Abstract
Purpose
We measured the whole-body distribution of IV-injected [11C]GSK215083, a new 5-HT6 antagonist PET tracer, as a function of time in adult subjects, in order to determine the radiation exposure.
Procedures
After injection with a single bolus of [11C]GSK215083 (range 330–367 MBq; mean 346 MBq), PET emission data were acquired for approximately 120 min in six subjects (three males and three females). Five organs were identified as exhibiting uptake above background. For these, regions of interest were delineated on emission images, and time–activity curves (TAC) generated. Residence times were calculated as the area under the curve of the TAC, normalized to injected activities and standard values of organ volumes. Dosimetry calculations were then performed using the computer program OLINDA/EXM 1.0.
Results
The mean effective dose averaged over both males and females (±standard deviation) was estimated to be 7.7 ± 1.0 μSv/MBq (male 7.0 ± 0.4; female 8.5 ± 0.6). For the effective dose equivalent, the corresponding values are 7.8 ± 1.2 μSv/MBq (male 6.8 ± 0.5; female 8.9 ± 0.1). The organ receiving the highest dose was the lung, with an average equivalent dose of 25.6 ± 6.9 μSv/MBq (male 20.8 ± 5.6; female 30.4 ± 4.4).
Conclusion
The estimated radiation dose for [11C]GSK215083 is consistent with those for other neuroreceptor ligands labeled with carbon-11. The somewhat higher dose estimate for females compared to males may reflect the difference in observed residence times and representative differences in the male and female phantoms used for dosimetry calculations. Based on conventionally accepted dose limits, [11C]GSK215083 may be used for multiple PET scans in the same subject.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The 5-hydroxytryptamine 6 receptor (5-HT6 R) is a comparatively recent addition to the serotonin receptor family, identified by two independent groups in 1993, using molecular cloning [1, 2]. 5-HT6 R is a seven-transmembrane domain guanine nucleotide-binding protein (G protein)-coupled receptor, which is positively coupled to adenylate cyclase. The 5-HT6 R is localized in the striatum, hippocampus, and cortex [1, 2] and is virtually absent outside the CNS, though 5-HT6 messenger RNA has been found in the peripheral blood mononuclear cells of rhesus macaque monkeys [3] and the lymphoid tissue and lymphocytes of rats [4]. The physiological significance of these findings is unclear, and no reports of similar findings in humans are available.
The 5-HT6 R activity modulates acetylcholinergic [5–8] and dopaminergic neurotransmission [8], and has been implicated in spatial learning and memory [9–11]. It has been proposed as a drug target for a number of neuropsychiatric conditions [12], with an increasing focus on the treatment of cognitive disorders [13].
11C-GSK215083 [11C-N-methyl]3-[(3-fluorophenyl)sulfonyl]-8-(4-methyl-1-piperazinyl) quinoline, is a selective positron emission tomography (PET) radioligand for the in vivo measurement of central 5-HT6 R availability [14, 15]. The aim of this study was to collect whole-body human biodistribution data to estimate the radiation exposure following intravenous administration of [11C]GSK215083.
Subjects and Methods
Subjects
The study population consisted of six healthy subjects (three males and three females, age 18–31; mean 25.5 years; SD 4.42). Suitability for participation including the absence of clinically significant illness or disease was assessed by interview, physical examination, electrocardiogram, vital signs measurements, routine blood tests, pregnancy test, and a drug and alcohol screen. This study was approved by the research ethics board for human subjects at the Centre for Addiction and Mental Health, Toronto, Canada.
Radiochemistry
[11C]-methylation of the normethyl precursor SB 792988A (hydrochloride salt) with [11C]-iodomethane was carried out inside an HPLC sample loop using our previously described “loop” method [16, 17]. The precursor (1.0 mg) was dissolved in a mixture of DMF (77 μl) and aqueous sodium bicarbonate (1 N, 7 μl), vortexed for 1 min and reacted on the loop for 1 min at RT with trapped [11C]-CH3I. Following HPLC purification (Phenomenex LunaC18, 250 × 10 mm; 40/60 CH3CN/H2O + 0.1 N amm. formate, 8 ml/min), volatiles were removed under vacuum in a rotary evaporator at 70°C. Upon formulation in buffered saline, [11C]-GSK215083 was obtained in 20–25% radiochemical yield (uncorrected for decay, from [11C]-CO2) in 24 min post end-of-bombardment.
PET/CT Scanning
Scans were performed using a Siemens-Biograph HiRez XVI PET camera system (Siemens Molecular Imaging, Knoxville, TN, USA), the performance characteristics of which have been described previously [18]. Following the acquisition of a scout view for accurate positioning, subjects underwent transmission CT (140 kVp, 30 mA, scan range 104 cm) After a single intravenous bolus injection of [11C]GSK215083 (injected activity 330–367 MBq; mean 346; SD 15), serial whole-body PET scans were acquired from head to midthigh for a period of up to 120 min, as a series of overlapping bed positions with a progressive increase of scan duration per bed position (positions 1 and 2, 15 s; 3 and 4, 30 s; 5 and 6, 60 s; 7, 120 s; 8, 180 s; 9, 240 s). Each subject underwent nine whole-body PET acquisitions with eight or nine bed positions per whole-body acquisition depending upon their height (two subjects having nine bed positions per scan), resulting in an acquisition duration of 510 or 750 s per whole-body scan. Subjects remained in position on the scanning bed for the duration of the scanning period. The difference between the time in the scanner and the duration of image acquisition is due to the time required to move between bed positions and the time between the end of one whole-body acquisition and the start of the next.
Data were normalized, gap filled, dead time corrected, arc corrected (both radial and axial), and converted from 3D to 2D using FORE [19] prior to reconstruction using the direct inverse Fourier transform algorithm [20]. A Gaussian filter was applied postreconstruction with an ‘FWHM’ of 5 mm in the x, y, and z directions. The attenuation correction was derived from a CT-derived mu-map, and the 3D scatter correction was model based [21].
Regions of Interest
Summed images (0–120 min) were visually inspected, tissues exhibiting uptake above background uptake were identified, and regions of interest (ROIs) were delineated on corresponding emission images. ROIs were drawn manually on these organs using Analyze software (Mayo Clinic) to obtain the mean activity concentration in each region and each pass (within subject, the same ROIs were applied to data from all passes). Included organs were the brain, heart, liver, lung, and stomach. For each subject summed images for different time ranges were used to draw the ROIs depending on the observed uptake in the different organs. For regions with low uptake, such as the lung early add images were used in conjunction with the CT to guide the definition of the ROI. The location of all organs was verified using the CT image. Finally the position of each ROI in each subject was visually inspected for all frames to ensure that the ROI was in the corresponding organ
Residence Time and Absorbed Dose Calculations
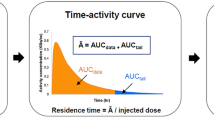
Mean residence times (MRTs) for each organ were calculated as follows (Eq. 1):
where AUC is the area under the curve of the nondecay corrected time–activity curve (TAC) and V organ is the tabulated organ volume as used in the OLINDA/EXM® version 1.0 [22].
Depending on the shape of the TAC, two cases were distinguished (see Fig. 2): for monotonically decaying TACs (e.g., lungs and heart), a two exponential model \( \left( {{\text{TAC}} = {A_1}{e^{{ - t\beta 1}}} + {A_2}{e^{{ - t\beta 2}}}} \right) \) was fitted to the TAC and the resulting parameters were used to analytically calculate the AUC \( \left( {{\text{AUC}} = {A_1}/{\beta_1} + {A_2}/{\beta_2}} \right) \). For nonmonotonic TACs the AUC was calculated using trapezoidal integration over the duration of the TAC plus a term that represents solely radioactive decay starting from the last point in the TAC.
The residence time of the remainder (assumed to be homogeneously distributed in the body) was obtained by subtracting the sum of all defined organ residence times from the inverse of the decay constant for 11C. MRTs for the organs mentioned above were entered into the dose estimation software OLINDA|EXM® version 1.0 [22] to calculate the radiation dose to individual organs, and resulting effective dose equivalents and effective doses (ED).
Since ICRP 103 tissue-weighting factors (W T) are not implemented in OLINDA/EXM 1.0, the male and female EDs are based on W T from ICRP-60. Extraction of organ dose data from OLINDA/EXM 1.0 and application of W T from ICRP 103 are possible but not straightforward as phantoms used in OLINDA/EXM 1.0 lack some of the organs used in the ICRP 103 scheme.
Results
Subject demographic data and 11C-GSK215083 injection parameters are given in Table 1. Whole-body PET images from a representative subject are displayed in Fig. 1, and time activity curves in Fig. 2. Mean residence times are displayed in Table 2. Organ absorbed doses, effective dose equivalent, and effective dose are displayed in Table 3. In all subjects, the heart and lung decreased monotonically, whereas the brain, stomach, and liver were unimodal, reaching peak values approximately 3, 15, and 20 min after injection.
The mean effective dose averaged over both males and females (±standard deviation) was estimated to be 7.7 ± 1.0 μSv/MBq (male 7.0 ± 0.4; female 8.5 ± 0.6). For the effective dose equivalent, the corresponding value is 7.8 ± 1.2 μSv/MBq (male 6.8 ± 0.5; female 8.9 ± 0.1).
Discussion
We estimated the radiation dosimetry of 11C-GSK215083 from data acquired with whole-body PET in six human subjects. The observed biodistribution most likely reflects rapid distribution in the intravascular compartment, followed by pulmonary uptake and disposition in tissues according to their blood perfusion rate and affinity for the tracer; data suggest distribution to the liver followed by excretion via the GI tract.
The estimated radiation doses are consistent with those for other neuroreceptor ligands labeled with carbon-11 [23]. The somewhat higher doses in females compared to males reflect the difference in observed residence times and representative differences in the male and female phantoms used for dosimetry calculations.
Restrictions on radiation exposures differ from country to country, though most are based on the recommendations of the ICRP [24, 25]. European Union member states have adopted the guidance in ICRP Publication 62 [26]. Following the World Health Organization categorisation of risk levels, effective dose constraints no greater than 10 mSv per study are recommended where research subjects do not receive a direct benefit from participation. As a result dose constraints implemented for PET research studies in healthy volunteers are typically somewhat lower than ICRP-recommended occupational effective dose limits (20 mSv per year, averaged over 5 years, not exceeding 50 mSv in a single year).
For studies performed in the USA, the maximum allowable single dose to the whole-body, blood-forming organs, lens of the eye, or gonads is 30 mSv, with a maximum annual dose of 50 mSv. The maximum allowable single and annual doses to all other organs are 50 and 150 mSv, respectively [27]. In the case of [11C]GSK215083, the lung is the organ with the highest equivalent dose (the “critical organ”); for a single study, the lung is dose limiting, in that the RDRC organ limit of 50 mSv would be reached before the whole-body limit of 30 mSv. For cumulative exposures, in males the maximum annual injected activity is driven by the whole-body limit of 50 mSv; whereas in females who have a slightly higher lung exposure, the lung remains dose limiting, in that the 150-mSv organ dose limit would be exceeded before the 50-mSv whole-body limit (Table 4).
When planning studies using PET–CT exposure from CT scanning should be considered. In this study subjects were scanned from the from the top of the head to the midthigh for the purpose of anatomical localisation and attenuation correction; the resulting exposure from the CT was estimated at 5.1 mSv, in a typical PET study involving a single head CT for attenuation correction exposure would be in the region of 0.2 mSv (depending on the scanner and protocol used). Recent work suggests that injected doses of ca. 370 MBq of [11C]GSK215083 (ED 2.8 mSv) will be sufficient to quantify central 5-HT6 receptors (Parker C., submitted). In conclusion [11C]GSK215083 may be used in multiple PET scans in the same subject within conventionally accepted dose limits, facilitating its use in receptor occupancy studies to support drug development.
References
Ruat M, Traiffort E, Arrang JM, Tardivel-Lacombe J, Diaz J, Leurs R et al (1993) A novel rat serotonin (5-HT6) receptor: molecular cloning, localization and stimulation of cAMP accumulation. Biochem Biophys Res Commun 193(1):268–276
Monsma FJ, Shen Y, Ward RP, Hamblin MW, Sibley DR (1993) Cloning and expression of a novel serotonin receptor with high affinity for tricyclic psychotropic drugs. Mol Pharmacol 43(3):320–327
Yang G, Qiu C, Zhao H, Liu Q, Shao Y (2006) Expression of mRNA for multiple serotonin (5-HT) receptor types/subtypes by the peripheral blood mononuclear cells of rhesus macaques. J Neuroimmunol 178(1–2):24–29
Stefulj J, Jernej B, Cicin-Sain L, Rinner I, Schauenstein K (2000) mRNA expression of serotonin receptors in cells of the immune tissues of the rat. Brain Behav Immun 14(3):219–224
Bourson A, Borroni E, Austin RH, Monsma FJ, Sleight AJ (1995) Determination of the role of the 5-ht6 receptor in the rat brain: a study using antisense oligonucleotides. J Pharmacol Exp Ther 274(1):173–180
Bourson A, Boess FG, Bös M, Sleight AJ (1998) Involvement of 5-HT6 receptors in nigro-striatal function in rodents. Br J Pharmacol 125(7):1562–1566
Bentley JC, Bourson A, Boess FG et al (1999) Investigation of stretching behaviour induced by the selective 5-HT6 receptor antagonist, Ro 04–6790, in rats. Br J Pharmacol 126(7):1537–1542
Lacroix LP, Dawson LA, Hagan JJ, Heidbreder CA (2004) 5-HT6 receptor antagonist SB-271046 enhances extracellular levels of monoamines in the rat medial prefrontal cortex. Synapse 51(2):158–164
Woolley ML, Bentley JC, Sleight AJ, Marsden CA, Fone KC (2001) A role for 5-ht6 receptors in retention of spatial learning in the Morris water maze. Neuropharmacology 41(2):210–219
Rogers DC, Hagan JJ (2001) 5-HT6 receptor antagonists enhance retention of a water maze task in the rat. Psychopharmacology (Berl) 158(2):114–119
Perez-García G, Meneses A (2005) Oral administration of the 5-HT6 receptor antagonists SB-357134 and SB-399885 improves memory formation in an autoshaping learning task. Pharmacol Biochem Behav 81(3):673–682
Woolley ML, Marsden CA, Fone KCF (2004) 5-ht6 receptors. Curr Drug Targets CNS Neurol Disord 3(1):59–79
Upton N, Chuang TT, Hunter AJ, Virley DJ (2008) 5-HT6 receptor antagonists as novel cognitive enhancing agents for Alzheimer’s disease. Neurotherapeutics 5(3):458–469
Martarello L, Cunningham VJ, Matthews JC, Rabiner E, Jakobsen S, Gee AD (2005) Radiolabelling and in vivo evaluation of [11C]GSK215083 as potential PET radioligand for the 5-HT6 receptor in the porcine brain. J Cereb Blood Flow Metab 25(S1):S598
Parker CA, Cunningham V, Martarello L et al (2008) Evaluation of the novel 5-HT6 receptor radioligand, [11C]GSK-215083 in human. NeuroImage 41(Supplement 2):T20
Wilson AA, DaSilva JN, Houle S (1994) Facile radiolabelling and purification of 2β-[O-11CH3]-carbomethoxy-3β-aryltropanes: radiotracers for the dopamine transporter. J Label Compd Radiopharm 34:759–765
Wilson AA, Garcia A, Jin L, Houle S (2000) Radiotracer synthesis from [(11)C]-iodomethane: a remarkably simple captive solvent method. Nucl Med Biol 27(6):529–532
Brambilla M, Secco C, Dominietto M, Matheoud R, Sacchetti G, Inglese E (2005) Performance characteristics obtained for a new 3-dimensional lutetium oxyorthosilicate-based whole-body PET/CT scanner with the National Electrical Manufacturers Association NU 2–2001 standard. J Nucl Med 46(12):2083–2091
Defrise M, Kinahan PE, Townsend DW, Michel C, Sibomana M, Newport DF (1997) Exact and approximate rebinning algorithms for 3D-PET data. IEEE Trans Med Imaging 16(2):145–158
Hamill JJ, Hawman EG (1995) Evaluating a frequency–space SPECT reconstruction algorithm. SPIE vol. 2622. Proc Opt Eng Midwest 95:785–791
Watson CC, Newport D, Casey ME, de Kemp RA, Beanlands RS, Schmand M (1997) Evaluation of simulation-base scatter correction for 3D PET cardiac imaging. IEEE Trans Nucl Sci 44(1):90–97
Stabin MG, Sparks RB, Crowe E (2005) OLINDA/EXM: the second-generation personal computer software for internal dose assessment in nuclear medicine. J Nucl Med 46(6):1023–1027
van der Aart J, Hallett WA, Rabiner EA, Passchier J, Comley RA (2011) Radiation dose estimates for carbon-11-labelled PET tracers. Nucl Med Biol. 2011 Oct 25.[Epub ahead of print]
ICRP (1991) 1990 Recommendations of the International Commission on Radiological Protection. Ann ICRP 21(1–3):1–201
ICRP (2007) The 2007 Recommendations of the International Commission on Radiological Protection. ICRP Publication 103. Ann ICRP 37(2–4):1–332
ICRP (1991) Radiological Protection in Biomedical Research. A report of Committee 3 adopted by the International Commission on Radiological Protection. Ann ICRP 22(3):1–28, v–xxiv
“Radioactive drugs for certain research uses.” Code of Federal Regulations Title 21, Part 361.1 2011. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=361.1 Accessed 22 June 2011
Acknowledgments
The authors would like to gratefully acknowledge the help of the following staff at the CAMH PET center who were instrumental in conducting this piece of work: Peter Bloomfield, Jeannie Fong, Armando Garcia, Winston Stableford, and Min Wong. In addition we would like to acknowledge the support of Jan Passchier of GlaxoSmithKline for the many helpful conversations over the years concerning the role of radiation protection in our research studies.
Conflict of Interest
At the time this work was conducted. RAC, CS, WH, NK, EAR and ML were employees of and owners of stock/options in GlaxoSmithKline. AAW and SH have received grants from GlaxoSmithKline.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Comley, R.A., Salinas, C., Mizrahi, R. et al. Biodistribution and Radiation Dosimetry of the Serotonin 5-HT6 Ligand [11C]GSK215083 Determined from Human Whole-Body PET. Mol Imaging Biol 14, 517–521 (2012). https://doi.org/10.1007/s11307-011-0523-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-011-0523-1