Abstract
Introduction
Improving feed utilization in cattle is required to reduce input costs, increase production, and ultimately improve sustainability of the beef cattle industry. Characterizing metabolic differences between efficient and non-efficient animals will allow stakeholders to identify more efficient cattle during backgrounding.
Objectives
This study used an untargeted metabolomics approach to determine differences in serum metabolites between animals of low and high residual feed intake.
Methods
Residual feed intake was determined for 50 purebred Angus steers and 29 steers were selected for the study steers based on low versus high feed efficiency. Blood samples were collected from steers and analyzed using untargeted metabolomics via mass spectrometry. Metabolite data was analyzed using Metaboanalyst, visualized using orthogonal partial least squares discriminant analysis, and p-values derived from permutation testing. Non-esterified fatty acids, urea nitrogen, and glucose were measured using commercially available calorimetric assay kits. Differences in metabolites measured were grouped by residual feed intake was measured using one-way analysis of variance in SAS 9.4.
Results
Four metabolites were found to be associated with differences in feed efficiency. No differences were found in other serum metabolites, including serum urea nitrogen, non-esterified fatty acids, and glucose.
Conclusions
Four metabolites that differed between low and high residual feed intake have important functions related to nutrient utilization, among other functions, in cattle. This information will allow identification of more efficient steers during backgrounding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Improving the feed efficiency of cattle is vital to increase the amount of beef produced per unit of feed, thus increasing production and decreasing input costs for producers in the United States. Feed is the largest cost to producers in the beef industry, representing approximately 60% of overall cost of production (Holmgren and Feuz 2015; Norton 2005). As resources become more limited and feed costs increase, the overall cost of production is likely to grow in subsequent decades (Lawrence et al. 2008). Therefore, the selection and optimization of economically important phenotypes, such as feed efficiency, must be evaluated.
Feed efficiency is moderately heritable (Berry and Crowley 2013), and genetic selection has been used to improve the efficiency of beef herds (Arthur et al. 2001). However, non-genetic factors such as the gut microbiome (Myer et al. 2017) and the environment (Mader and Davis 2004) also contribute to efficiency. Additionally, feed intake and body weight gain, key components of efficiency, are themselves polygenic traits with complex patterns of inheritance (Elzo et al. 2012). Methods beyond genetic selection are therefore needed to optimize efficiency in beef cattle. Biochemically, efficiency results from composite changes in metabolism of macronutrients that increase the yield of energy from feed. Markers of this variation in metabolism that results in difference in efficiency could therefore be used as phenotypic traits for selection. However, relatively little is known at the molecular level about the basis for efficiency.
Metabolomics is a tool that produces a snapshot of cellular metabolism by comprehensively profiling metabolite abundance in biological samples. Accordingly, metabolomic profiling provides insight into nutrient utilization in humans and animals. It provides finer resolution of factors affecting phenotypic variation in growth and physiological parameters (Fontanesi 2016). Because metabolites are the result of combined endogenous and exogenous production, metabolomic studies reveal information beyond that provided solely from genetics or genomics, revealing relationships between animal genetics and physiological phenotypes (Fontanesi 2016). With respect to beef cattle, metabolomics is a dual-purpose tool that can both increase the fundamental understanding of efficiency and identify metabolites that are potential biomarkers for selection.
While previous studies have focused on phenotypic or genetic selection for feed efficient animals, understanding molecular-level changes that distinguishes animals that utilize feed more efficiently could provide valuable insight for the selection of efficient animals. In this study, we hypothesized that steers that differ in feed efficiency would have different levels of metabolites associated with nutrient utilization. The objective was to determine serum metabolites that differed between low and high feed efficient animals, utilizing untargeted metabolomics.
2 Materials and methods
This study was approved and carried out in accordance with the recommendations of the Institutional Animal Care and Use Committee at the University of Tennessee, Knoxville.
Fifty purebred Angus steers were used in this study. Steers from the University of Tennessee Institute of Agriculture Plateau Research and Education Center (precht and Williams 2012) in Crossville, TN were 7 months of age and weighed 264 ± 2.7 kg at the start of the trial. Two weeks after weaning, steers were acclimated in the GrowSafe feeding system (GrowSafe Systems Ltd., Airdrie, Canada) for 10 days prior to measuring feed intake. Steers were fed a step up receiving diet for approximately 14 days before receiving a growing ration (11.57% crude protein and 76.93% total digestible nutrients on a dry matter basis) with 28 mg monensin/kg DM. After the acclimation period, body weight (Krysl and Hess 1993) was measured at 7 day intervals and feed intake was continuously measured using the GrowSafe© system for a 60 day feed efficiency trial. As a measure of feed efficiency, at the end of the feeding period, steers were ranked by residual feed intake (RFI) based on performance and feed intake measured from day 0 to day 60 (Koch et al. 1963). The average value of RFI (actual dry matter intake (DMI) vs. expected DMI) and standard deviation (Elzo et al. 2012) were calculated for individual animals and were divided into two groups of low (n = 14), and high (n = 15) RFI. High RFI was defined as RFI ≥ 0.5 SD above the mean; and Low RFI was RFI ≤ − 0.5 SD below the mean.
Weekly, approximately 9 mL of blood was collected from the coccygeal vein into serum separator tubes (Corvac, Kendall Health Care, St. Louis, MO) and centrifuged at 4 °C for 20 min at 2000xg. Serum was transferred via aspiration to plastic tubes and frozen at −80 °C for further analysis.
2.1 LC–MS analysis
Serum samples (50 μL) from each steer were extracted for metabolomic analysis using 0.1% formic acid in acetonitrile:water:methanol (2:2:1), as described previously (Kamphorst et al. 2011). Metabolites were separated using a Synergy Hydro-RP column (100 × 2 mm, 2.5 μm particle size). Mobile phases consisted of A: 97:3 H2O:MeOH with 11 mM tributylamine and 15 mM acetic acid and B: MeOH. The gradient consisted of the following: 0.0 min, 0% B; 2.5 min 0% B; 5.0 min, 20% B; 7.5 min, 20% B; 13 min, 55% B; 15.5 min, 95% B; 18.5 min, 95% B; 19 min, 0% B, and 25 min, 0% B. Flow rate was set to a constant 0.200 mL/min and the column temperature was kept at 25 °C. The autosampler tray was kept at 4 °C and 10 μL of sample was injected into the Dionex UltiMate 3000 UPLC system (Thermo Fisher Scientific, Waltham, MA). Electrospray ionization was used to introduce the samples into an Exactive Plus Orbitrap MS (Thermo Fisher Scientific, Waltham, MA), using an established method (Kamphorst et al. 2011; Lu et al. 2010). Representative extracted ion chromatograms (EIC) and compound list with mass accuracies and retention times are available in Supplementary Information (Supplementary 1; Supplementary 2).
2.2 Data analysis
Raw files obtained from Xcalibur MS software (Thermo Electron Corp., Waltham, MA) were converted into the mzML format using ProteoWizard (Chambers et al. 2012). The converted files were imported into MAVEN (Metabolomic Analysis and Visualization Engine for LC–MS Data), a software package (Clasquin et al. 2012). Peaks for the known metabolites were picked in MAVEN, which automatically performs non-linear retention time correction and calculates peak areas across samples, using a preliminary mass error of ± 20 ppm and retention time window of 5 min. The UTK Biological and Small Molecule Mass Spectrometry Core (BSMMSC) has replicated and expanded the method of Rabinowitz and coworkers (Lu et al. 2010) and final metabolite annotations were made using a library of 263 retention time-accurate m/z pairs taken from MS1 spectra. The annotation parameters have been verified previously with pure standards as part of establishing the method. For a metabolite to be annotated as a known compound, the eluted peak had to be found within 2 min of the expected retention time, and the metabolite mass had to be within ± 5 ppm of the expected value. Metabolite identities were confirmed using the MAVEN software package (Clasquin et al. 2012), and peak areas for each compound were integrated using the Quan Browser function of the Xcalibur MS Software (Thermo Electron Corp., Waltham, MA). Metabolomics data were pre-processed and analyzed using Metaboanalyst (Xia et al. 2015). Variables with missing values for > 20% of samples were removed from the dataset prior to statistical analyses. Missing data values were imputed using K-Nearest Neighbors (Stacklies et al. 2007). Peak areas were normalized by median values, transformed logarithmically, and scaled using Pareto scaling prior to statistical testing. Metabolites differing significantly (P < 0.05) between low- and high-RFI steers were identified using t-test. Multiple testing was addressed by setting a false discovery rate of 5%, using the method of Benjamini-Hochberg (Benjamini and Hochberg 1995). Orthogonal partial least squares discriminant analysis (O-PLS-DA) was used to visualize separation between the two groups of steers based on serum metabolite profiles. The metrics R2Y and Q2 were used to evaluate the fit and prediction power, respectively, of the O-PLS-DA model. Validity of model estimates was evaluated using permutation testing (van Velzen et al. 2008). Sample labels were randomly assigned 1000 times, and new models were fitted and estimates of R2Y and Q2 were calculated for each random permutation. Values of R2Y and Q2 from the original model were compared to the distribution of values from the permutations to calculate empirical p-values for R2Y and Q2.
Serum samples were analyzed using a 96-well EPOCH 2 microplate reader (BioTek Instruments, Winooski, VT) with commercial kits for non-esterified fatty acids (NEFA; Wako Chemicals, Richmond, VA; sensitivity of 0.01 mmol/L), glucose (Thermo Electron Corp., Waltham, MA; sensitivity of 0.3 mg/dL), and serum urea nitrogen (SUN; Thermo Electron Corp., Waltham, MA; sensitivity of 2.0 mg/dL). The intra- and interassay CV were, respectively, 4.08 and 3.73% for serum NEFA, 0.53 and 4.31% for serum glucose, 0.30 and 0.35% for SUN. Measurements were tested for normality using the PROC UNIVARIATE command in SAS 9.4 (SAS Institute, Cary, NC) and did not follow normal distribution. Non-parametric one way ANOVA was calculated using Wilcoxon rank-sum test in SAS 9.4. Statistical significance was set at P ≤ 0.05.
3 Results
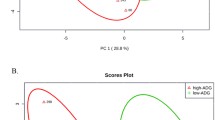
A total of 109 known metabolites were identified in serum samples from low- and high-RFI steers. Multivariate analysis (O-PLS-DA) was used to visualize the extent to which serum metabolomes discriminated steers with low versus high RFI. As shown in Fig. 1, steers tended to separate according to RFI, based on lack of overlap of Hotelling’s 95% confidence intervals. Statistically significant differences in metabolite abundance between the two groups of animals were identified by t-test. After controlling for multiple testing, four metabolites (homocysteine, pantothenate, carnitine and glutamate) differed significantly between low- and high-RFI steers (FDR < 0.05; Fig. 2). Each of the four metabolites was present at lower levels in serum of high- versus low-RFI animals (Table 1).
2D score plot from orthogonal PLS-DA of serum metabolomes from low (green plus symbol) and high (red triangle) RFI steers. Model fitting based on R2Y (0.98) and Q2 (0.41) was validated using permutation testing (P = 0.004 and 0.058, respectively) using 1000 permutations. Ellipses represent 95% confidence intervals
Circulating serum glucose, NEFA, and SUN concentrations did not differ significantly between low- and high-RFI steers (P ≥ 0.05; Table 2).
4 Discussion
Understanding the mechanisms driving the variation in feed efficiency in animals of similar genetic backgrounds could lead to substantial innovations in agriculture. Complex phenotypes such as feed efficiency are attributed to many confounding factors that are not completely understood. We hypothesized that steers differing in feed efficiency during a backgrounding phase would produce different levels of metabolites associated with nutrient utilization. Using LC–MS to perform untargeted metabolomics, it was found that metabolites differed between low- and high-RFI steers were pantothenate, carnitine, homocysteine, glutamine (Table 1), while no differences were observed in circulating serum NEFA, SUN, or glucose concentrations between the two groups (P ˃ 0.05). Delineating discrepancies in metabolite production in steers of dissimilar feed efficiencies may provide critical information critical for understanding factors associated with diverse feed efficiency phenotypes.
Pantothenate, or vitamin B5, was greater in low-RFI steers. Ruminants get PA in several ways; via absorption directly from feed, endogenous synthesis, or microbial production (Wegner et al. 1940). PA is required by all living organisms as it is necessary for the production of coenzyme A (Ball 2006; Begley et al. 2001; Bender 2003; McGinn et al. 2004; Pietrzik et al. 2008). Coenzyme A is key in intermediary metabolism, necessary for many amino acid, carbohydrate, and fat metabolism reactions (Leonardi et al. 2005). This coenzyme typically acts as a carrier protein, binding to acetyl and acyl groups for carbohydrate and fat metabolism, respectively (Leonardi et al. 2005). Acetyl-CoA introduces an acetate group into the tricarboxylic acid (TCA) cycle, while acyl-CoA assists with transport of fatty acids into and out of mitochondria (Begley et al. 2001; Leonardi et al. 2005). Coenzyme A can also be esterified to propionate, the most common glucogenic precursor in healthy and normally functioning cattle (Yost et al. 1977). Propionyl-CoA is converted to succinyl-CoA through a series of enzymatic reactions, which can then enter the TCA cycle (Young 1977). Increased coenzyme A acts as an inhibitory agent in the formation of coenzyme A from PA, but carnitine can reverse this inhibition (Miller et al. 2001). Because carnitine was also greater in low-RFI steers, it may help to counteract the inhibitory processes of the increased concentrations of PA or coenzyme A (Miller et al. 2001). Ruminants are unique in that gluconeogenesis is the primary source of glucose in the ruminant (Freetly and Klindt 1996; Bergman et al. 1974; Young 1977), compared to the non-ruminant, which relies on direct glucose absorption from the diet (Nafikov and Beitz 2007; Young 1977). In amphibians and monogastric species, supplementation of PA results in increased muscle performance, though results are inconsistent (Litoff et al. 1985; Miller et al. 2001; Nice et al. 1984; Shock and Sebrell 1944; Smith et al. 1987). Although physiology of monogastric and ruminant species differ greatly, increased concentrations of PA may increase muscle performance and growth of animals (Karasov and Douglas 2013).
Carnitine concentrations were elevated in low-RFI steers. Carnitine provides several important functions related to metabolism in the body, including import of long chain fatty acids (LCFA) into the mitochondria for β-oxidation via interaction with the coenzyme A attached to the fatty acid (Bremer 1983). Carnitine is also involved in controlling the acetyl-CoA to coenzyme A ratio, transporting long and medium chain fatty acids into the mitochondria, and mediating energy-associated molecules (Arslan 2006). Carnitine is synthesized endogenously, but several precursors and cofactors are required, including lysine, methionine, vitamin C, niacin, vitamin B12, choline, and reduced iron (Bremer 1983). Increasing carnitine in dairy cow hepatic cells stimulated LCFA β-oxidation in vitro (Drackley et al. 1991), and this has been demonstrated in vivo in other species, including beef cattle (Cetin et al. 2003; Greenwood et al. 2010). Carnitine supplementation in vivo also increased plasma glucose concentrations (Cetin et al. 2003; Greenwood et al. 2001). Increased carnitine and subsequent metabolism may result in more efficient nutrient utilization (Karisa et al. 2014). In our study, though no significant differences were reported between and low- and high-RFI steers in serum glucose concentrations, low-RFI steers had numerically greater glucose concentrations than high-RFI steers. Greater carnitine levels may have led to increased concentration of glucose in the steers in this study, though additional studies should be conducted to confirm.
Low-RFI steers also had greater concentrations of homocysteine than high-RFI steers. Homocysteine is an intermediate metabolite in the interconversion pathway of methionine and cysteine and concentrations differ based on physiological need (Lehninger 1977). Homocysteine supplementation increases methionine synthase, the enzyme responsible for conversion of homocysteine to methionine, in vitro (Ortiou et al. 2004). Abomasal infusion of homocysteine increased plasma concentrations of methionine in wethers (Amos et al. 1974). Methionine is required for protein synthesis and other metabolic processes in ruminants (Lehninger 1977; Seymour 2016) and can be an indicator of energy balance in cattle (Pedernera et al. 2010). Methionine is usually the first limiting amino acid, particularly in growing steers (Titgemeyer and Merchen 1990; Richardson and Hatfield 1978). Though no differences were observed between low- and high-RFI steers in methionine or cysteine using the methods described in this study, greater concentrations of homocysteine may indicate increased metabolic reactions involving methionine as a methyl donor (Ditscheid et al. 2005) or cysteine. It could also indicate more substrate availability for methionine-dependent reactions (Amos et al. 1974; Ortiou et al. 2004).
Greater concentrations of glutamine were measured in low-RFI steers. In livestock species, the goal of production is to promote muscle synthesis to increase available animal protein for consumption. In growing ruminants, glutamine may act as an anabolic mediator, increasing muscle growth (Lobley et al. 2001); at the very least, it has been linked to decreased catabolism of muscle (Reecy et al. 1996). Glutamine synthetase, the enzyme responsible for synthesizing glutamine from glutamate and ammonia, increases during time of backgrounding and weaning in steers, but decreases during the finishing stage (Matthews et al. 2016). This could be indicative of decreased need for glutamate for muscle growth, which is responsible for approximately half of circulating glutamine (Matthews et al. 2016). Glutamine is a very potent cause of protein swelling, which leads to protein synthesis (Bequette 2003). Glutamine is also a gluconeogenic amino acid, and it has been hypothesized that greater circulating levels of some gluconeogenic amino acids may moderate apparent energy status of beef cattle, and affect feed intake (Karisa et al. 2014).
A lack of differences in glucose, NEFA, SUN was unexpected given their use as markers of health and nutrient status in cattle (Adewuyi et al. 2005; Gleghorn et al. 2004; Ørskov et al. 1999). Attempts to correlate NEFA, SUN, and glucose with other metabolites, such as carnitine, have yielded inconsistent results (Adewuyi et al. 2005; Greenwood et al. 2001; Kelly et al. 2010). Glucose is highly regulated in ruminants and is constantly being produced via gluconeogenesis (Young 1977). Glucose concentrations can remain the same in growing steers regardless of diet (Seal et al. 2007). The tight regulation of glucose in ruminants may explain the lack of differences found between low- and high-RFI steers in this study. NEFA concentrations are used to infer information about the nutrient status of cattle (Bowden 1971). NEFA in blood is a result of mobilization of adipose tissue, particularly during times of fasting or high energy requirements, such as lactation (Bowden 1971; Reid and Hinks 1962). However, because the steers used in this study were growing steers, they would have little need to mobilize adipose tissue and would have lower concentrations of NEFA in serum. SUN is used in ruminants and other production species as an indicator of protein intake (Preston et al. 1965), nitrogen utilization (Egan and Kellaway 1971; Kohn et al. 2005), and nitrogen intake (Nolan et al. 1970). It is currently regarded as one of the most effective measurements of protein status in ruminants (Herdt 2000). In dairy cows, SUN concentrations increase after feeding (Miettinen and Juvonen 1990). Blood sample collections occurred prior to morning feeding in this study, which may partially account for the decreased concentrations in SUN.
Based on the findings of this study, Black Angus beef steers differing in feed efficiency exhibit varying levels of serum metabolites associated with nutrient utilization and energy status. The majority of the metabolites that were found to be in greater blood circulation in low-RFI steers are associated with energy usage, including pantothenate, glutamine, carnitine, and homocysteine. In addition, these four metabolites are intimately related, as they either inhibit or facilitate nutrient metabolism reactions. Determining causes of divergence in the production of these metabolites may, in part, account for some of the variation in feed efficiency of growing Black Angus beef steers. Subsequent analyses utilizing different techniques, such as transcriptomics, could bridge the gap between correlation and causation. Additional analyses should also be conducted across breeds and species to determine metabolite biomarkers that may be unique to those animals. Further understanding of the mechanisms driving these trends will result in improved nutrient utilization, increased feed efficiency, and reduction of production costs.
References
Adewuyi, A., Gruys, E., & Van Eerdenburg, F. (2005). Non esterified fatty acids (NEFA) in dairy cattle. A review. Veterinary Quarterly, 27(3), 117–126.
Amos, H. E., Little, C. O., Digenis, G. A., Schelling, G. T., Tucker, R. E., & Mitchell, G. E. (1974). Methionine, DL-homocysteine thiolactone and N-acetyl-DL-methionine for ruminants1. Journal of Animal Science, 39(3), 612–617. doi:10.2527/jas1974.393612x
Arslan, C. (2006). L-carnitine and its use as a feed additive in poultry feeding a review. [Review]. Revue de médecine vétérinaire, 157(3), 134–142.
Arthur, P., Renand, G., & Krauss, D. (2001). Genetic and phenotypic relationships among different measures of growth and feed efficiency in young Charolais bulls. Livestock Production Science, 68(2), 131–139.
Ball, G. (2006). F. vitamins in foods. Analysis, bioavailability, and stability. Boca Raton: CRC Press.
Begley, T. P., Kinsland, C., & Strauss, E. (2001). The biosynthesis of coenzyme a in bacteria. Vitamins & Hormones, 61, 157–171. doi:10.1016/S0083-6729(01)61005-7
Bender, D. A. (2003). Nutritional biochemistry of the vitamins. Cambridge: Cambridge University Press.
Benjamini, Y., & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B, 57(1), 289–300.
Bequette, B. (2003). Amino acid metabolism in animals: An overview. Amino Acids in Animal Nutrition, 2, 103–124.
Bergman, E., Brockman, R., & Kaufman, C. (1974). Glucose metabolism in ruminants: Comparison of whole-body turnover with production by gut, liver, and kidneys. Federation Proceedings, 33(7), 1849–1854.
Berry, D., & Crowley, J. (2013). Cell biology symposium: Genetics of feed efficiency in dairy and beef cattle. Journal of Animal Science, 91(4), 1594–1613.
Bowden, D. (1971). Non-esterified fatty acids and ketone bodies in blood as indicators of nutritional status in ruminants: A review. Canadian Journal of Animal Science, 51(1), 1–13.
Bremer, J. (1983). Carnitine–metabolism and functions. Physiological reviews, 63(4), 1420–1480.
Cetin, M., Petek, M., Polet, U., & Yalcin, A. (2003). Effects of dietary carnitine supplementation on plasma carnitine and some serum biochemical parameters in lambs. Revue de médecine vétérinaire, 154(3), 195–198.
Chambers, M. C., Maclean, B., Burke, R., Amodei, D., Ruderman, D. L., Neumann, S., et al. (2012). A cross-platform toolkit for mass spectrometry and proteomics. Nature Biotechnology, 30(10), 918–920.
Clasquin, M. F., Melamud, E., & Rabinowitz, J. D. (2012). LC-MS data processing with MAVEN: A metabolomic analysis and visualization engine. Current Protocol Bioinformatics. doi:10.1002/0471250953.bi1411s37
Ditscheid, B., Funfstuck, R., Busch, M., Schubert, R., Gerth, J., & Jahreis, G. (2005). Effect of L-methionine supplementation on plasma homocysteine and other free amino acids: A placebo-controlled double-blind cross-over study. European Journal of Clinical Nutrition, 59(6), 768–775.
Drackley, J. K., Beitz, D., & Young, J. (1991). Regulation of in vitro metabolism of palmitate by carnitine and propionate in liver from dairy cows1. Journal of Dairy Science, 74(9), 3014–3024.
Egan, A., & Kellaway, R. (1971). Evaluation of nitrogen metabolites as indices of nitrogen utilization in sheep given frozen and dry mature herbages. British Journal of Nutrition, 26(3), 335–351.
Elzo, M., Lamb, G., Johnson, D., Thomas, M., Misztal, I., Rae, D., et al. (2012). Genomic-polygenic evaluation of Angus-Brahman multibreed cattle for feed efficiency and postweaning growth using the Illumina 3K chip. Journal of animal science, 90(8), 2488–2497.
Fontanesi, L. (2016). Merging Metabolomics, Genetics, and Genomics in Livestock to Dissect Complex Production Traits. Systems Biology in Animal Production and Health, Vol. 1, (pp. 43–62). Cham: Springer.
Freetly, H. C., & Klindt, J. (1996). Changes in gut and liver glucose, lactate, insulin, and oxygen flux in mature ewes during mesenteric or abdominal vena cava glucose infusion. The Journal of Nutrition, 126(4), 924–932.
Gleghorn, J. F., Elam, N. A., Galyean, M. L., Duff, G. C., Cole, N. A., & Rivera, J. D. (2004). Effects of crude protein concentration and degradability on performance, carcass characteristics, and serum urea nitrogen concentrations in finishing beef steers. Journal of Animal Science, 82(9), 2705–2717. doi:10.2527/2004.8292705x
Greenwood, R., Löest, C., Titgemeyer, E. C., & Drouillard, J. S. (2010). Effects of carnitine on performance of finishing steers. Kansas Agricultural Experimental Station Research Reports, 1, 38–39.
Greenwood, R. H., Titgemeyer, E. C., Stokka, G. L., Drouillard, J. S., & Löest, C. A. (2001). Effects of L-carnitine on nitrogen retention and blood metabolites of growing steers and performance of finishing steers. Journal of animal science, 79(1), 254–260. doi:10.2527/2001.791254x.
Herdt, T. H. (2000). Variability characteristics and test selection in herdlevel nutritional and metabolic profile testing. Veterinary Clinics of North America: Food Animal Practice, 16(2), 387–403.
Holmgren, L., & Feuz, D. (2015). 2015 Costs and Returns for a 200 Cow, Cow-Calf Operation. Northern Utah: Utah State University Extension. http://digitalcommons.usu.edu/extension_curall/711/.
Kamphorst, J. J., Fan, J., Lu, W., White, E., & Rabinowitz, J. D. (2011). Liquid chromatography-high resolution mass spectrometry analysis of fatty acid metabolism. Analytical Chemistry, 83(23), 9114–9122. doi:10.1021/ac202220b
Karasov, W. H., & Douglas, A. E. (2013). Comparative digestive physiology. Comprehensive Physiology, 3(2), 741–783. doi:10.1002/cphy.c110054
Karisa, B., Thomson, J., Wang, Z., Li, C., Montanholi, Y., Miller, S., et al. (2014). Plasma metabolites associated with residual feed intake and other productivity performance traits in beef cattle. Livestock Science, 165, 200–211.
Kelly, A. K., McGee, M., Crews, D. H., Fahey, A. G., Wylie, A. R., & Kenny, D. A. (2010). Effect of divergence in residual feed intake on feeding behavior, blood metabolic variables, and body composition traits in growing beef heifers 1. Journal of Animal Science, 88(1), 109–123. doi:10.2527/jas.2009-2196
Koch, R. M., Swiger, L. A., Chambers, D., & Gregory, K. E. (1963). Efficiency of feed use in beef cattle. Journal of Animal Science, 22(2), 486–494.
Kohn, R., Dinneen, M., & Russek-Cohen, E. (2005). Using blood urea nitrogen to predict nitrogen excretion and efficiency of nitrogen utilization in cattle, sheep, goats, horses, pigs, and rats. Journal of Animal Science, 83(4), 879–889.
Krysl, L., & Hess, B. (1993). Influence of supplementation on behavior of grazing cattle. Journal of Animal Science, 71(9), 2546–2555.
Lamprecht, E. D., & Williams, C. A. (2012). Biomarkers of antioxidant status, inflammation, and cartilage metabolism are affected by acute intense exercise but not superoxide dismutase supplementation in horses. Oxidative medicine and cellular longevity, 2012, 1–15. doi:10.1155/2012/920932.
Lawrence, J., Mintert, J., Anderson, J. D., & Anderson, D. P. (2008). Feed grains and livestock: Impacts on meat supplies and prices. Choices, 23(2), 11.
Lehninger, A. (1977). Biochemistry (2nd edn.). New York: Worth Publishers Inc.
Leonardi, R., Zhang, Y.-M., Rock, C. O., & Jackowski, S. (2005). Coenzyme A: Back in action. Progress in Lipid Research, 44(2), 125–153.
Litoff, D., Scherzer, H., & Harrison, J. (1985). # 17 Effects Of pantothenic acid supplementation on human exercise. Medicine & Science in Sports & Exercise, 17(2), 287.
Lobley, G. E., Hoskin, S. O., & McNeil, C. J. (2001). Glutamine in animal science and production. The Journal of Nutrition, 131(9), 2525S–2531S.
Lu, W., Clasquin, M. F., Melamud, E., Amador-Noguez, D., Caudy, A. A., et al. (2010). Metabolomic analysis via reversed-phase ion-pairing liquid chromatography coupled to a stand alone orbitrap mass spectrometer. Analytical Chemistry, 82(3), 3212–3221. doi:10.1021/ac902837x
Mader, T. L., & Davis, M. S. (2004). Effect of management strategies on reducing heat stress of feedlot cattle: Feed and water intake1. Journal of Animal Science, 82(10), 3077–3087. doi:10.2527/2004.82103077x
Matthews, J. C., Huang, J., & Rentfrow, G. (2016). High-affinity glutamate transporter and glutamine synthetase content in longissimus dorsi and adipose tissues of growing Angus steers differs among suckling, weanling, backgrounding, and finishing production stages1. Journal of Animal Science, 94(3), 1267–1275. doi:10.2527/jas.2015-9901
McGinn, S. M., Beauchemin, K. A., Coates, T., & Colombatto, D. (2004). Methane emissions from beef cattle: Effects of monensin, sunflower oil, enzymes, yeast, and fumaric acid1. Journal of Animal Science, 82(11), 3346–3356. doi:10.2527/2004.82113346x
Miettinen, P., & Juvonen, R. (1990). Diurnal variations of serum and milk urea levels in dairy cows. Acta Agriculturae Scandinavica, 40(3), 289–296.
Miller, J. W., Rogers, L. M., & Rucker, R. B. (2001). Pantothenic acid (p. 253). Washington, DC: ILSI Press.
Myer, P. R., Wells, J. E., Smith, T. P. L., Kuehn, L. A., & Freetly, H. C. (2017). Analysis of the gut bacterial communities in beef cattle and their association with feed intake, growth, and efficiency. Journal of Animal Science. doi:10.2527/jas2016.1059
Nafikov, R. A., & Beitz, D. C. (2007). Carbohydrate and lipid metabolism in farm animals. The Journal of Nutrition, 137(3), 702–705.
Nice, C., Reeves, A., Brinck-Johnsen, T., & Noll, W. (1984). The effects of pantothenic acid on human exercise capacity. Journal of Sports Medicine and Physical Fitness, 24(1), 26–29.
Nolan, J., Cocimano, M., & Leng, R. (1970). Prediction of parameters of urea metabolism in sheep from the concentration of urea in plasma. In Proceedings of the Australian Society of Animal Production (Vol. 8, pp. 22). Wagga Wagga, NSW, Australia: Australian Society of Animal Production.
Norton, M. (2005). Factors affecting beef and cattle producer prices movements. Monthly Laboratory Review, 128, 32.
Ørskov, E., Meehan, D., MacLeod, N., & Kyle, D. (1999). Effect of glucose supply on fasting nitrogen excretion and effect of level and type of volatile fatty acid infusion on response to protein infusion in cattle. British Journal of Nutrition, 81(05), 389–393.
Ortiou, S., Alberto, J.-M., Guéant, J.-L., & Merten, M. (2004). Homocysteine increases methionine synthase mRNA level in Caco-2 cells. Cellular Physiology and Biochemistry, 14(4–6), 407–414.
Pedernera, M., Celi, P., García, S. C., Salvin, H. E., Barchia, I., & Fulkerson, W. J. (2010). Effect of diet, energy balance and milk production on oxidative stress in early-lactating dairy cows grazing pasture. The Veterinary Journal, 186(3), 352–357.
Pietrzik, K., Golly, I., & Loew, D. (2008). Handbuch der Vitamine (für Prophylaxe Therapie u. Beratung). Munchen: Urban and Fischer Verlag.
Preston, R., Schnakenberg, D., & Pfander, W. (1965). Protein utilization in ruminants. 1. Blood urea nitrogen as affected by protein intake. Journal of Nutrition, 86, 281–288.
Reecy, J. M., Williams, J. E., Kerley, M. S., MacDonald, R. S., Thornton, W. H., & Davis, J. L. (1996). The effect of postruminal amino acid flow on muscle cell proliferation and protein turnover. Journal of Animal Science, 74(9), 2158–2169.
Reid, R., & Hinks, N. (1962). Studies on the carbohydrate metabolism of sheep. XVII. Feed requirements and voluntary feed intake in late pregnancy, with particular reference to prevention of hypoglycaemia and hyperketonaemia. Australian Journal of Agricultural Research, 13(6), 1092–1111.
Richardson, C., & Hatfield, E. (1978). The limiting amino acids in growing cattle. Journal of Animal Science, 46(3), 740–745.
Seal, C. J., Parker, D. S., & Avery, P. J. (2007). The effect of forage and forage–concentrate diets on rumen fermentation and metabolism of nutrients by the mesenteric- and portal-drained viscera in growing steers. British Journal of Nutrition, 67(3), 355–370. doi:10.1079/BJN19920041
Seymour, W. M. (2016). Role of methionine and methionine precursors in transition cow nutrition with emphasis on liver function. In Proceedings of the 2016 Florida ruminant nutrition symposium (pp. 11–15). Gainesville, FL: University of Florida.
Shock, N., & Sebrell, W. (1944). The effect of changes in concentration of pantothenate on the work output of perfused frog muscles. American Journal of Physiology–Legacy Content, 142(2), 274–278.
Smith, C. M., Narrow, C. M., Kendrick, Z. V., & Steffen, C. (1987). The effect of pantothenate deficiency in mice on their metabolic response to fast and exercise. Metabolism: Clinical and Experimental, 36(2), 115–121.
Stacklies, W., Redestig, H., Scholz, M., Walther, D., & Selbig, J. (2007). pcaMethods—A bioconductor package providing PCA methods for incomplete data. Bioinformatics, 23(9), 1164–1167. doi:10.1093/bioinformatics/btm069
Titgemeyer, E. C., & Merchen, N. R. (1990). The effect of abomasal methionine supplementation on nitrogen retention of growing steers postruminally infused with casein or nonsulfur-containing amino acids. Journal of animal science, 68(3), 750–757.
van Velzen, E. J., Westerhuis, J. A., van Duynhoven, J. P., van Dorsten, F. A., Hoefsloot, H. C., Jacobs, D. M., et al. (2008). Multilevel data analysis of a crossover designed human nutritional intervention study. Journal of Proteome Research, 7(10), 4483–4491. doi:10.1021/pr800145j.
Wegner, M., Booth, A., Elvehjem, C., & Hart, E. (1940). Rumen Synthesis of the Vitamin B Complex. Proceedings of the Society for Experimental Biology and Medicine, 45(3), 769–771.
Xia, J., Sinelnikov, I. V., Han, B., & Wishart, D. S. (2015). MetaboAnalyst 3.0—making metabolomics more meaningful. Nucleic Acids Research, 43(W1), W251–W257.
Yost, W. M., Young, J. W., Schmidt, S. P., & McGilliard, A. D. (1977). Gluconeogenesis in ruminants: Propionic acid production from a high-grain diet fed to cattle. The Journal of Nutrition, 107(11), 2036–2043.
Young, J. (1977). Gluconeogenesis in cattle: Significance and methodology1. Journal of Dairy Science, 60(1), 1–15.
Acknowledgements
This study was supported by Ascus Biosciences, Inc. (Grant No. A17-0146-003) and USDA-NIFA Hatch/Multistate Project W2010- TEN00493—Integrated Approach to Enhance Efficiency of Feed Utilization in Beef Production Systems. The authors thank Ashley Honey and the staff at the Plateau Research and Education Center in Crossville, TN for their technical assistance.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Information 1: Representative extracted ion chromatograms (EIC). Representative EIC included with m/z ratio and peak areas.
Supplementary Information 2: Compound list containing mass accuracies and retention times.
Rights and permissions
About this article
Cite this article
Clemmons, B.A., Mihelic, R.I., Beckford, R.C. et al. Serum metabolites associated with feed efficiency in black angus steers. Metabolomics 13, 147 (2017). https://doi.org/10.1007/s11306-017-1282-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-017-1282-z