Abstract
The principal objectives of the study were to develop metabolomics tool and to test its efficiency for discrimination and biochemical pattern recognition of genotypes and phenotypes of silver birch trees (Betula pendula Roth). In the experiment were used two birch genotypes (GT 2 and GT 5) that have been grown over seven years on the two open fields A and B. The metabolomics tool was applied also to study biochemical responses of the GT 2 to elevated (1.5 × ambient) concentration of ozone as an environmental stress factor. These trees were treated with ozone over seven years using an open-air exposure system. The developed metabolomics tool was based on the analyses of lipophilic and polar compounds of birch leaves with GC-MS and HPLC-DAD (polar phenolics only). The metabolome database included 331 chemical traits and was analyzed with descriptive and multivariate statistics. Application of cluster and principle component analyses clearly discriminated genetically different birch trees. In addition, the genotype clusters were further divided into two subclusters corresponding to trees from field A and field B. Formation of these phenotypes was due to the differences in some environmental conditions between the field A and field B. Biochemical discrimination between phenotypes of control and ozone-treated birch trees of GT 2 was found also. However, distances between these phenotypes, as well as between phenotypes of control trees from the field A and field B were found to be considerably smaller than between birch genotypes. Metabolites with the largest contribution to birch genotype/phenotype discrimination were determined and some were identified.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Development of novel analytical strategies is essential in order to advance our understanding of the molecular basis of woody plant adaptation to environmental stress factors (Wang et al. 2004; Vinocur and Altman 2005). Genomics, transcriptomics and proteomics can provide insights into the potential of a plant to interact with external perturbations. However, these technologies do not provide direct information on how changes in mRNA or proteins are coupled with changes in biological functions. The metabolites are the end products of gene expression and, therefore, the changes in their levels may thus be regarded as the ultimate response of plant to the environmental stress (Fiehn 2002).
There is a long tradition of and extensive knowledge on metabolite analysis, but only recently this analysis has grown into an “omics” level field––metabolomics (Trethewey 2001). Metabolomics is a set of analytical and bioinformatics methods for comprehensive and unbiased identification and quantification of all the low molecular weight compounds (metabolome) present in a specific biological sample from a plant grown under defined conditions. The principle objective of metabolomics is to define the biochemical changes in the plant object due to genetic modification or environmental stress.
Plant metabolites are chemically very heterogeneous. In addition to relatively simple carbohydrates, amino acids and organic acids, plants contain very complex secondary compounds such as phenolics, alkaloids and terpenoids. The huge chemical diversity of plant metabolome is the big problem for its analysis, but it can be solved satisfactory by combination of different methods of chromatography with mass spectrometry detection. For example, gas chromatography––mass spectrometry (GC-MS) is a technique suitable for the analysis of thermostable compounds (Fiehn et al. 2000; Roessner et al. 2001). However, for quantification of complex secondary compounds, HPLC system coupled with mass spectrometry detector or diode-array detector (DAD) is more useful (Sumner et al. 2003).
In plant metabolomics, in order to exclude effects of unnecesary environmental factors on the variability of metabolites, it is recommended that experimental plants are grown in the phytotron under highly controlled conditions (Sumner et al. 2003). However, this approach cannot be always applied to woody plants because of their big size or specific conditions of the long-term experiments that require growing of plants under the open-field conditions close to natural. In this case, local environmental conditions for growth of individual trees or leaves of the same tree could be quite different, which may affect the results of biochemical phenotyping of experimental trees and lead to difficulties in their interpretation. Therefore, there is a need to test the efficiency of the metabolomics tool to detect biochemical responses of mature woody plants to environmental stress factors in the long-term open-field experiments.
Birch is a rapidly developing model in Finland to study chemical ecology of woody plants. This plant has been employed in the research of biochemical responses and adaptation to both natural and anthropogenic environmental stress factors (herbivores, elevated concentrations of CO2 and ozone, air pollution, etc. (Haukioja et al. 1998; Loponen et al. 1998; Ossipov et al. 2001; Saleem et al. 2001; Oksanen 2003; Peltonen et al. 2005; Valkama et al. 2007). However, only a small number of target birch metabolites was recorded and quantified in the majority of earlier studies.
Therefore, the principal objectives of the study were to develop metabolomics tool and to test its efficiency for discrimination and biochemical pattern recognition of genotypes and phenotypes of silver birch trees (Betula pendula Roth). Two birch genotypes GT 2 and GT 5 have been grown over seven years in the open-field conditions and were used in the experiment. The metabolomics tool was applied also to study biochemical responses of one of the genotypes (GT 2) to elevated (1.5 × ambient) concentration of ozone as an environmental stress factor. These trees of GT 2 were treated with ozone over seven years using an open-air exposure system (Oksanen 2003; Karnosky et al. 2007).
2 Materials and methods
2.1 Plant object
One-year old micropropagated plants of two Betula pendula Roth genotypes (GT 2 and GT 5) were planted at 1.0-m spacing in randomised block design into two control fields (A and B) and two elevated-ozone fields (C and D); ten plants per genotype per exposure field in May 1996. It has been shown earlier that GT 2 is tolerant and GT 5 is sensitive to elevated concentration of ozone that is manifested most noticeably in the magnitude of ozone-induced reduction of tree growth and leaf-level net photosynthesis (Oksanen 2003). Many others ecological, physiological and biochemical differences of these genotypes have been described also (Saleem et al. 2001; Kontunen-Soppela et al. 2007; Valkama et al. 2007).
2.2 Open field conditions
The trees were grown in natural soil and exposed to ambient and elevated ozone using an open field exposure system situated at the Kuopio University Research Garden (62°13′ N, 27°35′ E) (Oksanen 2003; Karnosky et al. 2007).
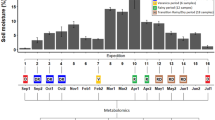
Volumetric soil moisture content was monitored 0.2 m below soil surface using a soil moisture sensor (Theta Probe, Delta-T Devices, Cambridge, UK) and supplemental water was added according to need (Oksanen 2003). The plants were fertilized weekly with 0.2% Superex-9 (N:P:K, 19:5:20, Kekkilä, Finland) until mid of July each year, corresponding to an nitrogen supply of 78 kg N/ha/year. The fertilizer was distributed around each tree in an area 40 cm in diameter. The pH and nutrient concentrations for Ca, P, K and Mg were analysed by Viljavuuspalvelu Oy (Mikkeli, Finland) separately for humus and mineral layers using four soil samples per each field at the beginning and end of each growing season (Oksanen 2003).
All the plants were measured for total stem height (H) and stem basal diameter (D) at ground level (2 cm above the soil surface) at the end of each growing season. Stem volume (dm3) was calculated from total stem height and stem basal diameter (D 2 H) (Oksanen 2003). The final destructive harvest was conducted before the bud opening on 16 May 2003. At harvest, all the trees were determined for final stem height and base diameter, the roots were excavated and dry masses were determined for branches (including buds), stem and roots separately (Oksanen 2003; Kontunen-Soppela et al. 2007).
Ozone was produced from pure oxygen with an ozone generator (Fisher OZ 500, Meckenheim, Bonn, Germany), and ozone-enriched air was injected into exposure fields at the vertical vent pipes from upwind direction. Ozone concentrations were continuously monitored with an ozone analyzer (Dasibi 1008-RS, Dasibi Environmental Corp., Glendale, CA, US) from four sampling points within the canopy in each exposure field. The ozone analyzer was calibrated against the reference standard UV Photometer SRP#11 (National Institute of Standards, USA) at the Finnish Meteorological Institute. The fumigations were carried out diurnally except during very low or high wind speeds or rain. Wind speed and direction was measured at the centre of each exposure field, at the top of the canopy. The target set for ozone enrichment was 1.5 times the ambient ozone level, based on trends and scenarios of ground-level ozone concentrations in Finland (Laurila et al. 2004).
2.3 Sampling of birch leaves
Samples for analysis of birch leaf metabolome were taken from six control trees of GT 2, six control trees GT 5, and eight ozone-treated trees of GT 2, which were not damaged by hare browsing during winter 1996–1997 years.
Each experimental tree was divided vertically into three segments (lower, middle and upper) and one representative branch per segment from the southern side of tree was selected. Three leaf samples from three branches per tree were collected from fully-expanded short-shoot leaves on 16 August 2002 between 10:30 and 13:30. A total of 6–8 leaves per sample were immediately placed in 5-ml plastic tube (Greiner, Labortechnik), frozen in liquid nitrogen and transported to the laboratory. At the laboratory the plant material was freeze-dried over 72 h, homogenized into a powder using an MM 301 vibration mill (Retsch GmbH & Co. KG) at a frequency of 30 Hz/s for 2 min and stored at −20°C.
2.4 Plant sample processing
Summary of developed birch leaf metabolomics tool is presented in Fig. 1.
Strategy of birch leaf metabolomics. Developed tool includes a sample extraction, a single fractionation step of leaf extract into lipophilic and polar phases, external silylation of metabolites, and their separation and quantification with GC-MS. HPLC-DAD is applied for analysis of polar phenolics only. The metabolite data of experimental trees are combined into raw database, normalized and compared using descriptive and multivariate statistics
2.4.1 Metabolite extraction
A sample of the birch leaf powder (30.0 ± 1.0 mg) was placed in 1.5-ml plastic Eppendorf tube (ET) of known weight. Then we added to each tube 50 μl of ribitol in 50% aqueous methanol (polar internal standard, 0.2 mg/ml), 50 μl of nonadecanoic acid methyl-ester in 100% acetone (non-polar internal standard, 2 mg/ml) and 0.65 ml of 80% aqueous acetone. The sample was allowed to stand for 30 min at room temperature with continuous stirring (1.5 units, Vortex, Genie 2). The homogenate was centrifuged for 10 min at 2500 g and the first portion of the extract was transferred into 2.0-ml plastic ET. The pellet was re-extracted again with 0.65 ml of 80% acetone and then twice with the same volume of 100% acetone. All extracts were combined and acetone was removed in a vacuum concentrator (Concentrator 5301, Eppendorf AG, Germany). The resulting aqueous phase was frozen and lyophilized over 24 h. Two samples from control trees GT 2 have been lost during their extraction.
2.4.2 Fractionation of extract
The lyophilized extract was dissolved in 1.0 ml of the chloroform/methanol/water mixture (50/30/20, v/v) and vortexed for 25 min. Then 0.5 ml of 20% aqueous methanol was added to the extract. The resulting biphasic system was vortexed for 10 min and then centrifuged for 10 min at 2900 g (+4°C) to separate the two solvent phases (chloroform/methanol and methanol/water). Chloroform/methanol fraction of lipophilic compounds (about 0.5 ml) was transferred into 1.5-ml ET and organic solvents were evaporated in the vacuum concentrator (Concentrator 5301, Eppendorf AG, Germany). Methanol/water fraction of polar compounds was concentrated in the vacuum concentrator to remove methanol and the water phase was frozen and lyophilized. Dry residue of polar compounds was dissolved in 0.7 ml of water, filtered through a 0.45 μm PTFE filter and frozen. Samples of lipophilic and polar compounds were stored in the freezer at −20°C.
2.5 Metabolome analysis
2.5.1 HPLC-DAD analysis of polar phenolics
Individual phenolic compounds were studied in the polar fraction with HPLC-DAD system (Merck-Hitachi, Tokyo, Japan) that included a pump L-7100, a diode array detector L-7455, a programmable autosampler L-7250, and an interface D-7000. The column used was Superspher 100 RP-18 (75 × 4 mm i.d., 4 μm, Merck, Germany). A 0.1 ml of polar fraction was transferred into vials for HPLC autosampler with 0.15-ml inserts and closed by caps with septa. Two solvents were used: (A) 0.05 M H3PO4; (B) acetonitrile. The elution profile was: 0–3 min, 98% A (isocratic); 3–22 min, 2–20% B in A (linear gradient); 22–30 min, 20–30% B in A (linear gradient). Flow rate was 1 ml/min; detection at 280 nm. The acquisition of UV spectra (230–500 nm) was done automatically at the apex of each peak. A selected set of samples was also analyzed with HPLC-ESI-MS as in Salminen et al. (1999). Individual phenolics were identified on the basis of their UV and mass spectra.
About 0.05–0.1 ml of polar fraction was used for quantification of soluble proanthocyanidins (condensed tannins) (Ossipova et al. 2001).
2.5.2 GC-MS analysis of lipophilic and polar metabolites
For GC-MS analysis, lipophilic compounds were dissolved in a 0.5 ml mixture of methanol/chloroform (1/1, v/v). A 0.3 ml was taken and transferred into 1.5-ml ET, and organic solvents were evaporated in vacuum. Dry residue was dissolved in a 50 μl mixture of pyridine-chloroform (1/1, v/v) and 50 μl of N-methyl-N-(trimethylsilyl) trifluoroacetamide (MSTFA) with 5% of trimethylchlorosilane (TMCS) as a catalyst (Sigma-Aldrich) and incubated for 1 h at 50°C and 24 h at room temperature. TMS derivatives of lipophilic compounds were stable for at least 48 h.
For derivatization of polar compounds, a two-step procedure was used: carbonyl moieties were first protected using methoximation, and thereafter acidic protons were exchanged for trimethylsilyl groups (TMS). A 0.4 ml of polar fraction (total volume 0.7 ml) was transferred into 1.5-ml ET and freeze-dried. About 80 μl of methoxyamine hydrochloride in pyridine (20 mg/ml) was added to the dry polar compounds and vortexed for 20 min with low speed. Then the solution was incubated at 50°C for 1 h and cooled for 20 min to the room temperature. A 80 μl MSTFA with 5% of TMCS was added, mixed and incubated at 50°C for 1 h. TMS derivatives of polar compounds were stable for at least 3–44 h.
TMS derivatives of lipophilic and polar compounds were transferred into Perkin-Elmer autosampler vials with 0.2-ml glass inserts, closed by caps with septa, and analyzed with the Perkin-Elmer GC-MS system (GC AutoSystem XL with TurboMass Gold quadrupole mass spectrometer, Norwalk, CT, USA). Mass spectrometer was used in the electron impact mode (EI+); the data acquisition scan time was 0.45 s and the scan range from 50 to 620 m/z. The column was a Perkin-Elmer capillary column (PE-5MS, 30 m, 0.25 mm i.d., film 0.25 μm) and helium was used as a carrier gas with a flow rate 1.0 ml/min.
The injection volume of lipophilic compounds was 2.0 μl and the split ratio 15:1. The injector temperature was 300°C and the inlet line and the MS source were held at 310°C and 200°C, correspondingly. Oven temperature was programmed as follows: initial temperature 110°C (hold 2.0 min) followed by temperature increase of 4°C/min up to 260°C, and then 1.5°C/min to final temperature 310°C, which was held for 17.17 min. Total run time of analysis was 90 min.
The injection volume of polar compounds was 1.0 μl and the split ratio 20:1. The injector temperature was 280°C and the inlet line and the MS source were held at 290°C and 200°C correspondingly. Oven temperature was programmed as follows: initial temperature 70°C (2.0 min) followed by temperature increase of 5°C/min up to 250°C, and then 20°C/min to final temperature 290°C, which was held for 17 min. Total run time of analysis was 60 min.
2.6 Raw data processing
Raw data of GC-MS analysis were processed by TurboMass Gold V.4.3 software (Perkin-Elmer). Peak area calculation was performed according to the following procedure. First, two chromatograms with the highest number of detected polar and lipophilic metabolites were chosen as master chromatograms. After automatic deconvolution of mass spectra of coeluting peaks with AMDIS software (Halket et al. 1999), the unique mass fragments for peak area integration and retention times for all individual compounds were determined. Many of the detected compounds were identified by using database searches (NIST and NBS) and mass spectra of reference compounds. On the basis of this information, two methods for quantification of lipophilic compounds and polar compounds were developed and peak areas of all individual metabolites in all samples were recorded. The results were exported to an Excel spreadsheet and relative contents of metabolites were calculated by normalizing their peak areas to the response of internal standards, and, further, to 1 g of dry mass of leaf sample.
For quantification of polar phenolics, HPLC raw data of all samples were exported into COWTool software (COWTool version 1.1., Department of Biotechnology, DTU, Denmark) in text format and subjected to baseline correction to reduce the noise level. Afterwards chromatograms derived from each sample were aligned against the master chromatogram of polar phenolics to correct retention time differences between different samples. Processed raw data were exported to an Excel spreadsheet and used for determination of retention time and height of all phenolic peaks. Relative content of individual phenolics was calculated as a peak height per 1 g of dry mass of leaf sample.
Relative contents of all metabolites in Excel format were combined in a table where the first column contains the metabolite names and the first row––sample names. Database normalization was done with web-based MetaGeneAlyse software (Daub et al. 2003; http://metagenealyse.mpimp-golm.mpg.de). First, each value of the row was normalized to the largest value within each row, and then the vector normalization (Euclidean normalisation) was used for columns (samples). Descriptive statistics (Student’s t-test) and multivariate analyses of database were done with SPSS 12.0.1 (SPSS Inc.).
3 Results and discussion
3.1 Metabolomics tool development
Successful deployment of metabolomics for genotype/phenotype discrimination requires the development of rapid, reliable, and efficient assays which will allow the detection of as many metabolites as possible and preferably at high-throughput rates. Although the desire is to have a single analysis that captures all metabolites in a short time, there is no single analysis that will be appropriate for all metabolites with a high degree of sensitivity and resolution. Therefore, for analysis of birch leaf metabolome, we applied combination of two techniques: GC-MS for analysis of thermostable compounds, and HPLC-DAD for polar phenolics (Fig. 1).
Extract of birch leaves represents a very complex mixture of metabolites with different chemical properties and at varying concentrations. It is thus difficult to have a good resolution of metabolites in a single GC-MS analysis of total extract. Therefore, we separated the total extract into fractions of polar and lipophilic compounds and analyzed these fractions separately (Fig. 1).
Characteristic GC-MS traces of lipophilic and polar fractions of birch leaves are shown on Fig. 2. We have recorded and quantified 283 lipophilic and polar metabolites. About 97 metabolites were identified at some detail, 124 metabolites––as molecular type (e.g., “monosaccharide”, “triterpenoid”), and 62 metabolites are not identified yet (supporting information can be downloaded from website http://users.utu.fi/ossipov/index.htm).
Characteristic GC-MS traces of lipophilic (a) and polar (b) metabolites of birch leaves. 283 metabolites are recorded and quantified in the lipophilic and polar fractions of extract. 97 metabolites are identified at some detail, 124 metabolites––as molecular type, and 62 metabolites are not identified. In addition, 48 individual phenolic compounds are quantified in polar fraction with HPLC-DAD
Phenolic segment of the birch leaf metabolome was analyzed with HPLC-DAD method that was developed for a different birch species (Ossipov et al. 1995). Thirteen major phenolics of birch leaves were previously isolated with preparative HPLC and identified with 1H-NMR, 13C-NMR and MS techniques (Ossipov et al. 1995, 1996). To confirm the results of the previous study of silver birch (Ossipov et al. 1996) and to identify minor phenolics, we applied HPLC-DAD and HPLC-ESI-MS detection (Salminen et al. 1999). Altogether, 31 individual phenolics (from total of 48) were identified in the polar fraction on the basis of their UV-spectra and MS-spectra (molecular ions and specific fragment ions) (data available on the website http://users.utu.fi/ossipov/index.htm).
The final result of the analytical part of the study was a metabolome database formation that is a simple 2D matrix with metabolites in rows and samples in columns. The next essential step is the database normalization. We used the unit and vector normalization which are recommended before applying multivariate statistics (Daub et al. 2003).
3.2 Variation of metabolite contents
Biological variation of metabolites of genetically identical plants of Arabidopsis thaliana grown under identical conditions was found to be on average 40% (Fiehn et al. 2000). Similar results were obtained in our experiment for two birch genotypes GT 2 and GT 5 (Fig. 3). Biological variation of most metabolites of birch leaves within genotype ranged between 10 and 50%. However, about thirty metabolites had coefficients of variation (CV) from 50 to 90%, and five metabolites––from 90 to 140% (see Fig. 3). High within-genotype variation of some metabolites may be due to plastic biochemical responses of leaves to their local micro-environments (e.g., differences in light-levels, temperatures, spatial orientation, contact with neighbours, local herbivore damage, etc.) (de Kroone et al. 2005).
Scatter plot of coefficients of variation (CV) of 331 metabolites in the leaves of two birch genotypes. CV (%) = Standard deviation/Mean value * 100. CV of most metabolites within genotype ranged between 10 and 50%, about 30 metabolites––between 50 and 90%, and five metabolites––between 90 and 140%. The largest CVs are displayed by two triterpenoids and three phenolic compounds: 1. Dammarane-3,12-diol, 20, 24-epoxy (code L126; see supporting information in the table of birch leaf metabolites on website http://users.utu.fi/ossipov/index.htm); 2–3. Two unknown polar phenolics (PP-9 and PP-23); 4. Unknown triterpenoid with characteristic fragment m/z 199 in mass spectrum (L119); 5. Unknown lipophilic phenolic compound (L37)
The largest CVs in birch leaves were displayed by two triterpenoids and three phenolics (one lipophilic and two polar) (Fig. 3, # 1–5). Levels of these secondary compounds are affected by many environmental factors. For example, induced accumulation of triterpenoids in B. pendula leaves was observed as a biochemical response of trees to manual defoliation (Valkama et al. 2005). Active accumulation of hydrolysable tannins was recorded also after damage of mountain birch leaves by Epirrita autumnata larvae (Ossipov, unpublished observation). Therefore, in the long-term open-field experiment with woody plants local influence of environmental factors may increase variation of some metabolites that will affect the results of biochemical phenotyping of trees and lead to difficulties of their interpretation.
3.3 Genotype discrimination and biochemical pattern recognition
At the first step of the statistical analysis of birch metabolome database, biochemical differences between control trees of GT 2 and GT 5 were determined with Student’s t-test. We found that GT 2 and GT 5 significantly differed (P < 0.01) in levels of 151 out of 331 quantified metabolites (data not shown). However, multivariate data mining tool that include hierarchical cluster analysis (HCA) and principal component analysis (PCA) enable to make more thorough examination of biochemical differences between birch genotypes and to determine the unintended phenotypic changes in the metabolome of individual tree or group of trees due to the local effects of some environmental factors.
HCA uses the metabolome database to calculate Euclidean distances between groups of samples (clusters) (Taylor et al. 2002). By examining the HCA dendrogram of the birch database, we found two major clusters corresponding to GT 2 and GT 5 (Fig. 4). The genotype clusters of both GT 2 and GT 5 were further divided into two subclusters corresponding to phenotypes of control trees from field A and field B. Discrimination of samples into subclusters was very effective, because only two samples of GT 2 from the field B (GT2T95-1 and GT2T94-3) were placed in the subcluster of the field A (Fig. 4). Overall, the largest distance metrics was found between the metabolomes of the two birch genotypes. Distances between the phenotypes of control trees from the fields A and B for both GT 2 and GT 5 were found to be considerably smaller than between birch genotypes.
Hierarchical cluster analysis of metabolite database of control trees of birch genotype 2 and genotype 5 that grown over seven years on two open fields A and B with ambient air conditions. Dendrogram obtained is divided into two major clusters of samples corresponding to different genotypes. The clusters of both genotypes are further divided into two subclusters corresponding to samples from field A and field B
The comparative study of environmental conditions on the fields A and B has shown that there were no significant differences between the fields for soil moisture content, temperature or leaf-level PAR recorded during photosynthesis (Oksanen 2003). The soil parameters of all fields satisfied to optimal growth conditions for birch (Ingestad 1962). There were almost no pest insects nor problems in pathogens infections in neither fields. However, soil analyses in August 2001 has shown some differences between field A and field B in soil pH––6.1 and 6.7 (field A and B correspondingly), and concentrations of Ca––1320 and 1480 mg/l, P––3.1 and 5.9 mg/l, K––107 and 88 mg/l, and Mg––241 and 151 mg/l (Oksanen, unpublished observation). In addition, soil type in field A was a bit more clay-containing. Therefore, we assume that the biochemical differences between the phenotypes of control trees from field A and field B for both GT 2 and GT 5 could be due to the local differences in soil properties between the fields.
PCA is concerned with the transformation of a large set of related variables (metabolites) into a new, smaller set of uncorrelated variables (principal components) (Jolliffe 1986). Plotting the first components against each other gives the two-dimensional projection of the data with the highest amount of variance. Application of PCA algorithms to the birch metabolome database analysis confirmed the results of HCA. Two major clusters were found on the score plot PC 1 vs. PC 2 and PC 1 vs. PC 3 (Fig. 5a, b). They were identical to the two genotypes of control trees (GT 2 and GT 5) that were discriminated in PC 1. Discrimination of trees from field A and B of both genotypes was occurred also but in different PCs. Phenotypes of GT 2 from field A and B were discriminated in PC 2 and PC 1, but phenotypes of GT 5––in PC 3 (Fig. 5a, b).
Principal component analysis of metabolite database of control trees of two birch genotypes GT 2 and GT 5 that grown over seven years on two open fields A and B with ambient air conditions. Two major clusters of samples are found on the score plot PC 1 vs. PC 2 and PC 1 vs. PC 3. They are discriminated in PC 1 and identical to the two genotypes of trees. Discriminations of samples from field A and B of both genotypes are occurred also but in different PCs. Two phenotypes of GT 2 from field A and B are discriminated in PC 2 and PC 1, and two phenotypes of GT 5––in PC 3
The metabolome database clustering with PCA is a useful approach for identification of biochemical traits with largest contribution to genotype/phenotype discrimination. The most important metabolites are determined by analyzing the contribution of each metabolite to the calculation of PC scores (Choi et al. 2004). Among the 331 metabolites from the initial database of birch metabolome, 37 metabolites had the largest positive or negative loading values for PC 1 (>0.4 and <−0.4) (Table 1). Differences in the mean contents of these metabolites between genotypes were highly significant also in the Student’s t-test analysis (P < 0.001, Table 1).
Leaves of the GT 2 contained 2–8 times higher levels of some lipophilic compounds (9 out of 18 metabolites) and polar compounds (8 out of 11 metabolites) as compared to the leaves of GT 5 (Table 1). In turn, leaves of GT 5 had relatively high levels of polar phenolics: myricetin-glycosides (# 32–34) and kaempferol-glycosides (# 36, 37). Therefore, metabolomes of two genotypes of birch trees are quite different, and it is not surprising that their biochemical phenotypes from field A and field B were discriminated with different metabolites (see Fig. 5).
3.4 Biochemical response of birch trees to elevated concentration of ozone
Long-term growth of GT 2 trees on the fields C and D with elevated concentration of ozone reduced dry mass of roots by 26.9%, dry mass of stem by 38.6%, dry mass of branches by 57.5%, and total dry mass of trees by 38.6% (Kontunen-Soppela et al. 2007). Ozone treatment of the trees resulted also in significant changes in the contents of 110 metabolites (P < 0.01 in Student’s t-test, data not shown).
PCA was applied for more thorough study of biochemical differences between metabolomes of control and ozone treated trees. As the result, three clusters of samples were found on the score plot PC 1 vs. PC 2 (Fig. 6). Control and ozone treated phenotypes of GT 2 trees were discriminated in PC 1, but phenotypes of control trees from field A and field B––in PC 2. In contrast to control trees, there were no significant differences between the metabolomes of GT 2 trees from the fields C and D with elevated level of ozone. We assume that ozone as a stronger stress factor suppresses and/or masks changes in the metabolome of birch leaves due to the local effects of some environmental factors.
Principal component analysis of metabolite database of control and ozone-treated trees of GT 2 that grown over seven years on two open fields A and B with ambient air conditions and on two open fields C and D with elevated (1.5 × ambient) concentration of ozone in air. Three clusters of samples are found on the score plot PC 1 vs. PC 2. They are identical to the two phenotypes of control trees from field A and B, and the phenotype of ozone-treated trees from fields C and D. Discrimination of samples of ozone-treated trees from field C and D are not found
Control and ozone treated trees of GT 2 are discriminated in PC 1 (Fig. 6), and 18 metabolites with the largest contribution to the calculation of the PC 1 and discrimination of these phenotypes were determined (Table 2). Elevated concentration of ozone increased levels of some lipophilic compounds such as 1-dotriacontanol, squalene, octadecanoic acid derivative and pentacyclic triterpenoid dammaran-3-ol,20,24-epoxy, and decreased levels of tetramethyl-2-hexadecen-1-ol and triterpenoid-1 with major characteristic m/z fragment 199 (Table 2). In contrast, levels of polar compounds, such as organic acids (malic and quinic) and carbohydrates (arabinose, fructose and galactose) were decreased in response to ozone exposure (Table 2).
Induced accumulation of phenolic compounds in the leaves of birch trees under elevated concentration of ozone is a very well-known phenomenon (Lavola et al. 1994; Saleem et al. 2001; Kontunen-Soppela et al. 2007; Valkama et al. 2007). In our experiment we found also that treatment of birch trees by ozone increases the levels of chlorogenic acid and of three flavonoid-glycosides (Table 2). The accumulation of phenolic antioxidants is often regarded as a result of biochemical adaptation of birch trees to oxidative stress (Lavola et al. 1994; Peltonen et al. 2005). However, total content of flavonoid-glycosides and content of chlorogenic acid were 1.4-fold higher in the leaves of sensitive to ozone GT 5 than in the leaves of tolerant to ozone GT 2. Therefore, high level of phenolics in birch leaves may not be indicative of tree tolerance to this stress factor. An alternative explanation of phenolic accumulation in the leaves of GT 2 under chronic ozone exposure could be acceleration of birch leaves senescence (Pell et al. 1997; Riikonen et al. 2004). This is quite possible, because accumulation of phenolics in the leaves is accompanied by decrease in the carbohydrate concentration (see Table 2), net photosynthesis and growth of trees, and by significantly increased stomatal conductance rate and the onset of visible foliar injuries (Oksanen 2003).
In the experiment with ozone, we have found a group of metabolites, which had very low contribution to PC 1, but their levels in control and ozone-treated trees differed 1.5 to 2.5-fold (Student’s t-test: alanine––P < 0.05; hexadecanoic acid, (oxy)-propyl ester, 9-octadecenoic acid derivative, flavonoid aglycone-1 and glycerol––P < 0.001). However, the contents of these metabolites in the control trees from the field A and field B were very different too (data not shown). Therefore, difference in the contents of the metabolites with low contribution to PC 1 cannot be regarded as a result of ozone-triggered biochemical changes. This is result of unintended changes in the metabolome of individual tree or group of trees due to the local effects of some other environmental factors.
4 Concluding remarks
An essential condition of metabolomics application to study biochemical responses of plants to the environmental stress is the minimization of the influence of factors that are not the subject of the study. The problem could be solved by using the phytotron with strictly controlled environmental parameters. However, this approach cannot be applied to mature woody plants because of their big size. Therefore, the experimental trees are often placed in the open fields, where they are affected by some study stress factor, and it is assumed that the effects of other environmental factors on the experimental trees are the same and stable. However, the variance in the intensity of these factors within the tree, between the trees within the experimental field, or between the different fields increases the phenotypic variation in tree metabolites and leads to difficulties in the result interpretation. Application of the metabolomics tool to genotype/phenotype discrimination and biochemical pattern recognition of birch trees grown over seven years in the open-field conditions has shown that the unintended changes in the metabolome of individual tree or group of trees due to the local effects of some environmental factors could be determined and eliminated with multivariate statistics.
The problem of discrimination of woody plant phenotypes is more complex than genotype discrimination, because the differences between metabolomes of phenotypes are considerably smaller than between metabolomes of genotypes. Moreover, metabolite profiles of different genotypes of trees are different, and it is not surprising that their biochemical responses to the same environmental factor could be quite different too (Valkama et al. 2005; Kontunen-Soppela et al. 2007; Fig. 5, this study). Therefore, application of metabolomics tool to study the biochemical responses of forest trees to environmental stresses is unlikely to produce reliable results because any individual tree in the forest is, as a rule, a different genotype. We assume that this conclusion could be important for ecologists working with natural populations of woody plants.
References
Choi, H.-K., Choi, Y., Verberne, M., Lefeber, A. W. M., Erkelens, C., & Verpoorte, R. (2004). Metabolic fingerprinting of wild type and transgenic tobacco plants by 1H NMR and multivariate analysis technique. Phytochemistry, 65, 857–864.
Daub, C. O., Kloska, S., & Selbig, J. (2003). MetaGeneAlyse: Analysis of integrated transcriptional and metabolite data. Bioinformatics, 19, 2332–2333.
Fiehn, O. (2002). Metabolomics––the link between genotypes and phenotypes. Plant Molecular Biology, 48, 155–171.
Fiehn, O., Kopka, J., Dörmann, P., Altman, T., Trethewey, R., & Willmitzer, L. (2000). Metabolite profiling for functional genomics. Nature Biotechnology, 18, 1157–1161.
Halket, J., Przyborowska, A., Stein, S., Down, S., & Chalmers, R. (1999). Deconvolution gas chromatography/mass spectrometry of urinary organic acid––potential for pattern recognition and automated identification of metabolite disorders. Rapid Communications in Mass Spectrometry, 13, 279–284.
Haukioja, E., Ossipov, V., Koricheva, J., Honkanen, T., Larsson, S., & Lempa, K. (1998). Biosynthetic origin of carbon-based secondary compounds: Cause of variable responses of woody plants to fertilization? Chemoecology, 8, 133–139.
Ingestad, T. (1962). Macro element nutrition of pine, spruce and birch seedling in nutrient solutions. Meddelande Statens Skogsforskningsinstitut, 51, 1–133.
Jolliffe, I. T. (1986). Principal component analysis. New York: Springer-Verlag.
Karnosky, D. F., Werner, H., Holopainen, T., Percy, K., Oksanen, T., Oksanen, E., Heerdt, C., Fabian, P., Nagy, J., Heilman, W., Cox, R., Nelson, N., & Matyssek, R. (2007). Free-air exposure systems to scale up ozone research to mature trees. Plant Biology, 9, 181–190.
de Kroone, H., Huber, H., Stuefer, J., & van Groenendael, J. (2005). A modular concept of phenotypic plasticity in plants. The New Phytologist, 166, 73–82.
Kontunen-Soppela, S., Ossipov, V., Ossipova, S., & Oksanen, E. (2007). Shift in birch leaf metabolome and carbon allocation during long-term open-field ozone exposure. Global Change Biology, 13, 1053–1067.
Laurila, T., Tuovinen, J.-P., Tarvainen, V., & Simpson, D. (2004). Trends and scenarios of ground-level ozone concentrations in Finland. Boreal Environment Research, 9, 167–184.
Lavola, A., Julkunen-Tiitto, R., & Pääkkönen, E. (1994). Does ozone stress change the primary or secondary metabolites of birch (Betula pendula Roth)? The New Phytologist, 126, 637–642.
Loponen, J., Ossipov, V., Lempa, K., Haukioja, E., & Pihlaja, K. (1998). Concentrations and among-compound correlations of individual phenolics in white birch leaves under air pollution stress. Chemosphere, 36, 1445–1456.
Oksanen, E. (2003). Responses of selected birch (Betula pendula). clones to ozone change over time. Plant, Cell & Environment, 26, 875–886.
Ossipov, V., Haukioja, E., Ossipova, S., Hanhimäki, S., & Pihlaja, K. (2001). Phenolic and phenolic-related factors as determinants of suitability of mountain birch leaves to an herbivorous insect. Biochemical Systematics and Ecology, 29, 223–240.
Ossipov, V., Nurmi, K., Loponen, J., Haukioja, E., & Pihlaja, K. (1996). HPLC separation and identification of phenolic compounds from leaves of Betula pubescens and Betula pendula. Journal of Chromatography. A, 721, 59–68.
Ossipov, V., Nurmi, K., Loponen, J., Prokopiev, N., Haukioja, E., & Pihlaja, K. (1995). HPLC isolation and identification of flavonoids from white birch Betula pubescens leaves. Biochemical Systematics and Ecology, 23, 213–222.
Ossipova S., Ossipov, V., Haukioja, E., Loponen, J., & Pihlaja, K. (2001). Proanthocyanidins from mountain birch leaves: Quantitation and properties. Phytochemical Analysis, 12, 128–133.
Pell, E. J., Schlagnhaufer, C. D., & Arteca, R. N. (1997). Ozone-induced oxidative stress: mechanisms of action and reaction. Physiologia Plantarum, 100, 264–273.
Peltonen, P. A., Vapaavuori, E., & Julkunen-Tiitto, R. (2005). Accumulation of phenolic compounds in birch leaves is changed by elevated carbon dioxide and ozone. Global Change Biology, 11, 1305–1324.
Riikonen, J., Lindsberg, M.-M., Holopainen, T., Oksanen, E., Lappi, J., Peltonen, P., & Vapaavuori, E. (2004). Silver birch and climate change: variable growth and carbon allocation responses to elevated concentrations of carbon dioxide and ozone. Tree Physiology, 24, 1227–1237.
Roessner, U., Luedemann, A., Brust, D., Fiehn, O., Linke, T., Willmitzer, L., & Fernie, A. (2001). Metabolic profiling allows comprehensive phenotyping of genetically or environmentally modified plant system. Plant Cell, 13, 11–29.
Saleem, A., Loponen, J., Pihlaja, K., & Oksanen, E. (2001). Effects of long-term open-field ozone exposure on leaf phenolics of European silver birch (Betula pendula Roth). Journal of Chemical Ecology, 27, 1049–1062.
Salminen, J.-P., Ossipov, V., Loponen, J., Haukioja, E., & Pihlaja, K. (1999). Characterisation of hydrolysable tannins from leaves of Betula pubescens by high-performance liquid chromatography––mass spectrometry. Journal of Chromatography. A, 864, 283–291.
Sumner, L., Mendes, P., & Dixon, R. (2003). Plant metabolomics: Large-scale phytochemistry in the functional genomics era. Phytochemistry, 62, 817–836.
Taylor, J., King, R. D., Altmann, T., & Fiehn, O. (2002). Application of metabolomics to plant genotype discrimination using statistics and machine learning. Bioinformatics, 18, 241–248.
Trethewey, R. (2001). Gene discovery via metabolic profiling. Current Opinion in Biotechnology, 12, 135–138.
Vinocur, B., & Altman, A. (2005). Recent advances in engineering plant tolerance to abiotic stress: Achievements and limitations. Current Opinion in Biotechnology, 16, 123–132.
Valkama, E., Koricheva, J., Ossipov, V., Ossipova, S., Haukioja, E., & Pihlaja, K. (2005). Delayed induced responses of birch glandular trichomes and leaf surface lipophilic compounds to mechanical defoliation and simulated browsing. Oecologia, 146, 385–393.
Valkama, E., Koricheva, J., & Oksanen, E. (2007). Effects of elevated O3, alone and in combination with elevated CO2, on tree leaf chemistry and insect herbivore performance: A meta-analysis. Global Change Biology, 13, 184–201.
Wang, W., Vinocur, B., Shoseyov, O., & Altman, A. (2004). Role of plant heat-shock proteins and molecular chaperones in the abiotic stress responses. Trends in Plant Science, 9, 244–252.
Acknowledgements
This work was funded by the Academy of Finland, projects 201073 (VO) and 51758 (EO). We thank Timo Oksanen for ozone fumigations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ossipov, V., Ossipova, S., Bykov, V. et al. Application of metabolomics to genotype and phenotype discrimination of birch trees grown in a long-term open-field experiment. Metabolomics 4, 39–51 (2008). https://doi.org/10.1007/s11306-007-0097-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11306-007-0097-8