Abstract
Introduction
Oxygen is essential for metabolic processes and in the absence thereof alternative metabolic pathways are required for energy production, as seen in marine invertebrates like abalone. Even though hypoxia has been responsible for significant losses to the aquaculture industry, the overall metabolic adaptations of abalone in response to environmental hypoxia are as yet, not fully elucidated.
Objective
To use a multiplatform metabolomics approach to characterize the metabolic changes associated with energy production in abalone (Haliotis midae) when exposed to environmental hypoxia.
Methods
Metabolomics analysis of abalone adductor and foot muscle, left and right gill, hemolymph, and epipodial tissue samples were conducted using a multiplatform approach, which included untargeted NMR spectroscopy, untargeted and targeted LC–MS spectrometry, and untargeted and semi-targeted GC-MS spectrometric analyses.
Results
Increased levels of anaerobic end-products specific to marine animals were found which include alanopine, strombine, tauropine and octopine. These were accompanied by elevated lactate, succinate and arginine, of which the latter is a product of phosphoarginine breakdown in abalone. Primarily amino acid metabolism was affected, with carbohydrate and lipid metabolism assisting with anaerobic energy production to a lesser extent. Different tissues showed varied metabolic responses to hypoxia, with the largest metabolic changes in the adductor muscle.
Conclusions
From this investigation, it becomes evident that abalone have well-developed (yet understudied) metabolic mechanisms for surviving hypoxic periods. Furthermore, metabolomics serves as a powerful tool for investigating the altered metabolic processes in abalone.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The key goal of intermediary metabolism is to uphold adenosine triphosphate (ATP) supply to ensure that living cells can grow, reproduce, and respond to stresses (Salway 2004). In unstressed circumstances, basal metabolism is dominated by anabolic activity that synthesizes macromolecules leading to cell growth, division and repair (Garrett and Grisham 2010). However, during hypoxic conditions, catabolic processes are activated to ensure the availability of energy for mechanisms related to survival, recovery and maintenance of cell homeostasis (Sokolova et al. 2012). Energy levels are partially managed through suppression of energy consuming reactions (oxy-conforming) and activation of oxygen-independent metabolic pathways, like phosphagen breakdown and anaerobic glycolysis (Storey 2005). Invertebrates have well defined anaerobic metabolic pathways capable of energy production during hypoxia (Gäde et al. 1984; Gäde and Grieshaber 1986; Fields and Storey 1987; Gäde 1988; Grieshaber et al. 1993; Carroll and Wells 1995; O’omolo et al. 2003; Storey 2004; Müller et al. 2012; Liu et al. 2014). Despite this, knowledge on the holistic metabolic response of abalone during hypoxic conditions is scarce. The interplay of anaerobic pathways and the mechanisms behind the many accumulated anaerobic end-products in marine invertebrates are understudied, not to mention the interplay between different tissues.
Abalone are single-shelled, marine mollusks, which have large commercial value and in South Africa, Haliotis midae farming generates the largest aquaculture-based income (Britz and Venter 2016). Consequently, abalone aquaculture is an important research field, with a central focus on increasing farming outputs and reducing animal losses. Hypoxia is responsible for significant losses to the aquaculture industry as it not only results in animal mortalities, but also causes a significant decline in growth (in surviving animals). Environmental hypoxia is typically experienced on abalone farms when animals are transported out of water for an extended period of time, such as during size grading procedures (Hooper et al. 2014), or during periods of reduced water flow or higher water temperatures. Moreover, hypoxia also occurs in the natural marine environment, which severely impacts growth and survival. This was previously demonstrated when harmful algal blooms caused massive abalone stock losses in South Africa, attributed to anoxia (Pitcher and Calder 2000; Mouton 2017). Considering this, a better understanding of the biochemical responses of abalone is crucial to assist with recovery or to prevent metabolic resource depletion when abalone are subjected to hypoxic episodes (Cook 2014).
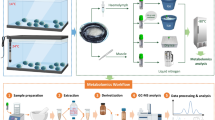
The aim of this study was to elucidate the metabolic response of H. midae subjected to environmental hypoxia; and to study the interplay of different abalone tissues using metabolomics. A multiplatform metabolomics approach (Fig. 1) was used to investigate the metabolite profiles in adductor muscle, foot muscle, epipodial tissue, hemolymph, left and right gill samples, and included the use of: untargeted nuclear magnetic resonance (NMR) spectroscopy; untargeted ultra-performance liquid chromatography–ion mobility-quadrupole time of flight spectrometry (UPLC–IM-QTOF); untargeted gas chromatography–time of flight spectrometry (GC–TOF); semi-targeted liquid chromatography–quadrupole time of flight mass spectrometry (LC–QTOF) analyses of butylated esters; semi-targeted gas chromatography–mass spectrometry-detector (GC–MSD) analyses of methylated fatty acids (FAMEs) and targeted liquid chromatography–tandem mass spectrometry (LC–MS/MS).
Summary of the experimental workflow. Adductor and foot muscle, right and left gill, epipodial tissue and hemolymph samples were collected after a hypoxia experiment and prepared for analysis with multiple analytical techniques. The results were processed and pathway interpretation performed to highlight the most affected metabolic pathways
2 Materials and methods
Detailed information regarding the abalone dissection procedure, chemicals and reagents, preparation of standards and solutions, sample preparation for specific platform analysis, sample analysis, data extraction processes and the procedures followed to identify detected compounds can be found in the online supplementary material.
2.1 Experimental setup and animals
Adult abalone, H. midae (n = 12) were collected during the winter season from a single raceway at HIK Abalone Farm (Pty) Ltd situated in Hermanus, South Africa. Animals of interest were transferred to a portable aquarium tank in circulating seawater as used by the farm. Following an acclimation period, control animals (n = 6) were removed and dissected. The remaining animals were exposed to environmental hypoxia, by covering the water surface area with a polystyrene sheet containing a small opening, through which 100% nitrogen was flushed slowly into the system (O’omolo et al. 2003). Abalone were removed after 6 h, at which point no attachment behavior was seen. These animals were dissected in the same manner as the control group to sample adductor and foot muscle, left and right gill, epipodial tissue and hemolymph samples, following the procedures stated in the online supplementary material (S.1).
2.2 Metabolite extraction
A subsection of each sampled tissue was removed with a scalpel (while on ice), transferred to a new tube and weighed for the purpose of pre-analysis normalization (Lindeque et al. 2013). Each sample was homogenized in bulk whereafter it was divided into five tubes for analysis on five different analytical platforms. A target sample weight of 100 mg for the right gill tissue, 200 mg for the left gill tissue, 150 mg for the epipodial tissue and 300 mg for the adductor and foot muscle tissue were used throughout the extraction process.
For every 10 mg of abalone tissue, 20 µL water, 50 µL methanol and 20 µL internal standard (2-acetamidophenol and 3-phenylbutyric acid, with a final concentration of 50 µg/mL) was added along with both a 3 and a 7-mm stainless steel bead to micro-centrifuge tubes. Samples were homogenized for 4 min at 30 Hz, using a Retch M400 vibration mill, whereafter 20 µL chloroform and 10 µL water were added to the homogenate. The samples were vortexed for 1 min and incubated on ice for 10 min, before they were centrifuged at 25,000×g for 10 min at 4 °C to give phase separation. Selected volumes of the aqueous and organic phases were transferred to a screw top vial (depending on the analytical platform) and dried under a gentle stream of nitrogen at 37 °C, together with an additional 100 µL platform-specific internal standard: for NMR analysis, 100 µL of the polar phase and 50 µL of the a-polar phase was transferred with nor-leucine internal standard (100 µg/mL). For UPLC–IM-QTOF and LC–QTOF analyses, 70 µL of the polar phase and 35 µL of the a-polar phase was transferred with nor-leucine internal standard (100 µg/mL). For GC–QTOF analysis 70 µL of the polar phase and 35 µL of the a-polar phase was transferred with nonadecanoic acid internal standard (100 µg/mL). For LC–MS/MS analysis 10 µL of the polar phase and 5 µL of the a-polar phase was transferred with nor-leucine internal standard (10 µg/mL). For GC–MSD analysis, nonadecanoic acid internal standard (100 µg/mL) and 150 µL of the a-polar phase of the tissue homogenate was transferred.
Hemolymph samples were prepared by adding 500 µL of the collected hemolymph to 1500 µL cold acetonitrile in micro-centrifuge tubes. The samples were vortexed and incubated on ice for 30 min, followed by centrifugation at 25,000×g for 10 min at 4 °C. The supernatant was aliquoted into several screw top vials (one for each analytical platform). All hemolymph samples were dried under a gentle stream of nitrogen at 37 °C after the addition of 100 μL instrument specific internal standards.
2.3 Sample preparation
For NMR spectroscopy analysis, dried sample extracts were re-dissolved in 500 µL ultra-pure water and centrifuged at 12,000×g for 10 min, whereafter the ultra-filtrate was transferred together with 50 µL 1.5 M potassium phosphate monobasic deuterated NMR buffer solution at pH 7.4 (Dona et al. 2014) to 5 mm NMR glass tubes. Sample preparation for compatibility with GC–TOF (Venter et al. 2016a), LC–MS/MS, LC–QTOF (Venter et al. 2017), GC–MSD (Willers et al. 2016) platforms were conducted as previously described. Extracts used for UPLC–IM-QTOF analysis were re-suspended in 110 µL water:acetonitrile (50:50), whereafter the samples were vortexed and incubated at room temperature for 30 min and centrifuged at 25,000×g for 10 min, after which the supernatant (100 µL) was transferred to vials for analysis. Details regarding the preparation methods can be found in the online supplementary material (S.1).
2.4 Sample analysis
Elaboration on the methods can be found in online supplementary material (S.1).
NMR spectroscopy analyses were performed on a Bruker Advance III HD NMR spectrometer following the parameters described previously (Irwin et al. 2016).
GC–TOF analyses were performed on a LECO Pegasus HT mass analyzer coupled to an Agilent 7890A GC in accordance to an in-house method.
LC–MS/MS analyses were performed in positive ion mode using an Agilent 1200 LC system coupled to an Agilent 6410 Triple Quadrupole. Selected metabolites were analyzed in multiple reaction monitoring (MRM) mode as described in the supplementary material (Table S.1).
LC–QTOF analyses were conducted using an Agilent 1200 LC system coupled to an Agilent 6510 QTOF mass analyzer operated in accordance to the method used by Venter et al. (2017).
GC–MSD analyses of FAMEs were performed with an Agilent GC–MSD instrument as described by Willers et al. (2016).
UPLC–IM-QTOF analyses were performed in accordance to an in-house method, using a Waters Acquity UPLC system coupled via an ESI interface to a Synapt G2-Si hybrid ion mobility-MS system.
2.5 Data analysis and processing
The raw data files of each platform were processed with their corresponding software to create a data matrix. Software and processing details are given in the online supplementary material (S.1). Supervised zero filtering was performed (Venter et al. 2015) so that features (unidentified chromatographic or NMR signals) detected in all the samples of at least one experimental group remained in the data matrix. The NMR data was normalized with the internal standard, 2-acetamidophenol, while GC–TOF data was normalized with 3-phenylbutyric acid. Mass spectrometry total useful signal (MSTUS) normalization (Warrack et al. 2009) was the preferred normalization method for the data acquired by UPLC–IM-QTOF, LC–QTOF, GC–MSD and LC–MS/MS analysis. Missing or zero values were replaced with half of the minimum positive value in the data, within MetaboAnalyst (Xia et al. 2015).
2.6 Data pre-treatment and statistical analysis
Data pre-treatment and statistical analyses were performed with the webserver MetaboAnalyst and MS Excel. The generalized logarithm (glog) was used to transform the data before statistical analysis (Lindeque et al. 2015). Univariate analyses were used to identify features that differed markedly between the control and experimental group. A feature was considered significant when it had a Student’s t test p value < 0.05 (FDR corrected) and effect size d value > 0.8 (Ellis and Steyn 2003). Effect size can be regarded as a scaled fold change, where the difference between the means of the two groups is divided by the maximum standard deviation of the two groups. The Pearson correlation of selected metabolites in different tissues was investigated in MetaboAnalyst. The list of metabolites used in this test is given in the online supplementary material (S.1).
2.7 Compound identification and pathway analysis
Features that differed markedly between the control and experimental group were identified by comparing the metadata provided by each analytical platform (such as accurate mass, fragmentation spectra, retention time, chemical shifts and collision cross section values) to several in-house, commercial and public libraries/databases. The online supplementary material (S.1) can be viewed for guidelines followed for instrument specific compound identifications. The important metabolites identified were ranked with the classification system previously published (Schymanski et al. 2014). Those metabolites that were confidently identified (Level 1–3) were used for pathway interpretation. Some metabolites were detected on more than one analytical platform, and when this occurred, the platform with the highest identification confidence level received preference, and if a duplicate metabolite still existed the metabolite with the best (smallest) p value was selected.
A metabolic map of the metabolic response of H. midae to environmental hypoxia was created. The metabolites are reported as relative compound intensities based on the tissue in which the findings were made. Manual pathway analysis was performed, where a pathway was considered important when most of the metabolites in the pathway were affected, and/or when shared metabolites in the pathway (i.e. metabolites connected with other pathways) were also considerably altered.
3 Results and discussion
Farmed abalone are rather homogeneous and have a very uniform genetic background enabling one to use limited numbers in experiments (as supported by power analysis). The number of animals analyzed in this study also agreed with the proposed sampling measures of the Metabolomics standards initiative (Sumner et al. 2007).
Those metabolites which differed significantly between the control and experimental group, and which were confidently identified, are listed in Table S.2 (Supplementary material—Table 2). Metabolites detected with elevated (↑) or decreased (↓) relative abundances (compared to the control group) were subsequently mapped (Fig. 2) and interpreted. The pathways that were affected by environmental hypoxia include: [A] carbohydrate metabolism (Glycolysis); [B] oxaloacetate–succinate pathway; [C] threonine–serine–glycine metabolism; [D] sulphur containing amino acid metabolism; [E] cysteine–taurine metabolism; [F] pyruvate–lactate/alanine pathway; [G] aspartate–succinate pathway; [H] pyruvate–opine pathway; [I] lysine–tryptophan metabolism; [J] glutaryl–CoA pathway; [K] branched chain amino acid metabolism; [L] ketone body metabolism; [M] propionyl–CoA pathway; [N] tricarboxylic acid (TCA) cycle; [O] alternative aspartate pathway; [P] purine and pyrimidine metabolism; [Q] urea cycle; [R] proline metabolism; [S] electron transport chain (ETC); [T] phenylalanine–tyrosine metabolism; [U] beta(ß)–oxidation; [V] fatty acid transport pathway; and [W] fatty acid synthesis. Significant metabolite concentration differences found in abalone adductor muscle (AM), foot muscle (FM), left gill (LG), right gill (RG), hemolymph (H) and epipodial tissue (E) samples after hypoxia are indicated in Fig. 2 where relative abundance are indicated as elevated (blue) or decreased (red).
3.1 ATP production and NAD+ recovery during environmental hypoxia
Evidence of phosphagen breakdown [Q] and anaerobic glycolysis [B, F and H] were found (Fig. 2) in most of the investigated tissues. Phosphagens and anaerobic metabolic pathways are responsible to uphold energy levels during hypoxia, through a process referred to as substrate-level-phosphorylation (Feala et al. 2009). Abalone typically use phosphoarginine as primary phosphagen for instant energy liberation (Grieshaber et al. 1993), which resulted in accumulated arginine in most tissues (epipodial tissue, adductor muscle and the gills). However, the presence of creatine in the foot muscle of hypoxic abalone suggests that energy is also maintained with phosphocreatine metabolism. Exposing abalone to environmental hypoxia resulted in reduced levels of arginine and creatine in the foot muscle samples, which is most likely due to the further breakdown pathways being up-regulated, or metabolic shuttles between tissues.
Initial energy replenishment through phosphagen breakdown is short-lived, and subsequently assisted by anaerobic pathways. As mitochondrial respiration and ATP production slow with reduced oxygen levels, anaerobic pathways are activated to counter the energy and redox (NADH:NAD+) imbalance, which is supported by the elevated levels of lactate, several opines, succinate and alanine. During prolonged hypoxia, the majority of ATP is produced by the phosphoglycerate kinase and pyruvate kinase reactions in the glycolysis pathway [B], which is only possible as long as cytoplasmic nicotinamide adenine dinucleotide (NAD+) levels are sustained. Three complementary glycolytic pathways are involved in NAD+ recovery during hypoxia in abalone, which are the: (1) glucose–lactate pathway [F], (2) glucose–opine pathway [H] and (3) glucose/aspartate–succinate pathway [G].
In an attempt to avoid metabolic shutdown and allow continued glycolysis during hypoxia, pyruvate is converted to lactate by oxidizing NADH to NAD+ (Whitney and Rolfes 2008), which is supported by the elevated lactate levels found in all of the abalone tissues investigated. During prolonged anaerobic metabolism, lactate synthesis will eventually exceed the clearance thereof, resulting in a reduction in intracellular pH (Green and Storey 2016), which in turn further limits ATP production over long periods of anaerobic stress. In order to prevent acidotic shock, an increase in cell buffer capacity is subsequently required, which is partially accomplished in H. midae by the production of alanine and opines.
Opines are anaerobic end-products, produced in H. midae through the condensation of pyruvate and several amino acids which converts NADH to NAD+ (Sato et al. 1993). A connection between the specific opines formed, and their associated amino acids, can be seen following environmental hypoxia [H]. Elevated levels of alanine were accompanied by an elevation in alanopine in the left and right gill samples. Reduced levels of glycine were accompanied by a reduction in strombine in the hemolymph. Elevated taurine in the adductor muscle, gills and hemolymph samples were also accompanied by elevated tauropine in the same tissue samples. Finally, octopine was elevated only in the left gill samples, which corresponded with elevated arginine. Similar associations were also recognized in other animals exposed to environmental hypoxia (Kreutzer et al. 1989). These results not only indicate the extent of the hypoxic stress induced during the environmental hypoxia experiment, but also the animal’s dependence on the opine metabolic pathway to sustain NAD+ levels and limit lactate-induced acidosis.
Reduced aspartate, accompanied by elevated concentrations of alanine and succinate in all of the tissues investigated, imply activation of the glucose/aspartate–succinate pathway [B] to sustain NAD+ levels. A synchronized depletion of aspartate accompanied by elevations in alanine and succinate in the foot and adductor muscles of other abalone were also previously reported in response to anoxia (Gäde 1988), confirming our findings. Succinate is the end product of the glucose–succinate pathway [B], the aspartate–succinate pathway [G] and the TCA cycle [N], during conditions of anaerobic ATP production in abalone.
3.2 Central carbon metabolism during environmental hypoxia
Several sugar-based metabolites (such as galactose, mannose and glucose) were generally elevated in H. midae, following hypoxic exposure (Fig. 2). As mentioned, glycolysis and interconnected carbohydrate pathways [A] become more important during hypoxia when anaerobic energy production is required, which result in the release of carbohydrates from glycogen stores and glycoproteins, and the up-regulation of gluconeogenesis (Roznere et al. 2014). This may lead to elevated carbohydrate metabolites, as seen in the abalone. Elevated TCA cycle [N] intermediates (citrate, aconitate, isocitrate and succinate) were detected possibly due to impaired mitochondrial respiration and down-regulation of the TCA cycle via isocitrate dehydrogenase, as a means to prevent metabolic energy waste as typically experienced in vertebrates (Garrett and Grisham 2010). The aforementioned redox imbalance is a major contributor to this down-regulation and the accumulation of the TCA cycle intermediates. Fortunately, however, alternative substrates, such as glutamate, can still be used to synthesize the much needed α-ketoglutarate and down-stream TCA cycle intermediates very effectively (Boardman et al. 2016) during hypoxia, preventing total TCA cycle shutdown.
3.3 Up-regulated protein and amino acid catabolism
The concentrations of numerous amino acids were significantly affected by environmental hypoxia (Table S.2). It is known that, during low energy states, the adenosine monophosphate (AMP) to ATP ratio increases, which subsequently allosterically activates AMP activated protein kinase (AMPK). AMPK signals the catabolism of proteins (and lipids), and inhibits anabolism and cell growth (Venter et al. 2016b). Considering the elevated levels of AMP detected in both the right gill and adductor muscle samples [Q], in addition to the elevated amino acid concentrations in these and other tissues, it becomes apparent that hypoxia results in pronounced protein catabolism (Fields 1983).
3.3.1 The role of ketogenic- and glucogenic amino acids
The glucogenic amino acids (arginine, glutamate, glutamine, glycine, histidine, methionine, proline and serine) were predominantly elevated in the adductor muscle of abalone during environmental hypoxia (Fig. 2). Typically, during stressed conditions, glucogenic amino acids are converted to alanine via the transamination of pyruvate [F] (Salway 2004). The detection of elevated alanine and pyruvate attests to this, as well as the elevated glutamate, synthesized by transamination of α-ketoglutarate. Elevated alanine is a key survival response as it assists in: the buffering of H+ ions, regulation of intracellular osmotic pressure, and serves as a substrate for alanopine production (Fujimori and Abe 2002; Garrett and Grisham 2010; Müller et al. 2012). Elevated concentrations of alanine were also seen in H. diversicolor, after exposure to a toxin stressor that induced an anaerobic state (Lu et al. 2017), supporting our results. Elevated concentrations of glycine and methionine detected in the adductor muscle samples as a result of hypoxia, were also previously reported (Lu et al. 2016). We additionally detected elevated serine concentrations, not previously reported, which is also an important energy substrate (for gluconeogenesis) as serine can be deaminated to form pyruvate (Salway 2004).
Aspartate, asparagine, glutamate and glutamine are well known metabolic fuels during anaerobic conditions (Storey 2005). Environmental hypoxia additionally resulted in reduced concentrations of both aspartate (all tissues investigated) and asparagine (hemolymph and left gill). In contrast, glutamate was increased in the epipodial tissue, adductor muscle and both gill samples. The reduced aspartate levels can be linked to its role in replenishing oxaloacetate during hypoxia [O], as was previously indicated for Apostichopus japonicus (Prathomya et al. 2017). Glutamate is an active intermediate of amino acid metabolism [N and R], and closely associated with the TCA cycle. As mentioned, amination of α-ketoglutarate produces glutamate, which in turn can be further aminated to yield glutamine. Glutamine in turn may serve as a nitrogen store, lowering ammonia toxicity during hypoxia (Venter et al. 2016b). Reduced histidine concentrations were also detected in the hemolymph samples of hypoxic H. midae, corresponding to the reduction of glutamate in the hemolymph. Histidine is metabolized to glutamate with the removal of one carbon group (Salway 2004), which links this also to the one-carbon metabolism and elevated methionine.
As seen in Fig. 2, environmental hypoxia resulted in significantly increased proline concentrations in the adductor muscle, left gill, right gill and epipodial tissue samples of H. midae (Table S.2) which is likely a result of reduced proline catabolism [R and Q] due to the redox imbalance. However, proline synthesis cannot be excluded as was previously seen in plants. During periods of dehydration, the proline anabolic pathways in plants are activated while the proline catabolic pathways are repressed (Hayat et al. 2012). Proline is thought to prevent dehydration, function as a molecular chaperon stabilizing the structure of proteins, maintain protein integrity and enhance the activities of different enzymes. It can be speculated that proline plays a similar role in marine animals. Furthermore, accumulated proline, buffer cytosolic pH and protects against the damaging effects of reactive oxygen species (Hochachka et al. 1973; Hayat et al. 2012; Vosloo et al. 2013). Evidence for this was found in green-lipped mussel subjected to temperature stress (Dunphy et al. 2015). Moreover, H. midae that were fed a l-proline supplemented feed for 1 month, and subjected to oxygen and temperatures stress, still displayed an increase in mass, suggesting some benefit for the animal despite the stressed conditions (Vosloo et al. 2013).
The levels of the ketogenic amino acids, leucine and lysine, were lower in the adductor muscle and left gill after exposure to environmental hypoxia. Both leucine and lysine are considered essential amino acids for vertebrate species, and can only be assumed to be the same for invertebrates. The results of this investigation support this hypothesis, as the concentrations of these, and other essential amino acids, were depleted after the prolonged hypoxia. Considering the up-regulated fatty acid oxidation and increased use of carnitine (Li et al. 2009) during hypoxic conditions, it is also reasonable to assume that lysine [I] was actively used to synthesize carnitine [J], which may contribute to the reduced lysine detected. Furthermore, leucine [K] and the other branched-chain amino acids are substrates for gluconeogenesis under conditions of high ATP demand (Mason et al. 2015).
3.3.2 Pathways preventing ammonia/nitrogen toxicity
The involvement of arginine and phosphoarginine in ATP production during hypoxia (Morash and Alter 2016) has already been discussed. However, since arginine showed the most significant increase in concentration in the adductor muscle of H. midae after exposure to environmental hypoxia (Table S.2), its involvement in connected pathways cannot be ignored. Significantly elevated concentrations of ornithine and citrulline were also found in the adductor muscle following environmental hypoxia, while elevated argininosuccinate was found in the foot muscle. This result implicates the urea cycle [Q] in maintaining the nitrogen/ammonia balance with increased amino acid catabolism. Only a small portion of the ammonia released from amino acid catabolism is used for the synthesis of non-essential amino acids (like alanine and glutamate), while the majority of the ammonia is excreted, either as pure ammonia or as urea (Nollens et al. 2004; Schmidt-Nielsen 2007). Previous literature on H. laevigata indicated that these organisms are extremely sensitive to ammonia exposure, which results in dramatically reduced growth and nutrient uptake (Harris et al. 1998). Thus the urea cycle in abalone plays an important role during stressed conditions such as hypoxia. In standard farming conditions, it was reported that energy loss in the form of ammonia excretion is negligible in H. midae, accounting for less than 1% of consumption (Barkai and Griffiths 1988). However, this is likely to be significantly more during hypoxia.
3.3.3 Detoxification through glycine conjugation
Several dipeptides (arginylglycine, alanylglycine, alanyllysine, cysteinylcysteine, isoleucylthreonine, valylglycine) were drastically increased in the hypoxic animals (Table S.2). The occurrence of dipeptides in cellular fluids can be largely ascribed to incomplete protein breakdown (Shen et al. 2008; Wishart et al. 2012). These peptides also play a key role in cell signaling, which may further modulate specific metabolic reactions. Interestingly, elevated alanylglycine and arginylglycine correlated with elevated alanine and arginine levels, hinting to possible phase II detoxification (specifically glycine conjugation). Alanine levels in the epipodial tissue (for example) correlated with alanylglycine levels (r = 0.9) in the environmental hypoxia group while they did not correlate in the control group. Similarly, arginine levels in the adductor muscle moderately correlated (r = 0.6) with arginylglycine levels in the hypoxic animals while no correlation was found in the controls. Also, lower isovalerylglycine levels corresponded to lower branched-chain amino acid levels.
In humans, it is well known that various states of organic aciduria produce large amounts of different acyl-CoAs, which in turn serve as substrates for glycine N-acyltransferase (GLYAT), and subsequently the synthesis of glycine conjugates (Loots et al. 2005). Considering that acyl-transferase enzymes can conjugate glycine to a variety of different accumulated amino acids (Badenhorst et al. 2014), the role of glycine conjugation in detoxifying accumulating intermediates and maintaining coenzyme A (CoA) levels seem highly probable in abalone based on our findings [L and M]; although additional evidence to confirm such activities in abalone is needed.
3.3.4 Amino acids as osmolytes
The osmolytes altered by environmental hypoxia included various non-essential amino acids (alanine, arginine, asparagine, aspartate, cysteine, glutamate, glutamine, glycine, proline, serine and tyrosine), opines, taurine, choline, betaine, dimethylglycine and sarcosine (Table S.2). This suggests a change in the osmotic regulation of abalone when exposed to oxygen poor environments. Taurine for example has been reported to be more related to osmoregulation than to energetics (Tripp-Valdez et al. 2017). Likewise, the elevated concentrations of the less metabolically active non-essential amino acids are believed to maintain osmoregulation in abalone (Rosenblum et al. 2005). Moreover, elevated opine levels also ensure that the intracellular osmotic pressure is maintained, via pH regulation (Harcet et al. 2013). A combination of trimethylamine oxide, betaine and sarcosine was also reported to contribute to intracellular osmolarity in marine invertebrates (Wilbur and Hochachka 2016). Considering this, the elevated levels of the aforementioned osmolytes in H. midae following hypoxic exposure, are most likely produced for the purpose to maintain intracellular osmolarity (in addition to energy production).
3.4 Lipid metabolism during environmental hypoxia
Very few lipid metabolites were altered after environmental hypoxia, suggesting that cellular energy production via lipid metabolism was limited. This was expected, since only a fraction of the fatty acids and sterols absorbed from the diet undergo de novo lipid synthesis in abalone (Venter et al. 2016b). The total lipid profiles (FAMEs analyses) did not show significant differences in the ratios of fatty acids within structural and store lipids after hypoxia; which can also be expected given the homogenous (pre-hypoxic) farming conditions during which lipid synthesis was most active. Moreover, the concentration of lipids (estimated from the FAMEs) were unchanged after hypoxia, which was rather surprising, but highly likely due to the relatively low lipid stores in abalone tissue previously reported (Vosloo and Vosloo 2010).
The types and amounts of free and bound fatty acids detected in the different tissues varied, similar to observations in H. rubra and H. laevigata (Grubert et al. 2004). Reduced concentrations of bound docosahexaenoate (22:6 n-3), eicosapentaenoate (20:5 n-3) and octadecatrienoate (18:3 n-6) were found in the left gill, right gill and adductor muscle, respectively (Table S.2). This suggests a higher fatty acid release rate (lipolysis) during hypoxia, specifically for the long chain fatty acids. On the other hand, the epipodial tissues contained higher amounts of bound docosahexaenoate and stearidonate (18:4 n-3) fatty acids, accompanied by lower levels of free palmitate, suggesting a lack of lipolysis activity. This contradicting finding was also seen in H. fulgens, where elevated concentrations of docosahexaenoate and eicosapentaenoate were detected in the muscle tissue following starvation stress (Durazo-Beltrán et al. 2004).
The fatty acylcarnitines in H. midae that were most affected included free carnitine, acetylcarnitine, butyrylcarnitine and palmitoylcarnitine, which were predominantly elevated (Table S.2). This can be explained by the inhibition of ß-oxidation [U], by the redox imbalance, resulting in elevated levels of fatty acids (such as palmitoleate) and their associated fatty acylcarnitines. Furthermore, it should be mentioned that carnitine conjugation of these accumulating fatty acids also plays a role in detoxification.
3.5 Interplay and cooperation of the different tissues
The largest metabolic effects were seen in the adductor muscle, followed by the epipodial tissue, then the left and right gill, the foot muscle and lastly, the hemolymph samples (Fig. 2). This diverse response to hypoxia can be attributed to the varying functionality and metabolism associated with the various organs from which the tissue was sampled. Additionally, much like higher organisms, we believe that metabolites are shuttled between various different organs (e.g. from hypoxic tissue to regions that have access to oxygen) where they can be more appropriately utilized. An example of this in higher-order animals is the lactate shuttle between fatigued muscles and liver.
The left gill of abalone is only used during hypoxic conditions to enhance surface area and oxygen uptake (Ragg and Taylor 2006). This is shown very aptly during this investigation, as comparison of the left gill of hypoxic animals to the inactive left gill in the control group resulted in numerous metabolic differences (not seen when the right gills were compared). With the accumulation of metabolites in the muscles during environmental hypoxia, it can be speculated that the left gill was recruited to assist with energy production and clearance of some accumulated metabolites. Since the gills are proximal to the immediate environment (and remaining oxygen) it can be reasoned that oxygen-dependent pathways are still partially functional. Thus the elevated metabolites detected in the gills may be a consequence of a combination of shuttled metabolites and their own metabolic shutdown. The idea that metabolites are shuttled to and from the gills are supported by the correlation of specific metabolites found in the gills (especially the left gill) and other tissues: for example, in the environmental hypoxic group, alanine levels in the left gill correlated with the levels in the hemolymph (r = 0.82), which was not the case in the control group. The same correlation was also found for lactate (r = 0.80) and several other accumulated metabolites.
Prior literature pertaining to the biochemistry and physiology of abalone epipodial tissue is rather scarce, but the results of this study showed that the epipodial tissue has similar metabolic profiles to the other muscles. It has been hypothesized that the epipodial tissue plays a role in oxygen uptake (Taylor and Ragg 2005). Hence, it might be possible that metabolites from the muscles are shuttled to the epipodial tissue during hypoxia, similar to the gills. Again this theory is supported by the correlation of metabolite levels between the tissues. Leucine and alanopine levels in the epipodial tissue and hemolymph correlated (r = 0.83 and 0.78 respectively) in the environmental hypoxic group while these metabolites did not correlate in the control group. Alanopine in the epipodial tissue also correlated moderately with that in the foot muscle (r = 0.73). Although it is possible that other factors contribute to this covariance, the involvement of metabolite shuttles cannot be dismissed. The aforementioned metabolic similarity of epipodial tissue to the muscle tissue also allows for an important opportunity for non-destructive sampling for health screening, without critical damage to the animal (Wasko et al. 2003; Slabbert and Roodt-Wilding 2006).
Of all the samples studied, the least number of significant differences were detected in the hemolymph during hypoxia. During extreme hypoxia, such as the environmental hypoxia, cellular metabolites may become depleted, resulting in reduced metabolite concentrations in the systemic fluid. Additionally, reduced pH and/or increased free ammonia could also influence metabolite channels and pumps. Considering this, the role of hemolymph as a sample for analyzing metabolic end-products, like ammonia for instance, becomes apparent. Metabolic end products produced during hypoxic conditions may be released into the hemolymph and absorbed by other tissues (organs) which are able to oxidize these products (metabolic shuttle), e.g. the metabolic profiles detected in the hepatopancreas (which receives large hemolymph volumes) of H. fulgens confirms the transport function of hemolymph (Tripp-Valdez et al. 2017). This was also the case according to the correlation analysis we performed. As in the previous sections, the concentrations of many compounds in the different tissues correlate with the hemolymph concentrations which highlight its transport/shuttle function that links the different tissues. Consequently, hemolymph represents the exo-metabolome since it contains metabolite information of how the intracellular metabolic network influences its external environment (by the uptake of extracellular metabolites and secretion of intracellular metabolites) (Dunn 2008). One of the end products of anaerobic respiration is lactate, which is known to cause a drop in extracellular and intracellular pH, resulting in a disturbed acid–base balance during hypoxia. It has been suggested that abalone hemolymph has very limited pH buffering capacity, and is consequently prone to metabolic acidosis during hypoxia (Morash and Alter 2016). However, it is important to note that hemocyanin of gastropod hemolymph displays a reverse Bohr Effect, where oxygen binds tighter at a low pH or higher carbon dioxide (CO2) partial pressure. This enables abalone to maintain oxygen saturation when clamping to surfaces during hypoxic conditions (Wells et al. 1998).
4 Conclusions
This is the first study to comprehensively describe the metabolic response of H. midae exposed to environmental hypoxia, using six complementary analytical platforms. The results demonstrate that environmental hypoxia created a metabolic imbalance in H. midae, with compromised energy levels, skewed NADH:NAD+ ratio and the accumulation of metabolites and toxic end products, such as ammonia. In response to the imbalances, the metabolism shifted to more catabolic processes in order to correct the energy and redox imbalances. Due to the large amounts of arginine detected, it can be deducted that these animals have large phosphoarginine pools able to regenerate ATP at the onset of hypoxia. Thereafter, energy production continued via anaerobic metabolic pathways associated with the breakdown of glycogen, proteins and lipids. For efficient anaerobic metabolism and energy production, recovery of NAD+ is crucial, which is predominantly achieved by the conversion of pyruvate to lactate, the conversion of pyruvate to opines, and the conversion of glucose and aspartate to succinate. It is apparent from the results that different tissues undergo different metabolic changes during hypoxia, which is likely linked to their metabolic activity (as seen with comparison to the left and right gills) and their proximity to the environment. We hypothesize that accumulated muscle metabolites are shuttled (via hemolymph) to the gills and epipodial tissue where available oxygen allows further breakdown of the metabolites or excretion into the environment. With these findings, it is now possible to predict metabolic responses in hypoxic conditions and intervene accordingly, by recovery diets or supplements.
References
Badenhorst, C. P. S., Erasmus, E., Van der Sluis, R., Nortje, C., & Van Dijk, A. A. (2014). A new perspective on the importance of glycine conjugation in the metabolism of aromatic acids. Drug Metabolism Reviews, 46(3), 343–361.
Barkai, R., & Griffiths, C. (1988). An energy budget for the South African abalone Haliotis midae Linnaeus. Journal of Molluscan Studies, 54(1), 43–51.
Boardman, L., Sørensen, J. G., Koštál, V., Šimek, P., & Terblanche, J. S. (2016). Chilling slows anaerobic metabolism to improve anoxia tolerance of insects. Metabolomics, 12(12), 176.
Britz, P., & Venter, S. (2016). Aquaculture review: South Africa. World Aquaculture, 47(4), 19–28.
Carroll, J. L., & Wells, R. M. (1995). Strategies of anaerobiosis in New Zealand infaunal bivalves: Adaptations to environmental and functional hypoxia. New Zealand Journal of Marine and Freshwater Research, 29(2), 137–146.
Cook, P. A. (2014). The worldwide abalone industry. Modern Economy, 5(13), 1181–1186.
Dona, A. C., Jiménez, B., Schäfer, H., Humpfer, E., Spraul, M., Lewis, M. R., et al. (2014). Precision high-throughput proton NMR spectroscopy of human urine, serum, and plasma for large-scale metabolic phenotyping. Analytical Chemistry, 86(19), 9887–9894.
Dunn, W. B. (2008). Current trends and future requirements for the mass spectrometric investigation of microbial, mammalian and plant metabolomes. Physical Biology, 5(1), 011001.
Dunphy, B. J., Watts, E., & Ragg, N. L. (2015). Identifying thermally-stressed adult green-lipped mussels (Perna canaliculus Gmelin, 1791) via metabolomic profiling. American Malacological Bulletin, 33(1), 127–135.
Durazo-Beltrán, E., Viana, M. A. T., D’Abramo, L. R., & Toro-Vazquez, J. F. (2004). Effects of starvation and dietary lipid on the lipid and fatty acid composition of muscle tissue of juvenile green abalone (Haliotis fulgens). Aquaculture, 238(1), 329–341.
Ellis, S., & Steyn, H. (2003). Practical significance (effect sizes) versus or in combination with statistical significance (p values): Research note. Management Dynamics: Journal of the Southern African Institute for Management Scientists, 12(4), 51–53.
Feala, J. D., Coquin, L., Zhou, D., Haddad, G. G., Paternostro, G., & McCulloch, A. D. (2009). Metabolism as means for hypoxia adaptation: Metabolic profiling and flux balance analysis. BMC Systems Biology, 3(1), 91.
Fields, J. H. (1983). Alternatives to lactic acid: Possible advantages. Journal of Experimental Zoology Part A: Ecological Genetics and Physiology, 228(3), 445–457.
Fields, J. H., & Storey, K. B. (1987). Tissue-specific alanopine dehydrogenase from the gill and strombine dehydrogenase from the foot muscle of the cherrystone clam Mercenaria mercenaria (Linn.). Journal of Experimental Marine Biology and Ecology, 105(2–3), 175–185.
Fujimori, T., & Abe, H. (2002). Physiological roles of free d-and l-alanine in the crayfish Procambarus clarkii with special reference to osmotic and anoxic stress responses. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 131(4), 893–900.
Gäde, G. (1988). Energy metabolism during anoxia and recovery in shell adductor and foot muscle of the gastropod mollusc Haliotis lamellosa: Formation of the novel anaerobic end product tauropine. The Biological Bulletin, 175(1), 122–131.
Gäde, G., Carlsson, K.-H., & Meinardus, G. (1984). Energy metabolism in the foot of the marine gastropod Nassa mutabilis during environmental and functional anaerobiosis. Marine Biology, 80(1), 49–56.
Gäde, G., & Grieshaber, M. K. (1986). Pyruvate reductases catalyze the formation of lactate and opines in anaerobic invertebrates. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry, 83(2), 255–272.
Garrett, R., & Grisham, C. (2010). Biochemistry, Brooks. (4th ed., pp. 511–812). Belmont, CA: Brooks Cole, Cengage Learning.
Green, S. R., & Storey, K. B. (2016). Regulation of crayfish, Orconectes virilis, tail muscle lactate dehydrogenase (LDH) in response to anoxic conditions is associated with alterations in phosphorylation patterns. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 202, 67–74.
Grieshaber, M., Hardewig, I., Kreutzer, U., & Pörtner, H.-O. (1993). Physiological and metabolic responses to hypoxia in invertebrates. In Reviews of physiology, biochemistry and pharmacology (Vol. 125, pp. 43–147). Berlin: Springer.
Grubert, M. A., Dunstan, G. A., & Ritar, A. J. (2004). Lipid and fatty acid composition of pre-and post-spawning blacklip (Haliotis rubra) and greenlip (Haliotis laevigata) abalone conditioned at two temperatures on a formulated feed. Aquaculture, 242(1), 297–311.
Harcet, M., Perina, D., & Pleše, B. (2013). Opine dehydrogenases in marine invertebrates. Biochemical Genetics, 51(9–10), 666–676.
Harris, J. O., Maguire, G. B., Edwards, S., & Hindrum, S. M. (1998). Effect of ammonia on the growth rate and oxygen consumption of juvenile greenlip abalone, Haliotis laevigata Donovan. Aquaculture, 160(3), 259–272.
Hayat, S., Hayat, Q., Alyemeni, M. N., Wani, A. S., Pichtel, J., & Ahmad, A. (2012). Role of proline under changing environments: A review. Plant Signaling & Behavior, 7(11), 1456–1466.
Hochachka, P. W., Fields, J., & Mustafa, T. (1973). Animal life without oxygen: Basic biochemical mechanisms. American Zoologist, 13(2), 543–555.
Hooper, C., Day, R., Slocombe, R., Benkendorff, K., & Handlinger, J. (2014). Histopathology and haemolymph biochemistry following anaesthesia and movement in farmed Australian abalone (Haliotis rubra × Haliotis laevigata). Aquaculture, 422, 202–210.
Irwin, C., van Reenen, M., Mason, S., Mienie, L. J., Westerhuis, J. A., & Reinecke, C. J. (2016). Contribution towards a metabolite profile of the detoxification of benzoic acid through glycine conjugation: An intervention study. PLoS ONE, 11(12), e0167309.
Kreutzer, U., Siegmund, B. R., & Grieshaber, M. K. (1989). Parameters controlling opine formation during muscular activity and environmental hypoxia. Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology, 159(5), 617–628.
Li, P., Mai, K., Trushenski, J., & Wu, G. (2009). New developments in fish amino acid nutrition: Towards functional and environmentally oriented aquafeeds. Amino Acids, 37(1), 43–53.
Lindeque, J. Z., Hidalgo, J., Louw, R., & van der Westhuizen, F. H. (2013). Systemic and organ specific metabolic variation in metallothionein knockout mice challenged with swimming exercise. Metabolomics, 9(2), 418–432.
Lindeque, J. Z., van Rensburg, P. J. J., Louw, R., van der Westhuizen, F. H., Florit, S., Ramírez, L., et al. (2015). Obesity and metabolomics: Metallothioneins protect against high-fat diet-induced consequences in metallothionein knockout mice. Omics: A Journal of Integrative Biology, 19(2), 92–103.
Liu, C., Shin, P., & Cheung, S. (2014). Comparisons of the metabolic responses of two subtidal nassariid gastropods to hypoxia and re-oxygenation. Marine Pollution Bulletin, 82(1), 109–116.
Loots, D. T., Erasmus, E., & Mienie, L. J. (2005). Identification of 19 new metabolites induced by ab normal amino acid conjugation in isovaleric acidemia. Clinical Chemistry, 51(8), 1510–1512.
Lu, J., Feng, J., Cai, S., & Chen, Z. (2017). Metabolomic responses of Haliotis diversicolor to organotin compounds. Chemosphere, 168, 860–869.
Lu, J., Shi, Y., Wang, S., Chen, H., Cai, S., & Feng, J. (2016). NMR-based metabolomic analysis of Haliotis diversicolor exposed to thermal and hypoxic stresses. Science of The Total Environment, 545, 280–288.
Mason, S., van Furth, A. M., Mienie, L. J., Engelke, U. F., Wevers, R. A., Solomons, R., et al. (2015). A hypothetical astrocyte–microglia lactate shuttle derived from a 1H NMR metabolomics analysis of cerebrospinal fluid from a cohort of South African children with tuberculous meningitis. Metabolomics, 11(4), 822–837.
Morash, A. J., & Alter, K. (2016). Effects of environmental and farm stress on abalone physiology: Perspectives for abalone aquaculture in the face of global climate change. Reviews in Aquaculture, 8(4), 342–368.
Mouton, A. (2017). Red tide devastates South African abalone farms. Aquaculture Magazine, 31, 32–35.
Müller, M., Mentel, M., van Hellemond, J. J., Henze, K., Woehle, C., Gould, S. B., et al. (2012). Biochemistry and evolution of anaerobic energy metabolism in eukaryotes. Microbiology and Molecular Biology Reviews, 76(2), 444–495.
Nollens, H. H., Keogh, J. A., & Probert, P. K. (2004). Haematological pathology of shell lesions in the New Zealand abalone, Haliotis iris (Mollusca: Gastropoda). Comparative Clinical Pathology, 12(4), 211–216.
O’omolo, S., Gäde, G., Cook, P., & Brown, A. (2003). Can the end products of anaerobic metabolism, tauropine and d-lactate, be used as metabolic stress indicators during transport of live South African abalone Haliotis midae. African Journal of Marine Science, 25(1), 301–309.
Pitcher, G., & Calder, D. (2000). Harmful algal blooms of the southern Benguela current: A review and appraisal of monitoring from 1989 to 1997. African Journal of Marine Science, 22, 255–271.
Prathomya, P., Prisingkorn, W., Jakovlić, I., Deng, F.-Y., Zhao, Y.-H., & Wang, W.-M. (2017). 1H NMR-based metabolomics approach reveals metabolic alterations in response to dietary imbalances in Megalobrama amblycephala. Metabolomics, 13(2), 17.
Ragg, N. L., & Taylor, H. H. (2006). Heterogeneous perfusion of the paired gills of the abalone Haliotis iris Martyn 1784: An unusual mechanism for respiratory control. Journal of Experimental Biology, 209(3), 475–483.
Rosenblum, E., Viant, M., Braid, B., Moore, J., Friedman, C., & Tjeerdema, R. (2005). Characterizing the metabolic actions of natural stresses in the California red abalone, Haliotis rufescens using 1H NMR metabolomics. Metabolomics, 1(2), 199–209.
Roznere, I., Watters, G. T., Wolfe, B. A., & Daly, M. (2014). Nontargeted metabolomics reveals biochemical pathways altered in response to captivity and food limitation in the freshwater mussel Amblema plicata. Comparative Biochemistry and Physiology Part D: Genomics and Proteomics, 12, 53–60.
Salway, J. G. (2004). Metabolism at a glance. Guildford: Wiley.
Sato, M., Takeuchi, M., Kanno, N., Nagahisa, E., & Sato, Y. (1993). Distribution of opine dehydrogenases and lactate dehydrogenase activities in marine animals. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry, 106(4), 955–960.
Schmidt-Nielsen, K. (2007). Animal physiology: Adaptation and environment. New York: Cambridge University Press.
Schymanski, E. L., Jeon, J., Gulde, R., Fenner, K., Ruff, M., Singer, H. P., et al. (2014). Identifying small molecules via high resolution mass spectrometry: Communicating confidence. Environmental Science and Technology, 48(4), 2097–2098.
Shen, Y., Hixson, K. K., Tolic, N., Camp, D. G., Purvine, S. O., Moore, R. J., et al. (2008). Mass spectrometry analysis of proteome-wide proteolytic post-translational degradation of proteins. Analytical Chemistry, 80(15), 5819–5828.
Slabbert, R., & Roodt-Wilding, R. (2006). Non-destructive sampling of juvenile abalone using epipodial tentacles and mucus: Method and application. African Journal of Marine Science, 28(3–4), 719–721.
Sokolova, I. M., Frederich, M., Bagwe, R., Lannig, G., & Sukhotin, A. A. (2012). Energy homeostasis as an integrative tool for assessing limits of environmental stress tolerance in aquatic invertebrates. Marine Environmental Research, 79, 1–15.
Storey, K. B. (2004). Adventures in oxygen metabolism. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 139(3), 359–369.
Storey, K. B. (2005). Functional metabolism: Regulation and adaptation. Hoboeken: Wiley.
Sumner, L. W., Amberg, A., Barrett, D., Beale, M. H., Beger, R., Daykin, C. A., et al. (2007). Proposed minimum reporting standards for chemical analysis. Metabolomics, 3(3), 211–221.
Taylor, H., & Ragg, N. (2005). The role of body surfaces and ventilation in gas exchange of the abalone, Haliotis iris. Journal of Comparative Physiology B, 175(7), 463–478.
Tripp-Valdez, M. A., Bock, C., Lucassen, M., Lluch-Cota, S. E., Sicard, M. T., Lannig, G., et al. (2017). Metabolic response and thermal tolerance of green abalone juveniles (Haliotis fulgens: Gastropoda) under acute hypoxia and hypercapnia. Journal of Experimental Marine Biology and Ecology, 497, 11–18.
Venter, L., van Rensburg, P. J. J., Loots, D. T., Vosloo, A., & Lindeque, J. Z. (2016a). Untargeted metabolite profiling of abalone using gas chromatography mass spectrometry. Food Analytical Methods, 9(5), 1254–1261.
Venter, L., Lindeque, Z., van Rensburg, P. J. J., Van der Westhuizen, F., Smuts, I., & Louw, R. (2015). Untargeted urine metabolomics reveals a biosignature for muscle respiratory chain deficiencies. Metabolomics, 11(1), 111–121.
Venter, L., Loots, D. T., Vosloo, A., Jansen van Rensburg, P., & Lindeque, J. Z. (2016b). Abalone growth and associated aspects: Now from a metabolic perspective. Reviews in Aquaculture, 2, 2016.
Venter, L., van Rensburg, P. J. J. , Loots, D. T., Vosloo, A., & Lindeque, J. Z. (2017). From untargeted LC–QTOF analysis to characterisation of opines in abalone adductor muscle: Theory meets practice. Journal of Chromatography B, 1071, 44–48.
Vosloo, D., van Rensburg, L., & Vosloo, A. (2013). Oxidative stress in abalone: The role of temperature, oxygen and L-proline supplementation. Aquaculture, 416, 265–271.
Vosloo, D., & Vosloo, A. (2010). Response of cold-acclimated, farmed South African abalone (Haliotis midae) to short-term and long-term changes in temperature. Journal of Thermal Biology, 35(7), 317–323.
Warrack, B. M., Hnatyshyn, S., Ott, K.-H., Reily, M. D., Sanders, M., Zhang, H., et al. (2009). Normalization strategies for metabonomic analysis of urine samples. Journal of Chromatography B, 877(5), 547–552.
Wasko, A. P., Martins, C., Oliveira, C., & Foresti, F. (2003). Non-destructive genetic sampling in fish. An improved method for DNA extraction from fish fins and scales. Hereditas, 138(3), 161–165.
Wells, R. M., Baldwin, J., Speed, S. R., & Weber, R. E. (1998). Haemocyanin function in the New Zealand abalones Haliotis iris and H. australis: Relationships between oxygen-binding properties, muscle metabolism and habitat. Marine and Freshwater Research, 49(2), 143–149.
Whitney, E., & Rolfes, S. R. (2008). Understanding nutrition. Belmont: Cengage Learning.
Wilbur, K. M., & Hochachka, P. W. (2016). The Mollusca: Environmental biochemistry and physiology (Vol. 2). New York: Academic Press.
Willers, C., van Rensburg, P. J. J., & Claassens, S. (2016). Can a metabolomics-based approach be used as alternative to analyse fatty acid methyl esters from soil microbial communities? Soil Biology and Biochemistry, 103, 417–428.
Wishart, D. S., Jewison, T., Guo, A. C., Wilson, M., Knox, C., Liu, Y., et al. (2012). HMDB 3.0—the human metabolome database in 2013. Nucleic Acids Research, 41(D1), D801–D807.
Xia, J., Sinelnikov, I. V., Han, B., & Wishart, D. S. (2015). MetaboAnalyst 3.0—making metabolomics more meaningful. Nucleic Acids Research, 43(W1), 251–257.
Acknowledgements
We gratefully acknowledge the North-West University and the Technology Innovation Agency of the Department of Science and Technology of South Africa for financial support. We also thank HIK Abalone Farm (Pty) Ltd, South Africa for supplying experimental animals and welcoming us to your farm.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
All of the research pertaining to the perturbations and abalone collection was done in accordance with institutional guidelines of the relevant institutional committees and granted Aquaculture Research Permit.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Venter, L., Loots, D.T., Mienie, L.J. et al. Uncovering the metabolic response of abalone (Haliotis midae) to environmental hypoxia through metabolomics. Metabolomics 14, 49 (2018). https://doi.org/10.1007/s11306-018-1346-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-018-1346-8