre-dose, end of infusion, and 3 h post infusion) 2, 3, and 4. For part 2, samples were obtained on days 8 (pre-dose, 5 min before end of infusion, and 6 h post infusion), 9, 15, and 22, week 5, and every 3 weeks thereafter. For the RO cohort, part 1 samples were obtained on days 1 and 8 (pre-dose and end of infusion) and 2 and 9. Part 2 samples were obtained on day 29 (pre-dose and end of infusion), day 30, week 8, and every 3 weeks thereafter. Serum patritumab concentrations were measured using a validated ELISA assay method by PPD Laboratories (Richmond, VA). The PK parameters were calculated by a non-compartmental analysis using the computer software WinNonlin (Ver5.2, Pharsight Corp., CA, USA).
Response
All subjects who received at least 1 cycle of patritumab monotherapy in part 2 were evaluable for disease response, by RECIST 1.1 [12]. Best response, overall survival (OS), and mortality status were documented.
Safety
The National Cancer Institute Common Terminology Criteria for Adverse Events (v4) were used to evaluate safety [13]. DLTs were any grade 3 or higher treatment-related hematologic or non-hematologic toxicity occurring within 7 days of administration of any dose of [64Cu]DOTA-patritumab, with the exception of grade 3 neutropenia unless accompanied by fever. An intolerable dose was defined as any dose of [64Cu]DOTA-patritumab resulting in an incidence of DLT in two of six evaluable subjects.
Statistical Evaluation
Sample size for the dosimetry cohort was empirically determined and was consistent with methods previously used in this type of first-in-man radiopharmaceutical study to determine biodistribution, safety, and tolerability [14, 15]. The RO cohort sample size was originally planned to include up to five cohorts each consisting of three evaluable subjects receiving day 8 doses of patritumab ranging from 0.3 to 20 mg/kg before the second administration of [64Cu]DOTA-patritumab. Descriptive statistics (continuous and categorical) were provided for safety, demographics, patient disposition, tumor uptake, apparent receptor occupancy, and radioactivity in the blood. Changes in tumor, blood, and liver activity before and after dosing with patritumab were evaluated using a paired t test. Spearman’s rank order correlation was used to assess the relationship between tumor tracer uptake at 24 h and tumor HER3 expression by IHC.
Results
Population Characteristics
Fifteen eligible participants were enrolled in the study. PET studies could not be performed in four subjects because of the failure of the radiopharmaceutical preparation. The remaining 11 participants (7 female, 4 male; median age 51 years, range 39–78 years) underwent PET studies (dosimetry cohort, n = 5; RO cohort, n = 6) and were evaluable for safety of labeled patritumab (Supplemental Figure 2). The tumor types were colonic, endometrial, pancreatic and papillary thyroid carcinoma (two subjects each), esophageal, non-small lung, and salivary gland carcinoma (one subject each). The Eastern Cooperative Oncology Group (ECOG) performance status was zero in seven and one in the remaining four subjects. Three subjects (8, 11, and 15) enrolled in the RO cohort were evaluable and three (10, 13, and 14) were considered non-evaluable for the apparent receptor occupancy analysis because they did not have detectable disease on baseline [64Cu]DOTA-patritumab PET/CT. Eight subjects (five dosimetry, three RO) received patritumab monotherapy in part 2 and were assessed for efficacy and safety of the unlabeled treatment.
Dosimetry
The residence times and dosimetry estimates for the five dosimetry Cohort subjects are summarized in Supplemental Tables 2 and 3. The average ED was 0.043 ± 0.004 mSv/MBq (0.16 ± 0.02 rem/mCi). The critical organ was the liver with an average radiation dose of 0.44 ± 0.08 mGy/MBq (1.61 ± 0.30 rad/mCi).
Tumor Uptake of [64Cu]DOTA-Patritumab
Individual participant tumor standardized uptake value (SUV) and T/B results for the dosimetry and RO cohorts (evaluable and non-evaluable) are summarized in Table 1. In the dosimetry cohort, the average tumor SUVmax (± SD) was 5.62 ± 4.51, 3.27 ± 1.70, and 2.98 ± 1.08 at 3, 24, and 48 h, respectively. The corresponding average T/B ratio was 0.82 ± 0.74, 0.83 ± 0.46, and 1.06 ± 0.33, respectively. Based on the dosimetry cohort results, the optimal time for imaging in the RO cohort was determined to be 24 h. The average tumor SUVmax at 24 h for all 11 participants in both cohorts was 3.36 ± 1.31. The average T/B ratio was 1.1 ± 0.75.
The median tumor H-score, as a measure of HER3-receptor expression, was 158 (range 35 to 290, the H-score was unavailable for one of the 11 subjects). There was no significant correlation between the H-score and tumor uptake of [64Cu]DOTA-patritumab at 24 h expressed as either SUVmax (R = 0.37, p = 0.29) or T/B ratio (R = 0.50, p = 0.14).
Apparent Receptor Occupancy
Of the six subjects in the RO cohort, three had essentially undetectable tumor uptake and three had detectable uptake. The tumor average SUVmax at 24 h after tracer infusion at baseline and after the unlabeled patritumab dosing in the three subjects who had both scans was 2.90 ± 0.72 and 4.01 ± 1.32, respectively (Table 2). The corresponding average T/B ratio for evaluable subjects was 1.00 ± 0.32 and 0.57 ± 0.17, respectively. Accordingly, the mean apparent receptor occupancy in these three subjects was 42.1 ± 3.9 %. A representative example is shown in Fig. 1. The tumor uptake of [64Cu]DOTA-patritumab after the administration of the unlabeled patritumab was slightly, but not significantly, greater than that at baseline. Blood activity was significantly greater, but liver activity was markedly and significantly decreased (Table 2).
Transaxial lung window (top) CT of the PET/CT demonstrates masses in both lung bases. Transaxial fused PET/CT (second row) and PET (third row) images, and reprojection (bottom) PET images of patient 8 (receptor occupancy cohort) at baseline (day 2) and after predosing (day 9) with unlabeled patritumab show a greater tracer uptake in the right lung base metastatic colon cancer after unlabeled patritumab, as well as higher blood pool activity and substantially decreased hepatic uptake. The measured apparent receptor occupancy was 41.3 %.
Because of the relatively low tumor uptake in the 11 subjects studied, the investigators decided to cease recruitment subjects to additional cohorts and close the trial.
Safety
Two participants in the RO cohort who received only [64Cu]DOTA-patritumab experienced adverse events (AEs). Subject 14 experienced grade 1 dizziness and subject 10 suffered a cerebral vascular accident associated with altered mental status that was considered “unlikely related” to the study agent. Overall, AEs related to treatment with the unlabeled patritumab were reported in seven of the eight subjects who received patritumab monotherapy. Diarrhea, reported in three subjects (38 %), was the most common drug-related AE during the study. No other drug-related AE was reported in more than three subjects. Other AEs included dizziness, fatigue, headache, hypertension, and weight loss (25 % each). In all 11 participants, there were only three AEs that were grade 3: abdominal infection, cardiac troponin 1 increase, and cognitive disturbance. All other AEs were grade 1 or 2. No grade 4 or 5 AEs were observed. Two individuals withdrew from the study because of non–drug-related AEs. No DLTs were reported. The maximum dose administered in this study was 18 mg/kg.
Pharmacokinetics
The mean serum patritumab concentrations vs. time is presented in Supplemental Figure 3 by dose. The pharmacokinetics of patritumab were similar to those observed for previous studies. [5].
Treatment Efficacy
Individual patient responses are included in Table 3. The three RO cohort subjects who had undetectable tumors were considered non-evaluable for response by RECIST because they did not receive unlabeled patritumab. The best response observed was SD in three participants in the dosimetry cohort. For subjects who were evaluable for efficacy (n = 8), the median time on study was 79 days (range: 49–178).
Discussion
This study was the first clinical evaluation of the tolerability, dosimetry, and apparent receptor occupancy of [64Cu]DOTA-patritumab. These results confirmed that a clinical PET strategy is feasible. [64Cu]DOTA-patritumab had an ED of 0.043 ± 0.004 mSv/MBq (0.16 ± 0.02 rem/mCi) and a critical organ dose to the liver of 0.44 ± 0.08 mGy/MBq (1.61 ± 0.30 rad/mCi). Labeled and unlabeled patritumab were well tolerated and the observed AEs with the latter were similar to the safety results noted in previous phase I studies of patritumab [5, 16].
In our limited patient population, we were able to demonstrate a reduction in the T/B ratio after subjects received unlabeled patritumab in a dose of 9 mg/kg. The T/B ratio in the three evaluable subjects was reduced from 1.00 ± 0.32 to 0.57 ± 0.17, indicating a mean apparent receptor occupancy at this dose level of 42.1 ± 3.9 %. Enrollment into this study was terminated prior to further dose-ranging studies with unlabeled patritumab because tumor uptake of [64Cu]DOTA-patritumab was not robust with a SUVmax at 24 h after tracer infusion of 3.36 ± 1.31 in all 11 subjects studied and because tumor uptake did not correspond with HER3 expression by calculated H-scores. We did not observe changes in the tumor-to-muscle uptake ratio (T/M) associated with unlabeled patritumab dosing [17].
Despite the modest imaging results, on final analysis of T/B ratios in our limited sample size, apparent receptor occupancy was demonstrated. The simple competitive binding assay approach we used admittedly does not address potential biological complexities related to the rate of blood clearance, changes in organ or tissue uptake or receptor internalization; therefore, final conclusions must be considered conservatively. From a drug development standpoint, it is encouraging that the administered dose of patritumab in the RO cohort (9 mg/kg), which is the same as the maintenance dose of patritumab in clinical trials, did affect the apparent [64Cu]DOTA-patritumab receptor occupancy. While not tested in this study, the patritumab standard loading dose (18 mg/kg) may further increase receptor occupancy. Because the study was discontinued, we were unable to demonstrate that specific serum concentrations of patritumab led to reductions in [64Cu]DOTA-patritumab tumor uptake. However, the observed results indicate that the clinically used doses of patritumab are potentially biologically relevant for HER3 targeting.
In our subjects, the activity in the tumor increased after the administration of the unlabeled antibody whereas in our previous animal studies, tumor uptake was decreased. The most likely explanation for the difference is the timing of the administrations of the unlabeled versus the labeled doses under the two circumstances. In the animal studies, the labeled agent and the unlabeled product were simultaneously injected. Accordingly, specific binding (and any nonspecific binding) in tumor, as well as any nonspecific binding in the liver (where the greatest fractional uptake of the tracer occurs) would both be expected to decrease to an approximate equivalent extent because of competition of labeled and unlabeled antibody for binding sites.
However, because of safety and logistical constraints, the higher dosage of unlabeled patritumab was infused first in the chemotherapy suite and the lower dosage of [64Cu]DOTA-patritumab was administered in the nuclear medicine facility. To account for the need to observe the patient for adverse events during the infusion of the unlabeled antibody and the distance between the two dosing locations, a 3-h-window (actual range 171–189 min) was provided in the protocol between the unlabeled patritumab administration and the [64Cu]DOTA-patritumab administration. As shown in Table 2, the administration of the unlabeled before the labeled antibody resulted in a substantial decrease in liver activity and a corresponding increase in blood activity and a lesser increase in tumor activity. Thus, despite the occupancy of tumor receptors by the unlabeled antibody, tumor activity increased because of the higher circulating levels of the labeled antibody in the blood. The substantial liver uptake of labeled monoclonal antibodies has been observed in many clinical studies [18–20]. A validated strategy, which is used during radioimmunoscintigraphy and radioimmunotherapy, to address this observation is to administer a high dose of naked antibody to reduce nonspecific uptake by the liver and other tissues of the labeled product allowing more of the labeled product to be systemically available for uptake by the tumor [21].
Another factor possibly contributing to the modest imaging results observed in this human PET study, in comparison to the animal microPET studies, is the potential for heterogeneous tumor HER3 overexpression in our human subjects versus the xenografts (mice injected with BxPC3 cells) [6]. The BxPC3 pancreatic cancer cell line used in the microPET study is known to have high levels of HER3 expression, whereas HER3 overexpression varies from 17 to 60 % in human tumors depending on the primary origin [20]. In the current study, patient HER3 status was obtained from archived tissue. Therefore, HER3 status of target lesions was unknown at the time of treatment. Adding to the complexity, is the low density of HER3 receptors on tumor cells [22].
Conclusions
This study confirmed that the administration of [64Cu]DOTA-patritumab and unlabeled patritumab is feasible and well-tolerated. In this study, we were able to demonstrate evidence of HER3 receptor occupancy by PET in a limited patient population. Early termination of the study did not allow us to determine whether a particular serum concentration or dose of patritumab would lead to an optimal level of receptor occupancy. However, clinically used doses of patritumab did affect tumor apparent HER3 receptor occupancy, indicating that the clinically used dose is potentially a biologically relevant dose. Clinical PET studies can therefore provide potentially valuable data for difficult-to-access target tumor tissues. Future studies to improve the characterization of patritumab HER3 receptor occupancy and its relationship with serum patritumab levels should consider design modifications, such as a reduction in the time between labeled and unlabeled antibody dosing and administering a loading dose of the unlabeled antibody to reduce liver uptake of the labeled product.
References
Gullick WJ (1996) The c-erbB3/HER3 receptor in human cancer. Cancer Surv 27:339–349
Gala K, Chandarlapaty S (2014) Molecular pathways: HER3 targeted therapy. Clin Cancer Res 20:1410–1416
Treder M, Hartmann S, Ogbagabriel S et al (2008) Fully human anti- HER3 monoclonal antibodies (mAbs) inhibit oncogenic signaling and tumor cell growth in vitro and in vivo [abstract LB-20]. Presented at American Association for Cancer Research Annual Meeting; April 12–16, 2008; San Diego, CA
Freeman D, Ogbagabriel S, Rothe M et al (2008) Fully human anti-HER3 monoclonal antibodies (mAbs) have unique in vitro and in vivo functional and antitumor activities versus other HER family inhibitors [abstract LB-21]. Presented at American Association for Cancer Research Annual Meeting; April 12,16, 2008; San Diego, CA
LoRusso P, Janne PA, Oliveira M et al (2013) Phase I study of U3-1287, a fully human anti-HER3 monoclonal antibody, in patients with advanced solid tumors. Clin Cancer Res 19:3078–3087
Sharp TL, Glaus C, Fettig H et al (2011) Pharmacological evaluation of 64CU–DOTA–AMG 888 (U3-1287) in control and tumor bearing mice using biodistribution and microPET imaging [Abstract T206]. World Molecular Imaging Congress, San Diego, CA
McCarthy DW, Shefer RE, Klinkowstein RE et al (1997) Efficient production of high specific activity 64Cu using a biomedical cyclotron. Nucl Med Biol 24:35–43
Lewis MR, Kao JY, Anderson AL et al (2001) An improved method for conjugating monoclonal antibodies with N-hydroxysulfosuccinimidyl DOTA. Bioconjug Chem 12:320–324
Wessels BW, Bolch WE, Bouchet LG et al (2004) Bone marrow dosimetry using blood-based models for radiolabeled antibody therapy: a multiinstitutional comparison. J Nucl Med 45(10):1725–33
Stabin MG, Sparks RB, Crowe E (2005) OLINDA/EXM: the second-generation personal computer software for internal dose assessment in nuclear medicine. J Nucl Med 46:1023–1027
Ishibashi H, Suzuki T, Suzuki S et al (2003) Sex steroid hormone receptors in human thymoma. J Clin Endocrinol Metab 88:2309–2317
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. US Department of Health and Human Services. May 28, 2009. Available at: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf Accessed March 7, 2014
Dijkers EC, Oude Munnink TH, Kosterink JG et al (2010) Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin Pharmacol Ther 87:586–592
Tamura K, Kurihara H, Yonemori K et al (2013) 64Cu-DOTA-trastuzumab PET imaging in patients with HER2-positive breast cancer. J Nucl Med 54:1869–1875
Wakui H, Yamamoto N, Nakamichi S et al (2014) Phase 1 and dose-finding study of patritumab (U3-1287), a human monoclonal antibody targeting HER3, in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol 73:511–516
Lockhart AC, Liu Y, Dehdashti F et al (2014) A phase 1 evaluation of 64Cu-DOTA-patritumab to assess dosimetry, receptor occupancy, and safety in advanced solid tumors. Cancer Res 74:5443
Rizvi SN, Visser OJ, Vosjan MJ et al (2012) Biodistribution, radiation dosimetry and scouting of 90Y-ibritumomab tiuxetan therapy in patients with relapsed B-cell non-Hodgkin’s lymphoma using 89Zr-ibritumomab tiuxetan and PET. Eur J Nucl Med Mol Imaging 39:512–20
Wiseman GA, White CA, Stabin M et al (2000) Phase I/II 90Y-Zevalin (yttrium-90 ibritumomab tiuxetan, IDEC-Y2B8) radioimmunotherapy dosimetry results in relapsed or refractory non-Hodgkin’s lymphoma. Eur J Nucl Med 27:766–77
Goldenberg EM (1993) Monoclonal antibodies in cancer detection and therapy. Am J Med 94:297–312
Rajendran JG, Gopal AK, Fisher DR et al (2008) Myeloablative 131I-tositumomab radioimmunotherapy in treating non-Hodgkin’s lymphoma: comparison of dosimetry based on whole-body retention and dose to critical organ receiving the highest dose. J Nucl Med 49:837–44
Buck E, Eyzaquirre A, Haley JD et al (2006) Inactivation of Akt by the epidermal growth factor receptor inhibitor erlotinib is mediated by HER-3 in pancreatic and colorectal tumor cell lines and contributes to erlotinib sensitivity. Mol Cancer Ther 5:2051–9
Acknowledgments
The authors wish to thank the subjects and their families for participating in this study, Daiichi-Sankyo for supporting this research, and Akita Biomedical for assistance in writing the manuscript. We thank Deborah Sultan, Hannah Luehmann, Tom Voller, and Stephen Moerlein for the radiopharmaceutical preparation. We also wish to recognize the important contribution of the late Dr. Michael Welch. He was the driving force behind this study and is greatly missed as a colleague and mentor.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study (ClinicalTrials.gov NCT01479023) was performed under an investigator-sponsored IND (114334). The study was approved by the Washington University Institutional Review Board and Radioactive Drug Research Committee prior to patient enrollment. Written informed consent was obtained for each participant.
[64Cu]DOTA-patritumab was prepared in compliance with the Good Manufacturing Practices by previously published methods in the Biologic Therapy Core Facility of the Siteman Cancer Center at Washington University.
Conflict of Interests
Drs. Lockhart, Liu, Dehdashti, Laforest, Welch, and Siegel received research support from Daiichi-Sankyo to conduct the preclinical and clinical components of this study. Drs. Desai, Mahmood, and Mendell were Daiichi-Sankyo employees at the time that this study was conducted.
Additional information
Michael J. Welch passed away during the preparation of this study.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 537 kb)
Rights and permissions
About this article
Cite this article
Lockhart, A.C., Liu, Y., Dehdashti, F. et al. Phase 1 Evaluation of [64Cu]DOTA-Patritumab to Assess Dosimetry, Apparent Receptor Occupancy, and Safety in Subjects with Advanced Solid Tumors. Mol Imaging Biol 18, 446–453 (2016). https://doi.org/10.1007/s11307-015-0912-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-015-0912-y