Abstract
Introduction
The application of metabolomic analysis in the pediatric nephrology field may offer an innovative approach to profile analysis of renal diseases.
Objective
We aimed to analyze the salivary the major metabolites in the saliva of children and adolescents with chronic kidney disease (CKD) before and after hemodialysis.
Method
Thirty-six children diagnosed with CKD and forty healthy children were recruited for the study. 1H-NMR spectra were analyzed using multivariate and univariate approaches.
Results
The CKD and the healthy control groups presented with similar numbers of dental caries (p > 0.05) as determined by the number of decayed, missing, or filled deciduous teeth (0.87 ± 2.2 and 0.67 ± 2.1, respectively) or permanent teeth (0.79 ± 1.30 and 0.90 ± 1.7, respectively). The amount of dental calculus was significantly higher in the CKD group than in the healthy control group (p < 0.001). Multivariate analyses using PLS-DA and O-PLS-DA demonstrated differences in the salivary metabolome of CKD patients before and after hemodialysis, as well as between post-dialysis CKD patients and healthy controls, suggesting that HD was not able to recover oral homeostasis. PLS-DA and OPLS-DA models showed satisfactory accuracy (ACC = 0.72) and prediction (0.64). On multivariate and univariate analyses, urea, acetate, ethanol, and fatty acid were significantly decreased in CKD saliva after hemodialysis. By contrast, saliva from the healthy controls had significantly higher levels of acetate and propionate and lower levels of ethanol, lactate, butyrate, phenylalanine, and creatinine than saliva from post-dialysis CKD patients.
Conclusion
Our results demonstrate that hemodialysis alters the expression of salivary metabolites; however, this alteration does not reestablish the healthy salivary metabolome, as the salivary metabolomic profile of healthy children is significantly different from that of children and adolescents with CKD, both before and after hemodialysis. The unique salivary characteristics of children with CKD may influence their oral health status.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Chronic kidney disease (CKD) is a disorder that significantly affects quality of life (Staples and Wong 2010; Wong et al. 2012), causing high mortality and severe morbidity, making it a critical global public health problem (Wong et al. 2006). End-stage renal disease, or renal failure, is the final stage of CKD and is less prevalent but more aggressive in pediatric patients than in adults. It is characterized by a low glomerular filtration rate (< 15 ml/min) and necessitates hemodialysis (HD), a therapy that aims to artificially replace the fluid and waste removal functions of the kidney. Using diffusion and ultrafiltration, HD maintains the body’s internal equilibrium of water and minerals, primarily sodium, potassium, chloride, calcium, phosphorus, magnesium, and sulfate. To monitor the patient during HD therapy, creatinine and urea levels are acceptable indicators of response to treatment (Desai et al. 2009).
Pediatric patients with CKD often have altered oral status, which result from changes in oral homeostasis. For example, these patients present with fewer caries but more calculus (Martins et al. 2012). Other oral clinical presentations of CKD include gingival hyperplasia; dry mouth; decreased salivary flow rate; delayed tooth eruption; and increased dental staining, calculus, tongue coating, oral ulcers, and enamel hypoplasia (Benmoussa et al. 2015; Martins et al. 2012). Pre- and post-dialysis levels of salivary biochemical markers, specifically urea, creatinine, sodium, potassium, and phosphate, have been shown to correlate with those in serum (Seethalakshmi et al. 2014).
Metabolomic profiling, using techniques such as nuclear magnetic resonance (NMR), allows the simultaneous and relative quantification of different metabolites in a biological sample (Nicholson and Lindon 2008; Zhang et al. 2012) without the need for prior purification. For this reason, NMR is an important metabolite assessment tool (Lindon et al. 2004). Changes in salivary metabolites are associated with the formation of dental caries (Fidalgo et al. 2015, 2013), periodontal disease (Aimetti et al. 2012), and systemic alterations (de Oliveira et al. 2016). We previously showed that the saliva of children with uncontrolled type 1 diabetes had glucose levels > 200 mg/dL, which is consistent with glycemia. In addition, lower molecular weight salivary components play a role in oral cavity homeostasis. To date, different metabolomic approaches have been used to evaluate the effect of HD on the biochemistry of blood and urine, including gas chromatography (Jankowski et al. 2001) and NMR (Fujiwara et al. 2015, 2009). Here, we aimed to analyze the major low molecular weight metabolites in the saliva of children and adolescents with CKD, before and after HD.
2 Materials and methods
This study was approved by the Local Ethical Committee (Comitê de Ética em Pesquisa do Hospital Federal de Bonsucesso - CEP-HFB 28/10) and a written informed consent was obtained from all participants or their respective legal representative.
2.1 Study population and sample collection
Thirty-six children diagnosed with CKD who were undergoing HD were initially identified from three pediatric nephrology centers of reference. The inclusion criteria for the CKD group were glomerular filtration rate < 15 ml/min and absence of metabolic syndromes and neurological and motor limitations. Subjects with orthodontic apparatuses were excluded from both the CKD and control groups. After applying the inclusion and exclusion criteria, 30 CKD patients (mean age 13.03 years ± 3.2 years; 15 males) were selected. For the healthy control group, 40 children and adolescents (mean age 20.3 years ± 3.5 years; 20 males) with non-contributory medical histories and who had not taken antibiotics in the 3 months prior to sample collection were selected for the study. The urea reduction rate (URR) for patients in the CKD group was obtained from the patients’ medical records.
Anamnesis was performed and oral findings such as dental caries (decayed-missing-filled relating to deciduous [dmft] and permanent [DMFT] teeth) and the presence of calculus (modified Volpe-Manhold index) were recorded (Manhold et al. 1965; WHO 1997).
For all CKD patients, saliva and serum samples were collected before and after a HD therapy session (HD-B and HD-A, respectively); saliva also was obtained from the healthy participants. For saliva sample collection, participants were required to expectorate 3 mL of unstimulated whole saliva into a plastic universal tube. They were asked to refrain from oral activities for one hour prior to saliva collection, which was done at approximately 10:30 am. Saliva samples were centrifuged at 10,000×g for 60 min at 4 °C, and the supernatants were stored at − 80 °C until NMR analyses. As a standard biofluid used to diagnose renal failure, serum was also collected from all participants. Blood (4 mL) was collected into BD vacutainer tubes with no additive and allowed to clot for 30 min at room temperature. Clotted blood was centrifuged at 1600×g for 15 min at 4 °C to remove red blood cells.
2.2 Sample preparation and NMR measurements
NMR spectra were acquired using a 600 MHz Avance III spectrometer (Bruker Biospin, Rheinstetten, Germany). Saliva samples were prepared by mixing 0.45 mL of salivary supernatant, 0.05 mL D2O (99.8%; to provide a field frequency lock), and 20 µM of 4,4-dimethyl-4-silapentane-1-sulfonic acid (DSS) for chemical shift reference, δ = 0.00 ppm. The ZGPR pulse was used for saliva samples at 298 K with 512 scans. Serum samples were prepared by diluting 1:3 in 0.9% NaCl solution: 130 µL of blood was combined with 340 µL of 0.9% NaCl solution and 50 µL of D2O. For blood samples, the Carr-Purcell-Meiboom-Gill (CPMG) pulse sequence using a T2 filter at 300 K was used to suppress signals from macromolecules.
After spectra acquisition, edge effects were evaluated by overlaying all spectra using Topspin (Bruker Biospin). The spectra and spectral regions that could not be corrected for phase and baseline were excluded from the analyses. Our assignment strategy includes the use of the Human Metabolome database (http://www.hmdb.ca/) (Wishart et al. 2007), and also previous studies (de Oliveira et al. 2016; Fidalgo et al. 2015, 2013; Silwood et al. 2002). 1H–1H total correlation (TOCSY) experiments were used to confirm, assign and resolve ambiguous metabolites and were acquired using 2,048 points X 256, spectral width of 12,019 Hz in each dimension and a mixing time of 70 ms.
The urea and creatinine reduction rates (URR and CRR, respectively) were calculated based on the 1H NMR spectra. The URR was calculated using the equation, [(HD-BUrea − HD-AUrea)/HD-BUrea] × 100, and the CRR was calculated using the equation, [(HD-BCreatinine − HD-ACreatinine)/HD-BCreatinine] × 100. Because the CPMG pulse sequence disturb the urea signal, urea quantification was performed using ZGPR pulse experiments for a subset of serum samples.
2.3 Statistical analyses
2.3.1 Clinical data
The clinical findings were analyzed using the SPSS statistical package (SPSS Inc., Chicago, IL, USA). Descriptive analyses were performed and the χ2 (Chi-squared) test with a confidence interval of 95% was reported.
2.3.2 NMR data
Metabolomic data were extracted using the AMIX statistical program (Bruker Biospin). Each NMR spectrum was analyzed by integrating bucket size regions of 0.03 ppm without the water region (for serum samples, 4.7–4.9 ppm and for saliva samples, 4.1–5.6 ppm). The regions that could not be aligned were excluded from the analysis (for serum samples: 1.44–1.48, 2.07–2.15, 2.39–2.46, 2.64–2.83, 3.55–3.60, 3.91-4.00, 6.8–7.20 and 7.5–8.3 ppm and for saliva samples, 1.4–1.5, 2.15–2.20, 3.5–3.6, and 6.5–7.9 ppm).
The saliva and serum spectral datasets were stored in separate matrices, with rows representing the subject samples and columns representing chemical shifts (242 buckets for serum and 179 buckets for saliva). For paired and unpaired comparisons, the bucket tables were normalized using the sum of intensities, and the Pareto scaling method (Ramadan et al. 2006) was used before applying the partial least squares discriminant analysis (PLS-DA) method. The PLS-DA used the initial input variables for each group that were stored in the y-table (0 and 1), and was performed using Metaboanalyst 3.0 software (http://www.metaboanalyst.ca/MetaboAnalysts). Orthogonal PLS-DA (O-PLS-DA) analyses were also performed using the Metaboanalyst 3.0 software (Xia et al. 2015).
Metaboanalyst 3.0 software was also used to obtain the predictive performance of the models; each model was evaluated for Q2 and Q2Y (fraction of Y predicted by model X for PLS-DA and O-PLS-DA, respectively), R2 and R2Y (modeling X variation fraction for PLS-DA and O-PLS-DA, respectively), and accuracy (ACC), for the purpose of cross-validation (van Velzen et al. 2008).
For each model, 1000 permutations were performed. The receiver operating characteristic (ROC) curve was generated by Monte-Carlo cross-validation using balanced sub-sampling. For each Monte-Carlo cross-validation, two-thirds of the samples were used to evaluate the feature importance. The area under the ROC curve (AUC) and the prediction (Prediction accuracy during training with 1000 permutation) matrix were obtained to assess sensitivity and the specificity. A 95% confidence interval was used for the models.
The variable importance in projection (VIP) scores was obtained for each comparison, and the corresponding metabolites were analyzed using peak intensity and univariate statistical analyses. All spectral regions (buckets) were subjected to univariate statistical analyses. NMR data distribution was also assessed using the Shapiro–Wilk test (p < 0.05); Mann–Whitney tests were performed for unpaired comparisons and Wilcoxon tests for paired comparisons. Linear regression was performed in order to evaluate the relationship between dental calculus formation and metabolites levels (SPSS Inc., Chicago, IL, USA); a p value < 0.05 was considered statistically significant.
3 Results
3.1 Saliva NMR spectra
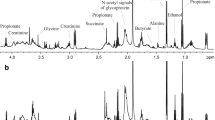
NMR spectra from the saliva of healthy participants and HD-B and HD-A from children and adolescents with CKD were assigned using the strategy described by Fidalgo et al. (2013) (Suppl Fig. S-1).
Multivariate statistical analyses were performed to evaluate the metabolite differences in samples before and after HD. Figure 1a, b show the PLS-DA and O-PLS-DA, respectively, of saliva samples before and after HD, demonstrating differences between HD-B and HD-A. Table 1 shows that the model presented satisfactory accuracy and prediction (ACC = 0.72; prediction = 0.64). Figure 1c shows the salivary metabolites with significant VIP scores. Levels of urea, ethanol, acetate, and fatty acid decreased significantly in saliva after HD; in contrast, levels of butyrate, lysine, lactate, and N-acetyl signals of glycoproteins were significantly increased.
a O-PLS-DA b PLS-DA; c plot of VIP scores from 1H NMR spectra of saliva from HD-B and HD-A; d PLS-DA; e O-PLS-DA; f plot of VIP scores from 1H NMR spectra of saliva from healthy participants and HD-B; g PLS-DA; h O-PLS-DA; and i plot of VIP scores from 1H NMR spectra of saliva from healthy participants and HD-A
The salivary metabolite profiles of patients with CKD before and after HD were compared with those of healthy children. Figure 1d, e show PLS-DA and O-PLS-DA, respectively, of saliva samples from healthy children; the metabolite profile of healthy subjects was clearly different from those of HD-B and HD-A. Figure 1f, i show the metabolites with significant VIP scores. Levels of ethanol, fatty acid, and creatinine were significantly higher in HD-B than in the saliva of healthy participants. Levels of butyrate, lactate, phenylalanine, and creatinine were significantly higher in HD-A than in the saliva of healthy participants. These data demonstrate that the salivary metabolites in HD-B and HD-A are not only distinctly different from those in healthy participants, but present in different concentrations as well (Fig. 1g, h).
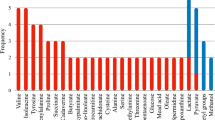
To further evaluate the metabolites with higher VIP scores, the peak intensity of NMR spectra from the saliva of ten CKD patients before and after HD were evaluated (Fig. 2). Creatinine was also included in this analysis because of its importance to the staging of CKD. The 3.01 ppm peak was chosen for this metabolite; the 4.05 ppm peak was not considered to eliminate the interference from the water resonance. Table 2 shows the metabolites that were significantly different in multivariate and univariate analyses. The table shows the salivary metabolites, chemical shift, peak intensity variation, and p-values.
Figure 3 illustrates the metabolic pathway analysis performed using the principal metabolites identified in the saliva of patients with CKD before and after HD. For this analysis, lactate, fatty acid, and N-acetyl signals of glycoproteins were used. Acetate was not included because it is a component of dialysis buffer while urea was also not included because its increased concentration is associated with renal failure. Ethanol and butyrate seem to be related to bacteria metabolism. The pathway analysis showed that the identified metabolites are related to pyruvate metabolism.
3.2 Serum NMR analysis
The serum of CKD patients was analyzed and the results were compared to those obtained using saliva. Suppl Fig. S-2A and B show representative spectra of HD-B and HD-A serum samples.
Supplementary Fig. S-3 shows a comparison of the PLS-DA and O-PLS-DA and the VIP scores between HD-B and HD-A. The majority of metabolites, including urea, creatinine, sugar region, acetate, formate, lactate, N-acetyl signals of glycoproteins, phenylalanine, valine, leucine, isoleucine, and lipid were significantly (p < 0.05) different on multivariate and univariate analyses (Suppl Table S-1).
Supplementary Fig. S-4 shows the changes observed between HD-B and HD-A. Sera from ten representative subjects showed a reduction in lipids, creatinine, urea, and lactate with an increase in valine, leucine, isoleucine, N-acetyl signals of glycoproteins, and acetate after HD.
Further, we measured the URR and CRR based on 1H NMR spectra (Suppl Mat Fig. S-2). Based on the NMR analysis that demonstrated a good ACC (Suppl Table S-3), urea (5.84 ppm) and creatinine (3.01 and 4.05 ppm) decreased after HD in the studied biofluids (Suppl Table S-2); similar trends for URRs were observed in saliva.
3.3 Oral assessment findings
All participants presented with a similar number of primary and permanent teeth (p = 0.283). No significant differences were found between the healthy participant and the CKD patient groups in number of caries (p = 0.492). The healthy group had a mean of 0.28 ± 1.16 teeth with dental caries (dmft = 0.67 ± 2.1 and DMFT 0.90 ± 1.7) and the CKD group had a mean of 0.52 ± 1.5 teeth with dental caries (dmft = 0.87 ± 2.2 and DMFT 0.79 ± 1.3). In terms of calculus, the healthy group had a mean of 0.67 ± 1.18 mm, varying from 0 to 7 mm of dental calculus, whereas the CKD group had a mean of 17.90 ± 19.61 mm (p < 0.001), varying from 0 to 59 mm of dental calculus.
We analyzed the metabolites levels and calculus formation in CKD children using linear regression but we could find no statistical association between the severity of dental calculus and the level of any metabolite (p = 0.260).
4 Discussion
The current study evaluated the effects of HD on the salivary metabolite profiles of children and adolescents with end-stage renal disease. PLS-DA and O-PLS-DA of 1H-NMR spectra demonstrated differences in the composition of the salivary metabolite profile and the concentrations of low molecular weight metabolites between the saliva of participants before HD and after HD. The salivary profiles were also compared to those of healthy participants. Importantly, children and adolescents with CKD had different salivary profiles compared to healthy children and HD was unable to recover normal salivary conditions. These findings may indicate that children and adolescents with CKD have specific clinical dental conditions.
No difference in the number of dental caries was found between patients with CKD and healthy participants, in accordance with other published studies (Epstein et al. 1980; Martins et al. 2008, 2012). A low prevalence of dental caries in patients with renal disease is attributed to increased salivary pH (Peterson et al. 1985), which also favors the formation of dental calculus (Gupta et al. 2015). The high pH decreases the population of cariogenic bacteria and, instead of dental demineralization, leads to dental calculus formation (Gupta et al. 2015). The presence of calculus in CDK patients has been associated to the presence of urea and changes in pH (Martins et al. 2012; Driessens et al. 1985). Our studies showed no correlation with urea levels, therefore, probably the pH, salt concentration or other oral component/condition could be related to the severity of calculus formation.
Previous studies investigating salivary markers associated with end-stage renal disease in adults have identified changes in cortisol, nitrite, uric acid, sodium, chloride, pH, amylase, and lactoferrin. Previous studies investigating salivary markers associated with end-stage renal disease in adults have identified changes in cortisol, nitrite, uric acid, sodium, cloride, pH, amylase, lactoferrin, creatinina, potassium and phosphate (Seethalakshmi et al. 2014; Arregger et al. 2008; Tomas et al. 2008; Walt et al. 2007).
The metabolomic strategy utilized in the present study allowed analysis of the full spectrum of metabolites to identify those that changed in biofluids collected before HD compared to those collected after HD. Using this approach, we observed changes in the levels of lactate, n-acetyl, butyrate, ethanol, fatty acid, and urea in the saliva of children and adolescents after HD compared to those in the saliva of children and adolescents before HD. Similar to our study, previous studies in adults support up to a 60% reduction in salivary levels of urea after HD compared to those before HD (Klassen and Krasko 2002).
Salivary butyrate has a role in bacterial metabolism; the increase in this metabolite after HD can be explained by changes in the metabolism of oral microorganisms (Huang et al. 2011) due to decreased food intake during HD, during which patients typically do not eat or drink.
A known complication of CKD is dyslipidemia related to hypertriglyceridemia, resulting from the accumulation of lipoprotein remnant particles. HD has been shown to lower low density lipoprotein levels (Kaysen 2007). In our study, salivary fatty acid levels decreased after HD, consistent with previous findings. On the other hand, the increased levels of salivary lactate and lysine after HD still need to be clarified.
In the current study, pathway analysis was performed using the principle metabolites that changed after HD—lactate, fatty acid, and N-acetyl signals of glycoproteins. Acetate and urea were excluded from the analysis because acetate is a component of the dialysis buffer (Fujiwara et al. 2009) and urea is associated with renal failure. Pathway analysis showed that the identified metabolites are related to pyruvate metabolism. Altered pyruvate metabolism is associated with several diseases, such as cancer, heart failure, neurodegeneration, and non-specific renal failure (Gray et al. 2014). Pyruvate is a critical molecule involved in numerous metabolic pathways. It is an end-product of glycolysis and important for mitochondrial ATP generation and for driving several major biosynthetic pathways (Hocher and Adamski 2017).
The present study found that salivary acetate levels decreased after HD. Dialysis solution consists of glucose, potassium-based electrolytes, and an acetate buffer, which is responsible for a temporary acidosis in HD patients. Currently, there is no clear explanation for why salivary acetate levels decreased after HD therapy.
In the present study, similar VIP scores were observed after HD in serum as were observed in saliva, suggesting that salivary levels of urea, fatty acid, and N-acetyl glycoproteins correlate with those in serum of children and adolescents undergoing HD. The vasculature around the salivary glands permits the transport of proteins, ions and metabolites from serum to saliva (Liu and Duan 2012; Wong 2006). Albumin, secretory IgA and transferrin are examples of serum proteins present in saliva. In the present study, similar VIP scores were observed after the HD procedure in both serum and saliva. Urea, fatty acid and N-acetyl glycoproteins metabolites appear to be interrelated in serum and saliva of children and adolescents undergoing HD.
Information regarding metabolites and metabolite changes can provide insight into the state of an individual’s metabolic profile, which reflects both genotypic and phenotypic information; this information can be used by health care providers for more accurate disease assessment and to determine individualized medical treatment (Moco et al. 2013). The application of metabolomics to the field of pediatric nephrology after further studies may represent an innovative approach for the early diagnosis and monitoring of renal diseases.
Abbreviations
- CKD:
-
Chronic kidney disease
- CRR:
-
Creatinine reduction rate
- CPMG:
-
Carr-Purcell-Meiboom-Gill
- dmft:
-
Decayed, missing, or filled deciduous teeth
- DMFT:
-
Decayed, missing, or filled permenant teeth
- HD:
-
Hemodialysis
- HD-A:
-
After hemodialysis
- HD-B:
-
Before hemodialysis
- NMR:
-
Nuclear magnetic resonance
- O-PLS-DA:
-
Orthogonal partial least squares discriminant analysis
- PLS-DA:
-
Partial least squares discriminant analysis
- TOCSY:
-
Total correlation spectroscopy
- URR:
-
Urea reduction rate
- VIP:
-
Variable importance in projection
References
Aimetti, M., Cacciatore, S., Graziano, A., & Tenori, L. (2012). Metabonomic analysis of saliva reveals generalized chronic periodontitis signature. Metabolomics, 8(3), 465–474.
Arregger, A. L., Cardoso, E. M., Tumilasci, O., & Contreras, L. N. (2008). Diagnostic value of salivary cortisol in end stage renal disease. Steroids, 73(1), 77–82.
Benmoussa, L., Renoux, M., & Radoi, L. (2015). Oral manifestations of chronic renal failure complicating a systemic genetic disease: Diagnostic dilemma. Case report and literature review. Journal of Oral and Maxillofacial Surgery. doi:10.1016/j.joms.2015.05.029
de Oliveira, L. R., Martins, C., Fidalgo, T. K., Freitas-Fernandes, L. B., de Oliveira Torres, R., Soares, A. L., Almeida, F. C., Valente, A. P., & de Souza, I. P. (2016). Salivary metabolite fingerprint of type 1 diabetes in young children. Journal of Proteome Research, 15(8), 2491–2499.
Desai, A. A., Nissenson, A., Chertow, G. M., Farid, M., Singh, I., Van Oijen, M. G., Esrailian, E., Solomon, M. D., & Spiegel, B. M. (2009). The relationship between laboratory-based outcome measures and mortality in end-stage renal disease: A systematic review. Hemodialysis International: International Symposium on Home Hemodialysis, 13(3), 347–359.
Driessens, F. C. M., Borggreven, J. M. P. M., Verbeeck, R. M. H., van Dijk, J. W. E., & Feagin, F. F. (1985). On the physicochemistry of plaque calcification and the phase composition of dental calculus. Journal Periodontal Research, 20, 329–336.
Epstein, S. R., Mandel, I., & Scopp, I. W. (1980). Salivary composition and calculus formation in patients undergoing hemodialysis. Journal of Periodontology, 51(6), 336–338.
Fidalgo, T. K. S., Freitas-Fernandes, L. B., Almeida, F. L., Valente, A. P., & Souza, I. P. R. (2015). Longitudinal evaluation of salivary profile from children with dental caries before and after treatment. Metabolomics, 11(3), 780–785.
Fidalgo, T. K. S., Freitas-Fernandes, L. B., Angeli, R., Muniz, A. M. S., Gonsalves, E., Santos, R., Almeida, F. L., Valente, A. P., & Souza, I. P. R. (2013). Salivary metabolite signatures of children with and without dental caries lesions. Metabolomics, 9(3), 657–666.
Fujiwara, M., Ando, I., Takeuchi, K., Oguma, S., Sato, H., Sekino, H., Sato, K., & Imai, Y. (2015). Metabolic responses during hemodialysis determined by quantitative (1)H NMR spectroscopy. Journal of Pharmaceutical and Biomedical Analysis, 111, 159–162.
Fujiwara, M., Kobayashi, T., Jomori, T., Maruyama, Y., Oka, Y., Sekino, H., Imai, Y., & Takeuchi, K. (2009). Pattern recognition analysis for 1H NMR spectra of plasma from hemodialysis patients. Analytical and Bioanalytical Chemistry, 394(6), 1655–1660.
Gray, L. R., Tompkins, S. C., & Taylor, E. B. (2014). Regulation of pyruvate metabolism and human disease. Cellular and Molecular Life Sciences: CMLS, 71(14), 2577–2604.
Gupta, M., Gupta, M., & Abhishek (2015). Oral conditions in renal disorders and treatment considerations: A review for pediatric dentist. Saudi Dental Journal, 27(3), 113–119.
Hocher, B., & Adamski, J. (2017). Metabolomics for clinical use and research in chronic kidney disease. Nature Reviews: Nephrology, 13(5), 269–284.
Huang, C. B., Alimova, Y., Myers, T. M., & Ebersole, J. L. (2011). Short- and medium-chain fatty acids exhibit antimicrobial activity for oral microorganisms. Archives of Oral Biology, 56(7), 650–654.
Jankowski, J., Hagemann, J., Yoon, M. S., van der Giet, M., Stephan, N., Zidek, W., Schluter, H., & Tepel, M. (2001). Increased vascular growth in hemodialysis patients induced by platelet-derived diadenosine polyphosphates. Kidney International, 59(3), 1134–1141.
Kaysen, G. A. (2007). Dialysis removes apolipoprotein c-i, improving very low-density lipoprotein clearance. Kidney International, 72(7), 779–781.
Klassen, J. T., & Krasko, B. M. (2002). The dental health status of dialysis patients. Journal of the Canadian Dental Association, 68(1), 34–38.
Lindon, J. C., Holmes, E., Bollard, M. E., Stanley, E. G., & Nicholson, J. K. (2004). Metabonomics technologies and their applications in physiological monitoring, drug safety assessment and disease diagnosis. Biomarkers: Biochemical Indicators of Exposure, Response, and Susceptibility to Chemicals, 9(1), 1–31.
Liu, J., & Duan, Y. (2012). Saliva: a potential media for disease diagnostics and monitoring. Oral Oncology, 48(7), 569–577.
Manhold, J. H., Volpe, A. R., Parker, L., & Adams, S. H. (1965). In vivo calculus assessment. Ii. A comparison of scoring techniques. Journal of Periodontology, 36, 299–304.
Martins, C., Siqueira, W. L., & Guimaraes Primo, L. S. (2008). Oral and salivary flow characteristics of a group of brazilian children and adolescents with chronic renal failure. Pediatric Nephrology, 23(4), 619–624.
Martins, C., Siqueira, W. L., Oliveira, E., Nicolau, J., & Primo, L. G. (2012). Dental calculus formation in children and adolescents undergoing hemodialysis. Pediatric nephrology, 27(10), 1961–1966.
Moco, S., Collino, S., Rezzi, S., & Martin, F. P. (2013). Metabolomics perspectives in pediatric research. Pediatric Research, 73(4 Pt 2), 570–576.
Nicholson, J. K., & Lindon, J. C. (2008). Systems biology: Metabonomics. Nature, 455(7216), 1054–1056.
Peterson, S., Woodhead, J., & Crall, J. (1985). Caries resistance in children with chronic renal failure: Plaque ph, salivary ph, and salivary composition. Pediatric Research, 19(8), 796–799.
Ramadan, Z., Jacobs, D., Grigorov, M., & Kochhar, S. (2006). Metabolic profiling using principal component analysis, discriminant partial least squares, and genetic algorithms. Talanta, 68(5), 1683–1691.
Seethalakshmi, C., Koteeswaran, D., & Chiranjeevi, V. (2014). Correlation of serum and salivary biochemical parameters in end stage renal disease patients undergoing hemodialysis in pre and post-dialysis state. Journal of Clinical Diagnostics Research, 8(12), CC12. doi:10.7860/JCDR/2014/10404.5306.
Silwood, C. J., Lynch, E., Claxson, A. W., & Grootveld, M. C. (2002). 1H and (13)C NMR spectroscopic analysis of human saliva. Journal of Dental Research, 81(6), 422–427.
Staples, A., & Wong, C. (2010). Risk factors for progression of chronic kidney disease. Current Opinion in Pediatrics, 22(2), 161–169.
Tomas, I., Marinho, J. S., Limeres, J., Santos, M. J., Araujo, L., & Diz, P. (2008). Changes in salivary composition in patients with renal failure. Archives of Oral Biology, 53(6), 528–532.
van Velzen, E. J., Westerhuis, J. A., van Duynhoven, J. P., van Dorsten, F. A., Hoefsloot, H. C., Jacobs, D. M., Smit, S., Draijer, R., Kroner, C. I., & Smilde, A. K. (2008). Multilevel data analysis of a crossover designed human nutritional intervention study. Journal of Proteome Research, 7(10), 4483–4491.
Walt, D. R., Blicharz, T. M., Hayman, R. B., Rissin, D. M., Bowden, M., Siqueira, W. L., Helmerhorst, E. J., Grand-Pierre, N., Oppenheim, F. G., Bhatia, J. S., et al. (2007). Microsensor arrays for saliva diagnostics. Annals of the New York Academy of Sciences, 1098, 389–400.
WHO. (1997). Oral health surveys: Basic methods, 4th edn. Geneva: WHO
Wishart, D. S., Tzur, D., Knox, C., Eisner, R., Guo, A. C., Young, N., Cheng, D., Jewell, K., Arndt, D., Sawhney, S., et al. (2007). Hmdb: The human metabolome database. Nucleic Acids Research, 35(Database issue), D521-D526.
Wong, C. J., Moxey-Mims, M., Jerry-Fluker, J., Warady, B. A., & Furth, S. L. (2012). Ckid (ckd in children) prospective cohort study: A review of current findings. American Journal of Kidney Diseases: The Official Journal of the National Kidney Foundation, 60(6), 1002–1011.
Wong, H., Mylrea, K., Feber, J., Drukker, A., & Filler, G. (2006). Prevalence of complications in children with chronic kidney disease according to kdoqi. Kidney International, 70(3), 585–590.
Xia, J., Sinelnikov, I. V., Han, B., & Wishart, D. S. (2015). Metaboanalyst 3.0–making metabolomics more meaningful. Nucleic Acids Research, 43(W1), W251-257.
Zhang, A., Sun, H., Wang, P., Han, Y., & Wang, X. (2012). Recent and potential developments of biofluid analyses in metabolomics. Journal of Proteomics, 75(4), 1079–1088.
Acknowledgements
The authors acknowledge the hemodialysis centers and the doctors Alberto Zagury and Gisella Pires Mello for their medical support.
Funding
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq (306214/2013; 303785/2014-4; 426265/2016-5), Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro – FAPERJ (201.278/2014; 620.012/2015; 202.902/2015), Centro Nacional de Ressonância Magnética Nuclear (CNRMN), and Instituto Nacional de Ciência and Tecnologia de Biologia Estrutural e Bioimagem (InBEB).
Author information
Authors and Affiliations
Contributions
All authors confirmed they have contributed to the intellectual content of this paper and have met the following 3 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This study analyzes collected data which involved human participants. This study was approved by the Local Ethical Committee (Comitê de Ética em Pesquisa do Hospital Federal de Bonsucesso - CEP-HFB 28/10).
Informed consent
Written informed consent was obtained from all participants or their respective legal representative.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Almeida, P.A., Fidalgo, T.K.S., Freitas-Fernandes, L.B. et al. Salivary metabolic profile of children and adolescents after hemodialysis. Metabolomics 13, 141 (2017). https://doi.org/10.1007/s11306-017-1283-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-017-1283-y