Abstract
Recent studies have shown that mesenchymal stem cells (MSCs) and their conditioned medium (CM) have potential therapeutic effects in animal models of neuropathic pain (NP). However, the mechanisms underlying these effects are not fully understood. Because of the leading involvement of purinergic receptors in the pathogenesis of NP, this study aimed to investigate the effect of MSCs-CM on the expression levels of P2X4 and P2X7 receptors in a rat model of NP induced by chronic constriction injury (CCI) of the sciatic nerve. CM was prepared from the rats’ bone marrow–derived MSCs culture. After that, NP rats were treated by intraperitoneal injection of CM, or Dulbecco’s modified Eagle’s medium (DMEM) 1 day before and 7 and 11 days after CCI surgery. The NP status was assessed in the treated animals using behavioral tests, including mechanical allodynia and thermal hyperalgesia, on days − 1, 3, 6, 9, 12, and 15 of the study. At the end of the study (Day 15), the animals were sacrificed, and the relative gene expression of P2X4 and P2X7 receptors were measured in the spinal cord using quantitative real-time PCR. The results demonstrated that in the CM-treated NP rats, mechanical allodynia and thermal hyperalgesia were significantly reduced compared with the DMEM-treated group. In addition, the expression levels of P2X4 and P2X7 receptors were noticeably prevented in the CM-treated group than the control group. These findings indicate that the antinociceptive effects of CM in the NP rats are partly mediated through preventing the upregulation of P2X4 and P2X7 receptors in the spinal cord.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuropathic pain (NP) is a chronic pain caused by damage to the central or peripheral nerves due to the growth of a cancerous tumor, inflammation, diabetes, viral infections, and ischemia [1, 2]. In this type of pain, behavioral symptoms such as unpleasant burning sensation and paresthesia, spontaneous pain, increased sensitivity to painful stimuli (hyperalgesia), and non-painful stimuli (allodynia) can be mentioned [3]. P2X4 and P2X7 purinergic receptors (P2X4R and P2X7R) are ATP-gated cation channels which expressed in the central nervous system predominantly on glial cells and contribute in glia-neuron and glia-glia interactions. These receptors have a pivotal role in the pathogenesis of NP [4]. Researchers have reported that depletion and targeting of P2X4R could prevent and reduce pain hypersensitivity in the animal models of pain [5, 6]. Studies have also shown that the intrathecal and systemic administration of P2X7R antagonist can prevent NP in rats [7, 8]. It has been shown that after nerve injury, the expression of P2X4 and P2X7 receptors is increased in glia cells [9]. Following the nerve damage, a large amount of ATP is released which in turn through P2X4 and P2X7 receptors leads to the activation of glia cells and secretion of proinflammatory cytokines such as IL-1β, TNF-α, and IL-6 [10, 11]. Studies have demonstrated that IL-1β and other inflammatory cytokines have a major role in the development and maintenance of NP. For instance, evidence shows that IL-1β can directly sensitize nociceptors, and TNF-α injection into the rat’s sciatic nerve can induce pain hypersensitivity similar to NP in human [12].
NP is a hard-to-treat syndrome and has a high morbidity and negative consequences on quality of life of the affected individuals. Tricyclic antidepressants, venlafaxine, duloxetine, and antiepileptic drugs such as gabapentin are common drugs for NP treatment with low efficacy and undesirable side effects. Hence, many efforts are being made to discover new anti-NP drugs [13].
Scientists have recently paid great attention to the study of stem cell biology, hoping that these cells will play an important role in the treatment of a number of incurable diseases. Among them, mesenchymal stem cells (MSCs) are a source of tissue repair due to their high proliferation and differentiation capacities and immunological properties. Under appropriate conditions, they can also differentiate into specialized cell types such as bone, cartilage, and fat [14, 15]. Studies have shown that bone marrow–derived MSCs transplantation is associated with decreased levels of inflammatory cytokines and chemokines such as CCL2, CCL5, CXCL10 and can play an important role in reducing pain in animals with spinal cord injury [16]. On the other hand, stem cells prevent the progression of destructive pathways, and result in harnessing of the signaling pathway of apoptosis and strengthening the survival and recovery pathways of the damaged and healthy neurons. Studies have shown that these cells improve neurological symptoms in the animal models probably by harnessing the mechanism of inflammation and apoptosis, increasing the expression of neurotrophic factors, as well as enhancing neurogenesis and angiogenesis [17].
Despite the advantages of MSCs transplantation, there are several concerns about cell-based therapies, such as the possibility of turning stem cells into tumor cells during long-term cultures, the possibility of tumor induction using autologous cells, and the possibility of infectious agent transmission from donors to recipients in allogeneic MSCs transplantation [18]. Due to these concerns, the use of conditioned medium (CM) of MSCs is a better option than the cells themselves. CM contains all the soluble factors secreted from MSCs, and it has been demonstrated that the injection of CM via tail vein can cause a powerful and long-term analgesic effects in mice with NP [19]. In one study, a single intravenous injection of CM from bone marrow–derived MSCs could completely reverse mechanical allodynia and heat hyperalgesia in mice with established diabetic neuropathy [20]. De Gregorio et al. reported that systemic administration of CM from human adipose-derived mesenchymal stem cells has antinociceptive, antiapoptotic, and angiogenic effects in BKS db/db mice with diabetic polyneuropathy [21]. In a study conducted by Brini and her colleagues, they showed that a single injection of CM from human adipose–derived mesenchymal stem cells exerts a rapid and long-lasting anti-neuropathic pain effects in streptozotocin-induced diabetic mice [22]. They also concluded that these effects are mediated through a decrease in proinflammatory (IL-1β, TNF-α, and IL-6) and an increase in anti-inflammatory cytokine (IL-10) levels in spinal cord of the mice [22].
Although recent studies have shown that MSCs and their CMs have potential therapeutic effects in the animal models of NP, the mechanisms underlying these effects are not fully understood. Because of the leading involvement of purinergic receptors in the pathogenesis of NP, this study aimed to investigate the effect of MSCs-CM on the expression levels of P2X4 and P2X7 receptors in the NP rats induced by chronic constriction injury (CCI) of the sciatic nerve.
Materials and methods
Animals
In this experimental study, 32 male Wistar rats weighing 200–300 g were purchased from the animals’ center of Mashhad University of Medical Sciences (Mashhad, Iran). They were kept in appropriate conditions (22 ± 2 °C and 12-h light/dark cycle) with free access to water and food. All experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Academy of Sciences (The National Academies Press, Washington, D.C, 8th edition) and the Basel Declaration. In addition, all methods were approved by the Research Ethics Committee of Arak University of Medical Sciences (ethical code: IR.ARAKMU.REC.1397.333).
Isolation, culture, and immunophenotyping of MSCs
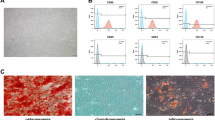
MSCs were isolated from the femurs and tibias of two rats by flushing the bone marrows with DMEM containing 10% FBS, 100 μg/ml streptomycin, and 100 U/ml penicillin. The resulting cell suspension was transferred to T175 cell culture flasks. In order to remove non-adherent cells, the culture medium was changed after 24 h. The cell cultures were daily inspected, and the medium was changed every 72 h. The cells were passaged to a new cell culture flask if they reached 80% confluency. In the second passage, the cells were selected for flow cytometric analysis in order to determine MSC identity. In brief, 100,000 cells were separately stained with fluorochrome-conjugated monoclonal antibodies against CD34, CD44, CD45, and CD90 cell surface markers (eBioscience, Inc., San Diego, CA, USA) along with appropriate isotype control antibodies and then analyzed using flow cytometry.
CM preparation
CM was collected from the MSC culture in passage 2 as follows: when the cells reached 80% confluency, the medium was changed with FBS-free DMEM culture medium, and the cells were incubated for 48 h in CO2 incubator. After this time, the supernatant of MSCs culture was harvested, filtered through a 0.22-μm filter, and frozen at − 80 °C until use.
Neuropathic pain induction
Neuropathic pain (NP) was induced in rats by CCI method as previously described [23]. Briefly, after anesthetizing the animals, the sciatic nerve was carefully separated from the surrounding connective tissue, and four loose knots were tied at 1-mm intervals using chromic gut stitching thread around the sciatic nerve. The sciatic nerve was then put back in place, the wound was disinfected, and the muscle and skin were sutured.
Experimental groups and treatments
NP rats were randomly divided into three groups (n = 8 in each group) as follows: CM treated (CM group), DMEM treated (DMEM group), normal saline treated (NP group). Healthy rats were considered as sham group. CM or DMEM culture medium (1 ml) was intraperitoneally injected into corresponding groups 1 day before and 7 and 11 days after CCI surgery. The NP and sham groups received normal saline at the mentioned times.
Assessment of pain status
To assess the NP status in the experimental animals, behavioral tests including von Frey and hot plate tests were performed on days − 1, 3, 6, 9, 12, and 15 of the study as previously described [23,24,25].
Mechanical allodynia was assessed using von Frey test. Briefly, a set of different von Frey filaments (2, 4, 6, 8, 15, 26, and 60 g; Stoelting, Wood Dale, IL, USA) was applied on the plantar surface of the injured hind paw, and the paw withdrawal threshold (PWT) was recorded. To prevent tissue damage, 60 g von Frey filament was considered as cut-off.
Thermal hyperalgesia was evaluated using hot plate test. In summary, each animal was placed on a hot plate (52–55 °C), and the time (s) it took the animal to withdraw its foot and lick it was measured as paw withdrawal latency (PWL). A maximum of 20 s was considered as cut-off to prevent tissue damage.
Measurement of the relative gene expression of P2X4 and P2X7 receptors
At the end of the study (Day 15), the animals were sacrificed, and the relative gene expression of P2X4 and P2X7 receptors was measured in the spinal cord using quantitative real-time PCR technique as previously described [23]. Briefly, each animal’s spinal cord was flushed out with cold normal saline, and RNA was extracted using RNX-Plus kit (Sinaclon, Iran) according to the kit protocol. Total RNA was then reverse-transcribed using the Prime Script™ RT reagent Kit (Takara Bio Inc. Japan) according to the manufacturer’s instruction. Finally, the expression levels of P2X4 and P2X7 receptors were measured based on SYBR Green method using RT-PCR technique on CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad, Philadelphia, PA USA). The specific primers were as follows: P2RX4, forward 5′-GACGTGGCGGACTATGTGAT-3′, reverse 5′-TCTTCCAGTCGCAACTCCAC-3′; P2RX7, forward 5′-CAAAGGTCAAGAGGTCCCGAGAC-3′, reverse 5′-GCCCTGCGGTTCTCTGGTAG-3′. The fold change of the gene expression was calculated using 2−ΔΔCt method in comparison with the GAPDH as reference gene.
Statistical analysis
All data were presented as mean ± SD. The data analysis was performed using SPSS 16 (IBM Corporation, Armonk, NY, USA). In case of the data normality, one-way ANOVA followed by post hoc Bonferroni test was used for data analysis; otherwise, the Kruskal-Wallis test was used. In all cases, P < 0.05 was considered as significant differences. All graphs were designed using GraphPad Prism 8 software.
Results
Bone marrow–derived cells expressed MSC CD markers
Bone marrow–derived MSCs were characterized using fluorochrome-conjugated monoclonal antibodies. The results of flow cytometry indicated that these cells were positive for CD44 and CD90 (rat MSCs markers) [26] and were negative for CD34 and CD45 (hematopoietic lineage surface markers) (Fig. 1).
CM treatment reduced mechanical allodynia and thermal hyperalgesia in NP rats
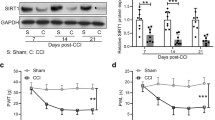
To assess the effect of MSCs-CM on the pain status in NP rats, mechanical allodynia and thermal hyperalgesia were evaluated using von Frey and hot plate tests on days − 1, 3, 6, 9, 12, and 15 of the study. Figure 2 represents the results of these tests in different days of the study. As shown in the Fig. 2a, allodynia was induced in the NP and DMEM groups on day 3 (P < 0.01 and P < 0.05 vs. the sham group, respectively) after CCI surgery, progressively exacerbated on day 6 (P < 0.01), and maintained until the end of the study period (P < 0.01). On the contrary, in the CM-treated group, which received a single dose of CM on day − 1 (a day before CCI surgery), allodynia development was prevented on days 3 and 6 (P > 0.05 vs. the sham group). However, on days 9 and 12, allodynia was evident in the CM-treated group (P < 0.05 vs. the sham group), but it was considerably milder than the NP and DMEM groups (P < 0.05). On day 15, there was a significant antinociceptive response to mechanical allodynia in the CM-treated rats compared with the control groups (P < 0.01). This antinociceptive response could be due to the CM which was injected on days 7 and 11 after CCI surgery. Collectively, these results indicate that a single injection of CM prior to CCI surgery can postpone allodynia development in rats, and that CM injection after the establishment of NP can significantly reduce it.
Effect of the conditioned medium of MSCs on the pain status of NP rats. NP rats were treated by intraperitoneal injection of CM or DMEM 1 day before and 7 and 11 days after CCI surgery. Mechanical allodynia and thermal hyperalgesia were measured in different days of the study using von Frey and hot plate tests and represented as a paw withdrawal threshold (PWT) and b paw withdrawal latency (PWL), respectively. Data are represented as mean ± SD; n = 8 rats per group. CM treatment could significantly reduce mechanical allodynia and thermal hyperalgesia in the CM group compared with the NP and DMEM groups. *P < 0.05 and **P < 0.01 vs. the sham group, #P < 0.05 and ##P < 0.01 vs. the NP and DMEM groups
Figure 2b represents the results of hot plate test. As shown in this figure, thermal hyperalgesia was induced in all NP groups on day 3 after CCI surgery and maintained until the end of the study period. However, from day 6 to the end of the study, thermal hyperalgesia was significantly milder in the CM-treated group than the NP and DMEM groups (P < 0.05). Based on these results, it can be concluded that CM treatment cannot completely prevent development of thermal hyperalgesia in CCI rats but can significantly reduce it.
CM treatment prevented the upregulation of P2X4 and P2X7 receptors in the spinal cord
At the end of the study (day 15), the animals were sacrificed, and the relative gene expression of P2X4 and P2X7 receptors was measured in the spinal cord using quantitative real-time PCR technique. The results showed that in the CM-treated group, the relative gene expression of P2X4 and P2X7 receptors was almost equal to that of the sham group (Fig. 3). In contrast, the level of P2X4 and P2X7 receptors mRNA was markedly upregulated (more than threefold) in the NP and DMEM groups compared with the sham and CM groups (P < 0.05). These results indicate that CM treatment can prevent the upregulation of P2X4 and P2X7 receptors in the spinal cord of CCI rats.
Effect of CM on the relative gene expression of P2X4 and P2X7 receptors. The expression levels of P2X4 and P2X7 receptors in the spinal cord of NP rats were measured using real-time PCR at the end of the study (Day 15). Data are represented as mean ± SD; n = 8 rats per group. The results showed that CM could significantly downregulate P2X4 and P2X7 receptors in the CM-treated group compared with the control groups (the NP and DMEM groups). *P < 0.05 vs. the sham group, #P < 0.05 vs. the NP and DMEM groups
Discussion
Studies have shown that following peripheral nerve injury (PNI), P2X4 and P2X7 purinergic receptors are significantly upregulated in the activated microglia of the spinal dorsal horn (SDH) [4, 27]. Therefore, this study aimed to investigate whether the analgesic effect of CM is mediated via regulating the expression of these two purinoceptors in the CCI model of neuropathic pain (NP). Interestingly, the results of this study showed that CM can inhibit the upregulation of P2X4 and P2X7 receptors in the spinal cord and significantly reduce mechanical allodynia and thermal hyperalgesia in NP rats.
It is well established that following PNI, large amounts of ATP are released in the spinal cord [4, 10]. In addition, nerve damage leads to the activation of SDH microglia and induces different signaling pathways in these cells [4]. In one of these pathways, activation of IRF8 and IRF5 transcription factors leads to an increase in the expression of different proteins such as P2X4 and P2X7 receptors [28]. Tsuda et al. have reported an increase in the expression of P2X4 receptor, starting 1 day after PNI, and reaches its maximum after 14 days [29]. ATP can enter activated microglia via P2X4 and P2X7 receptors and through activating of inflammasome complex and induces secretion of IL-1β and IL-18 proinflammatory cytokines from these cells [4]. Besides, activation of p38-MAPK signaling pathway leads to the production of various inflammatory factors such as IL-1β, TNF-α, IL-6, COX-2, and iNOS from microglia [30, 31]. Subsequently, these inflammatory factors lead to neuronal hyperexcitability and thereby the development and maintenance of the neuropathic pain symptoms (allodynia and hyperalgesia) [4].
In the present study, CM was injected on days − 1, 7, and 11 of the study. In the preventive scenario, CM injection a day before the CCI surgery completely prevented the development of mechanical allodynia. On the other hand, although thermal hyperalgesia was evident on day 3, it was gradually decreased significantly by the end of the study compared with the control groups. In the treatment scenario, after the development of neuropathic pain symptoms, two CM injections (on days 7 and 11) could significantly reduce mechanical allodynia and thermal hyperalgesia by the end of the study. On the final day (day 15), the expression levels of the P2X4 and P2X7 mRNA in the animals’ spinal cord were measured. The results showed that in the CM group, the expression of these receptors was almost equal to that of the sham group, whereas in the control groups, P2X4 and P2X7 receptors were significantly upregulated (more than threefold). Collectively, these results indicate that CM alleviates neuropathic pain symptoms possibly by preventing the upregulation of P2X4 and P2X7 receptors in the spinal cord. These findings are in agreement with Teng et al.’s study that reported two doses of intrathecally injected bone marrow–derived MSCs, reduced NP via the preventing of P2X4 receptor expression in microglia, in chronic compression of the dorsal root ganglion (CCD) model [32]. In other similar studies, a single dose of CM had a rapid and durable analgesic effect in NP animals [20, 22].
Gama et al. reported that CM from bone marrow–derived MSCs contains 21 proteins that include a range of cytokines, enzymes, and factors such as vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), and angiopoietin-1 which are considered as important analgesic mediators in neuropathic pain [19]. For example, VEGF has neuroprotective effects and also improves function, reduces pain, and reduces allodynia in the CCI model of NP [19]. CM also contains various bioactive agents with anti-inflammatory and neuroprotective properties. For instance, Chen et al. have reported that the analgesic effect of intrathecally injected bone marrow–derived MSCs is mediated through TGF-β secretion [33]. In another study, TNF-α-stimulated gene 6 protein (TSG-6) secreted from MSCs has been introduced as anti-neuropathic pain molecule [34]. Collectively, based on previous studies and the results of the present study, the molecular mechanisms of CM analgesic effects in the CCI model can be explained as follows. After CCI surgery, in addition to releasing large amounts of ATP, SETD7 protein increases in SDH microglial cells, which causes microgliosis in SDH [35]. In activated microglia, different signaling pathways are induced, which in one of these pathways, activation of IRF8 and IRF5 transcription factors leads to an increase in the expression of P2X4 and P2X7 purinergic receptors on the surface of microglia [28]. Then, ATP enters the microglia via these receptors, and by activating the inflammasome complex, it leads to the secretion of proinflammatory cytokines [4]. Studies have shown that neuroinflammation, in addition to disruption of the blood-spinal cord barrier (BSCB), has a key role in the development and maintenance of NP by activating nociceptors [36]. In contrast, anti-inflammatory agents in CM such as TGF-β and TSG-6 prevent microgliosis and activation of SDH microglial cells. For example, TSG-6 prevents the activation of microglia by inhibiting the TLR2/MyD88/NF-κB signaling pathway [34]. Therefore, it can be concluded that CM may prevent the upregulation of P2X4 and P2X7 purinergic receptors by inhibiting the activation of microglial cells and consequently reducing the secretion of inflammatory mediators from these cells and thereby alleviating NP symptoms. However, further studies are needed to reveal the exact molecular mechanisms by which CM prevents purinoceptor expression in microglia.
The reason why CM was injected three times in this study is because the bioactive agents in CM have a short half-life [19], and therefore, CM injection should be performed several times in order to maintain the therapeutic concentration of these agents in the target organ. Another important point that should be noted is that in this study, CM injection was performed inside the peritoneum cavity of the animals, while in similar studies, CM has been injected intravenously [19, 22] or intrathecally [34]. Evidence suggests that intraperitoneal injection is an easy and acceptable route to study the therapeutic effects of drugs in the laboratory animals. Due to the presence of extensive network of blood and lymphatic vessels in the peritoneal cavity, drugs are easily absorbed into systemic blood circulation and therefore reach to the target tissue [37]. It has been shown that in intraperitoneal injections, proteins greater than 5 kD (for example, most bioactive proteins responsible for the analgesic effects of CM such as TGF-β and TSG-6) mainly enter systemic blood circulation through lymphatic vessels and thereby reach the target organ [37]. In addition, intraperitoneal route allows for the injection of a larger volume of CM, so there is no need to concentrate CM before injection, while if it is injected intravenously or intrathecally, CM should be concentrated [19, 22, 34].
In summary, the results of this study demonstrate that CM from MSCs exerts its antinociceptive effects, in part, through preventing the upregulation of P2X4 and P2X7 receptors in the spinal cord of CCI rats.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Baron R (2006) Mechanisms of disease: neuropathic pain—a clinical perspective. Nat Clin Pract Neurol 2(2):95–106
Colloca L, Ludman T, Bouhassira D, Baron R, Dickenson AH, Yarnitsky D, Freeman R, Truini A, Attal N, Finnerup NB (2017) Neuropathic pain. Nat Rev Dis Primers 3(1):1–19
Gilron I, Baron R, Jensen T Neuropathic pain: principles of diagnosis and treatment. In: Mayo Clinic Proceedings, 2015. vol 4. Elsevier, pp 532–545
Inoue K, Tsuda M (2018) Microglia in neuropathic pain: cellular and molecular mechanisms and therapeutic potential. Nat Rev Neurosci 19(3):138–152
Tsuda M, Kuboyama K, Inoue T, Nagata K, Tozaki-Saitoh H, Inoue K (2009) Behavioral phenotypes of mice lacking purinergic P2X4 receptors in acute and chronic pain assays. Mol Pain 5:1744-8069-1745-1728
Ulmann L, Hatcher JP, Hughes JP, Chaumont S, Green PJ, Conquet F, Buell GN, Reeve AJ, Chessell IP, Rassendren F (2008) Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci 28(44):11263–11268
He W-J, Cui J, Du L, Zhao Y-D, Burnstock G, Zhou H-D, Ruan H-Z (2012) Spinal P2X7 receptor mediates microglia activation-induced neuropathic pain in the sciatic nerve injury rat model. Behav Brain Res 226(1):163–170
Honore P, Donnelly-Roberts D, Namovic MT, Hsieh G, Zhu CZ, Mikusa JP, Hernandez G, Zhong C, Gauvin DM, Chandran P (2006) A-740003 [N-(1-{[(cyanoimino)(5-quinolinylamino) methyl] amino}-2, 2-dimethylpropyl)-2-(3, 4-dimethoxyphenyl) acetamide], a novel and selective P2X7 receptor antagonist, dose-dependently reduces neuropathic pain in the rat. J Pharmacol Exp Ther 319(3):1376–1385
Makoto T, Hidetoshi TS, Kazuhide I (2012) P2X4R and P2X7R in neuropathic pain. Wiley Interdisciplinary Reviews: Membrane Transport and Signaling 1 (4):513–521
Ellis A, Bennett D (2013) Neuroinflammation and the generation of neuropathic pain. Br J Anaesth 111(1):26–37
Munoz FM, Patel PA, Gao X, Mei Y, Xia J, Gilels S, Hu H (2020) Reactive oxygen species play a role in P2X7 receptor-mediated IL-6 production in spinal astrocytes. Purinergic Signal:1–11
Leung L, Cahill CM (2010) TNF-α and neuropathic pain-a review. J Neuroinflammation 7(1):1–11
Xu L, Zhang Y, Huang Y (2016) Advances in the treatment of neuropathic pain. Adv Exp Med Biol 904:117–129. https://doi.org/10.1007/978-94-017-7537-3_9
Alipour R, Sadeghi F, Hashemi-Beni B, Zarkesh-Esfahani SH, Heydari F, Mousavi SB, Adib M, Narimani M, Esmaeili N (2010) Phenotypic characterizations and comparison of adult dental stem cells with adipose-derived stem cells. Int J Prev Med 1(3):164–171
Al-Massri KF, Ahmed LA, El-Abhar HS (2019) Mesenchymal stem cells therapy enhances the efficacy of pregabalin and prevents its motor impairment in paclitaxel-induced neuropathy in rats: role of Notch1 receptor and JAK/STAT signaling pathway. Behav Brain Res 360:303–311. https://doi.org/10.1016/j.bbr.2018.12.013
Honczarenko M, Le Y, Swierkowski M, Ghiran I, Glodek AM, Silberstein LE (2006) Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells 24(4):1030–1041. https://doi.org/10.1634/stemcells.2005-0319
Zhou H, Zhang H, Yan Z, Xu R (2016) Transplantation of human amniotic mesenchymal stem cells promotes neurological recovery in an intracerebral hemorrhage rat model. Biochem Biophys Res Commun 475(2):202–208
Patel AN, Genovese J (2011) Potential clinical applications of adult human mesenchymal stem cell (Prochymal®) therapy. Stem Cells Cloning 4:61
Gama KB, Santos DS, Evangelista AF, Silva DN, de Alcântara AC, dos Santos RR, Soares MBP, Villarreal CF (2018) Conditioned medium of bone marrow-derived mesenchymal stromal cells as a therapeutic approach to neuropathic pain: a preclinical evaluation. Stem Cells Int 2018
Evangelista AF, Vannier-Santos MA, de Assis Silva GS, Silva DN, Juiz PJL, Nonaka CKV, dos Santos RR, Soares MBP, Villarreal CF (2018) Bone marrow-derived mesenchymal stem/stromal cells reverse the sensorial diabetic neuropathy via modulation of spinal neuroinflammatory cascades. J Neuroinflammation 15(1):1–17
De Gregorio C, Contador D, Díaz D, Cárcamo C, Santapau D, Lobos-Gonzalez L, Acosta C, Campero M, Carpio D, Gabriele C (2020) Human adipose-derived mesenchymal stem cell-conditioned medium ameliorates polyneuropathy and foot ulceration in diabetic BKS db/db mice. Stem Cell Res Ther 11:1–21
Brini AT, Amodeo G, Ferreira LM, Milani A, Niada S, Moschetti G, Franchi S, Borsani E, Rodella LF, Panerai AE, Sacerdote P (2017) Therapeutic effect of human adipose-derived stem cells and their secretome in experimental diabetic pain. Sci Rep 7(1):9904. https://doi.org/10.1038/s41598-017-09487-5
Nazemi S, Jafari F, Amin B, Gholami O, Kafami M, Mojadadi M-S (2020) Effect of Umbelliprenin on antinociceptive activity of morphine in a rat model of neuropathic pain induced by chronic constriction injury of the sciatic nerve. Nat Prod J 10:1
Nazemi S, Rudsarabi H, Amin B, Farahani H, Azhdari Zarmehri H, Mojadadi M-S (2020) Anti-neuropathic pain effects of ethyl acetate extract of Ferula asafoetida oleo-gum-resin in streptozotocin-induced diabetic rats. Nat Prod J 10:1
Deuis JR, Dvorakova LS, Vetter I (2017) Methods used to evaluate pain behaviors in rodents. Front Mol Neurosci 10:284. https://doi.org/10.3389/fnmol.2017.00284
Mittal R, Ocak E, Zhu A, Perdomo MM, Pena SA, Mittal J, Bohorquez J, Eshraghi AA (2020) Effect of bone marrow-derived mesenchymal stem cells on cochlear function in an experimental rat model. Anat Rec 303(3):487–493
Tsuda M (2016) Microglia in the spinal cord and neuropathic pain. J Diabetes Investig 7(1):17–26. https://doi.org/10.1111/jdi.12379
Masuda T, Iwamoto S, Yoshinaga R, Tozaki-Saitoh H, Nishiyama A, Mak TW, Tamura T, Tsuda M, Inoue K (2014) Transcription factor IRF5 drives P2X4R+-reactive microglia gating neuropathic pain. Nat Commun 5(1):1–11
Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K (2003) P2X 4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature 424(6950):778–783
Jin S-X, Zhuang Z-Y, Woolf CJ, Ji R-R (2003) p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J Neurosci 23(10):4017–4022
Tsuda M, Mizokoshi A, Shigemoto-Mogami Y, Koizumi S, Inoue K (2004) Activation of p38 mitogen-activated protein kinase in spinal hyperactive microglia contributes to pain hypersensitivity following peripheral nerve injury. Glia 45(1):89–95
Teng Y, Zhang Y, Yue S, Chen H, Qu Y, Wei H, Jia X (2019) Intrathecal injection of bone marrow stromal cells attenuates neuropathic pain via inhibition of P2X 4 R in spinal cord microglia. J Neuroinflammation 16(1):1–15
Chen G, Park C-K, Xie R-G, Ji R-R (2015) Intrathecal bone marrow stromal cells inhibit neuropathic pain via TGF-β secretion. J Clin Invest 125(8):3226–3240
Yang H, Wu L, Deng H, Chen Y, Zhou H, Liu M, Wang S, Zheng L, Zhu L, Lv X (2020) Anti-inflammatory protein TSG-6 secreted by bone marrow mesenchymal stem cells attenuates neuropathic pain by inhibiting the TLR2/MyD88/NF-κB signaling pathway in spinal microglia. J Neuroinflammation 17:1–21
Shen Y, Ding Z, Ma S, Ding Z, Zhang Y, Zou Y, Xu F, Yang X, Schäfer MK, Guo Q (2019) SETD7 mediates spinal microgliosis and neuropathic pain in a rat model of peripheral nerve injury. Brain Behav Immun 82:382–395
Rosenberg GA (2012) Neurological diseases in relation to the blood–brain barrier. J Cereb Blood Flow Metab 32(7):1139–1151
Al Shoyaib A, Archie SR, Karamyan VT (2020) Intraperitoneal route of drug administration: should it be used in experimental animal studies? Pharm Res 37(1):12
Acknowledgments
We thank Mrs. Falanji for her help on RT-PCR analysis.
Funding
This work was supported financially by the deputy of research and technology at Arak University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Academy of Sciences (The National Academies Press, Washington, D.C, 8th edition) and the Basel Declaration. In addition, all methods were approved by the Research Ethics Committee of Arak University of Medical Sciences (IR.ARAKMU.1397.333).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Masoodifar, M., Hajihashemi, S., Pazhoohan, S. et al. Effect of the conditioned medium of mesenchymal stem cells on the expression levels of P2X4 and P2X7 purinergic receptors in the spinal cord of rats with neuropathic pain. Purinergic Signalling 17, 143–150 (2021). https://doi.org/10.1007/s11302-020-09756-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-020-09756-5