Abstract
Nucleoside di- and triphosphates and adenosine regulate several components of the mucocilairy clearance process (MCC) that protects the lung against infections, via activation of epithelial purinergic receptors. However, assessing the contribution of individual nucleotides to MCC functions remains difficult due to the complexity of the mechanisms of nucleotide release and metabolism. Enzymatic activities involved in the metabolism of extracellular nucleotides include ecto-ATPases and secreted nucleoside diphosphokinase (NDPK) and adenyl kinase, but potent and selective inhibitors of these activities are sparse. In the present study, we discovered that ebselen markedly reduced NDPK activity while having negligible effect on ecto-ATPase and adenyl kinase activities. Addition of radiotracer [γ 32P]ATP to human bronchial epithelial (HBE) cells resulted in rapid and robust accumulation of [32P]-inorganic phosphate (32Pi). Inclusion of UDP in the incubation medium resulted in conversion of [γ 32P]ATP to [32P]UTP, while inclusion of AMP resulted in conversion of [γ 32P]ATP to [32P]ADP. Ebselen markedly reduced [32P]UTP formation but displayed negligible effect on 32Pi or [32P]ADP accumulations. Incubation of HBE cells with unlabeled UTP and ADP resulted in robust ebselen-sensitive formation of ATP (IC50 = 6.9 ± 2 μM). This NDPK activity was largely recovered in HBE cell secretions and supernatants from lung epithelial A549 cells. Kinetic analysis of NDPK activity indicated that ebselen reduced the V max of the reaction (K i = 7.6 ± 3 μM), having negligible effect on K M values. Our study demonstrates that ebselen is a potent non-competitive inhibitor of extracellular NDPK.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nucleotides and nucleosides within the airway surface liquid (ASL) regulate key components of the mucociliary clearance (MCC) process that removes foreign particles and pathogens from the lung [1–3]. Adenosine-5′-triphosphate (ATP) and uridine-5′-triphosphate (UTP) activate the Gq-coupled P2Y2 receptor (P2Y2-R) that promotes mucin secretion from goblet cells [2]. The P2Y2-R expressed on ciliated cells, promotes ciliary beat frequency, protein kinase C-enhanced cystic fibrosis transmembrane conductance regulator (CFTR)-mediated Cl− secretion, and Ca2+-activated Cl− channel (CaCC) activity [3]. In addition, P2Y2-R activation results in inhibition of the epithelial sodium channel ENaC [3]. UDP promotes CaCC activity via activation of the P2Y6-R expressed on ciliated cells [3]. Adenosine generated from the hydrolysis of ATP in ASL activates the Gs-coupled A2B receptor (A2B-R) that promotes cyclic AMP-regulated CFTR activity and increases cilia beat frequency [3–5]. In the distal lung, ATP/UTP and/or adenosine (mainly via P2Y2-R and A2B-R, respectively) stimulate type II cell surfactant secretion [6], regulate alveolar ion transport and fluid clearance [7, 8], and contribute to alveolar remodeling and inflammation [9, 10].
Nucleotide/nucleoside levels in ASL and lung secretions reflect a balance between cellular nucleotide release and extracellular metabolism [11]. Early studies suggested that ATP, and to a lesser extent UTP and other nucleoside triphosphates (NTPs), are released from airway epithelial cells [11–13] and subsequently hydrolyzed to nucleoside di- and monophosphate (NDP and NMP, respectively) and nucleosides (e.g., adenosine) by action of cell surface NTPDases, NTP pyrophosphatase, alkaline phosphatase, and 5′-nucleotidase [11, 14, 15]. In addition, NDP kinase (NDPK) and adenyl kinase provide nucleotide interconversion activities in ASL [11, 16, 17].
Recently, a mathematical model based on the rates of ATP release and metabolism and adenosine accumulation rates in ASL predicted that adenosine-5′-diphosphate (ADP) and/or monophosphate (AMP) are released from airway epithelial cells, in addition to ATP [18]. Consistent with this prediction, we have recently demonstrated the presence of a nucleotide pool within secretory granules isolated from goblet cells, which was represented (in abundance order) by ADP > AMP >> ATP [19]. Direct release of ADP/AMP to ASL would selectively promote liquid secretion from ciliated cells, via adenosine formation and A2B-R activation. In addition, our recent demonstration that airway epithelial and other cells release UDP-sugars from Golgi-derived vesicles [20] implies that UDP, which accumulates in the Golgi as product of UDP-sugar-based glycosylation reactions, is released from cells via the secretory pathway [21, 22]. Since the P2Y6-R is expressed on ciliated cells (but not on goblet cells) [2], UDP release would selectively promote CaCC-mediated electrolyte transport and ciliary beat frequency without enhancing mucin secretion.
While ATP, UTP, ADP, UDP, AMP, and adenosine are naturally occurring molecules in ASL [13, 23–26], assessing the exact contribution of individual nucleotides to MCC functions remains difficult, due in part to the complexity of the mechanisms that control nucleotide release and concentrations in ASL. In particular, strong NDPK activity has been reported in conditioned medium from airway epithelial cells [11, 27]. The activity of extracellular NDPK, which catalyzes transphosphorylation reactions such as the reversible phosphorylation of UDP by ATP (ATP + UDP = ADP + UTP), surmounts that of nucleotidases under a number of conditions. NDPK-catalyzed transphosphorylating reactions provide a confusing factor in the assessment of the primary source of ASL nucleotides. Measuring the rates of release of NTPs, NDPs, and NMPs has been dampened by the lack of non-nucleotide molecules to inhibit nucleotide interconversion.
The organoselenium compound ebselen [1, 2-phenyl-1, 2-benzisoselenazol-3(2H)-1] has been reported to inhibit, albeit partially (∼60% inhibition, <100 μM ebselen), the hydrolysis of extracellular ATP in rat platelets [28]. More recently, we have illustrated that ebselen slightly delayed ATP metabolism on primary cultures of human airway epithelial cells [25], but the identity of the ATP metabolizing ecto-activity present in airway epithelia targeted by ebselen is not known. In the present study, by examining the effect of ebselen on the metabolism of extracellular ATP on airway epithelial cells, we discovered that ebselen is a potent, non-competitive full inhibitor of NDPK.
Materials and methods
Reagents
ADP, UDP, 2-phenyl-1, 2-benzisoselenazol-3(2H)-1 (ebselen), and luciferase from Photinus pyralis were obtained from Sigma (St. Louis, MO). For experiments using ebselen derivatives, ebselen and its analogues 2, 2′-diseleno benzanilide (EbSe2), 2, 2′-ditelluro benzanilide (EbTe2), 2-methyl seleno benzanilide (EbMe), and 2-benzyl seleno benzanilide (EbBz) were obtained as previously described [29]. Luciferin was obtained from BD PharMingen (Franklin Lakes, NJ). ATP and UTP were purchased from GE Healthcare (Hillsborough, NC). [γ 32P]ATP was obtained from Amersham Biosciences (Piscataway, NJ). All other reagents were from sources previously reported [11, 13, 20].
Cell culture and incubations
Polarized cultures of well-differentiated primary HBE cells (provided by the UNC Cystic Fibrosis Center Center Tissue Culture Core Lab) and A549 lung epithelial cells were grown on 12-mm Transwell supports (Costar) and on 24-well plastic plates, respectively, as previously described [13, 25, 30]. The cells were rinsed and 300 μl serum-free Dulbecco’s modified Eagle’s medium were added to the mucosal compartment of HBE cells or to A549 cell culture wells. After a 1 h pre-incubation period, cell cultures where incubated with drugs, as indicated below. Alternatively, pre-incubation medium was collected, centrifuged, and the supernatant used within 2 h to assess nucleotide metabolism activities in conditioned medium. Incubations were initiated by the addition of the indicated reagent to cultures or to 100-μl conditioned medium. After the desired incubation time at 37°C, samples from cultures and conditioned medium were heated for 2 min at 95°C to inactivate enzyme activities.
NDPK and adenyl kinase activities
NDPK catalyzes the reversible phosphorylation of NDPs by NTPs, while adenyl kinase catalyzes the reversible phosphorylation of AMP by ATP. NDPK activity was assessed via two alternative protocols, (reaction 1) as a function of UDP-dependent conversion of [γ 32P]ATP to [32P]UTP, or (reaction 2) as a function of UTP- and ADP-dependent formation of ATP:
Adenyl kinase activity was assessed either as a function of AMP-dependent conversion of [γ 32P]ATP to [32P]ADP (reaction 3) or as a function of ADP conversion to ATP (reaction 4):
The resulting 32P-labeled species were quantified by high-performance liquid chromatography (HPLC). ATP mass formation was assessed by the luciferin/luciferase assay. In experiments where NDPK activity was assessed as a function of UTP-dependent phosphorylation of ADP (reaction 2), ATP formation values obtained in the absence of UTP (as in reaction 4) were subtracted from those obtained in the presence of UTP.
HPLC analysis
[32P]ATP, [32P]UTP, [32P]ADP, and 32P-inorganic phosphate (32Pi) were separated by HPLC (Shimatzu) using 10-μm Hamilton PRP-X100 anion exchange column. The mobile phase (1 ml/min; 30% methanol, solvent A; 0.5 M NH4HCO3 (pH 8.5) in 30% methanol, solvent B) developed as follows: 75% A and 25% B from 0 to 5 min, 33% A and 67% B from 5 to 15 min, and the column was re-equilibrated to the initial conditions for additional 10 min. 32P-species were quantified on-line with a FLO-ONE 500TR Radiomatic analyzer (Packard), as described previously [11].
Measurement of ATP mass
ATP measurements were performed via a LB953 AutoLumat luminometer (Berthold), as previously described [11]. Calibration curves were generated at the end of each experiment, using known concentrations of ATP. None of the reagents used during incubations interfered with the luciferase reaction.
Data analysis
Kinetic parameters from substrate concentration–response relationships and inhibition constants were calculated using Sigma Plot v.10 data fitting analysis.
Results
Ebselen inhibits the UDP-dependent conversion of [γ 32P]ATP to [32P]UTP on WD-HBE cells
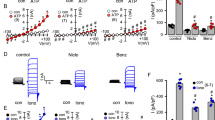
The fate of trace amounts of [γ 32P]ATP added to the mucosal medium bathing WD-HBE cell cultures was assessed by HPLC. Addition of [γ 32P]ATP to cells resulted in rapid and robust release (∼50% after 3 min) of the radiolabel as 32Pi (Fig. 1a (i and ii)). ATP hydrolysis was not affected by the inclusion of 30 μM ebselen in the incubation mix (Fig. 1a (iii)). Previously, we reported the presence of ecto-NDPK activity on various cell types, including airway epithelial cells [11, 27, 31]. Consistent with these reports, addition of radiotracer [γ 32P]ATP and excess mass of UDP (10 μM UDP) to WD-HBE cells resulted in the formation of [32P]UTP (Fig. 1a (iv)). The conversion of [γ 32P]ATP to [32P]UTP was markedly reduced by the presence of 30 μM ebselen (Fig. 1a (v)).
Ebselen inhibits the UDP-dependent conversion of [γ 32P]ATP to [32P]UTP on WD-HBE cells. HPLC analysis of [γ 32P]ATP metabolism on WD-HBE cells. Radiotracer [γ 32P]ATP (∼0.1 μCi) was added to cultures (mucosal addition) and the resulting [32P]-labeled species separated and analyzed by HPLC, as indicated in “Materials and methods”. a Representative HPLC tracings (n = 4) corresponding to samples incubated for T = 0 min (i), or 3 min in the presence of vehicle (ii), 30 μM ebselen (Ebs) (iii), 10 μM UDP (iv), or 30 μM ebselen and 10 μM UDP (v). The tracings represent the radioactivity (cpm) eluting from the HPLC column, recorded at 10-s intervals. b [γ 32P]ATP alone (i), [γ 32P]ATP and 10 μM UDP (ii), or [γ 32P]ATP and 10 μM AMP (iii) was added to cell cultures or to conditioned medium (Con. medium) for the indicated times, and the formation of 32Pi (i), [32P]UTP (ii), and [32P]ADP (iii) were quantified by HPLC (mean ± SD from two experiments performed in triplicates). The results are expressed as percent conversion of [32P]ATP to [32P]-product, relative to T = 0”. The area of each radioactive peak was calculated using the HPLC analysis software provided by the manufacturer
Ebselen-sensitive NDPK activity is recovered in the conditioned medium
ATP hydrolysis on airway epithelial cells reflected the action of cell surface ecto-ATPases [11]. Accordingly, release of 32Pi from [γ 32P]ATP was rapid and robust when the radiotracer was added to cells, but was negligible in the WD-HBE cell-conditioned medium (Fig. 1b (i)). Consistent with the data in Fig. 1a, ebselen had no effect on [γ 32P]ATP hydrolysis (Fig. 1b (i)). In contrast to ATPase activity, NDPK activity could be substantially recovered as a soluble enzyme, i.e., in the cell conditioned medium (Fig. 1b (ii), and [11]). Ebselen (30 μM) markedly reduced the conversion of [γ 32P]ATP to [32P]UTP (Fig. 1b (ii)).
In addition to NTPDase and NDPK activities, airway epithelia express ecto-adenyl kinase activity [16, 17], which reversibly phosphorylates AMP (as described above in reaction 3). Addition of [γ 32P]ATP together with 10 μM AMP resulted in rapid formation of [32P]ADP on both WD-HBE cell cultures and cell-conditioned medium (Fig. 1b (iii)). The adenyl kinase activity was markedly less robust than the NDPK activity and was not affected by ebselen (Fig. 1b (iii)).
Altogether, the data indicate that NDPK activity (1) is markedly inhibited by ebselen and (2) can be assessed in cell-free-conditioned medium.
Ebselen inhibits the UTP-dependent conversion of ADP to ATP
To further investigate the action of ebselen on NDPK activity, we adopted a non-radioactive protocol that quantifies the formation of ATP as a function of UTP and ADP in conditioned medium (see reaction 2 in “Materials and methods”). Conditioned medium from WD-HBE cell cultures contains low nanomolar concentrations of ATP (∼6 nM, Fig. 2a), which reflects the presence of endogenous ATP released from cells [13]. Addition of 10 μM ADP in the absence of UTP resulted in formation of ATP (∼50 nM ATP, Fig. 2a), consistent with an adenyl kinase-catalyzed reaction (see reaction 4 in “Materials and methods”). The ADP-dependent ATP formation (observed in the absence of exogenous UTP) was not affected by ebselen (Fig. 2a). Addition of 10 μM UTP to the medium in the absence of exogenous ADP resulted in minor ebselen-sensitive formation of ATP (∼20 nM ATP, Fig. 2a), likely reflecting phosphorylation of endogenous ADP by NDPK. Furthermore, a marked formation of ATP (∼200 nM) was observed when UTP and ADP were added together (Fig. 2a). Formation of ATP in the presence of both UTP and ADP was nearly abolished by ebselen (Fig. 2a). Figure 2b indicates that ebselen dose-dependently inhibited NDPK activity in WD-HBE cell secretions, displaying an IC50 value of 6.9 ± 2 μM.
Ebselen inhibits the UTP-dependent phosphorylation of ADP in conditioned medium from WD-HBE cells. a Conditioned medium from WD-HBE cells was incubated for 5 min with either vehicle or 30 μM ebselen in the absence (control) or presence of 10 μM ADP and/or 10 μM UTP. The formation of ATP was assessed by the luciferin–luciferase assay, as described in Methods. b Concentration–effect relationship for ebselen-inhibited UTP-dependent ATP formation (mean ± SD, n = 4); ATP values obtained in the absence of UTP were subtracted from the corresponding ADP/UTP data point
Ebselen is a potent, non-competitive inhibitor of NDPK
To characterize in greater detail the nature of ebselen inhibition on NDPK, conditioned medium from lung epithelial A549 cells was obtained. We chose using this fast-growing cell line as a source of secreted NDPK to bypass the limitations inherent to the low availability of primary cultures of HBE cells. Thus, protocols described above were adapted to non-polarized cultures of lung epithelial A549 cells. An initial assessment indicated that, like primary HBE cells, the conditioned medium of A549 cells displays robust NDPK activity that catalyzes the phosphorylation of UDP (10 μM) by [γ 32P]ATP in an ebselen-sensitive manner (Fig. 3). Also like in HBE cells, [γ 32P]ATP hydrolysis by A549 cell-conditioned medium was minor (<20% 32Pi accumulation) and only slightly affected by ebselen (Fig. 3).
Ebselen inhibits the phosphorylation of UDP in conditioned medium from A549 cells. A549 cell-conditioned medium was incubated with [γ 32P]ATP in the absence (control) or presence of 10 μM UDP. The percent conversion of [γ 32P]ATP to 32Pi or [32P]UTP was assessed as in Fig. 1. Ebselen (30 μM) or vehicle was added to samples 1 min prior incubations, as indicated. The data represent the mean (±SD) from two experiments performed in triplicate
Next, the effect of ebselen on the substrate concentration dependence for UTP- and ADP-promoted formation of ATP was assessed. Figure 4a illustrates the effect of UTP concentration on ATP formation, using 20 μM ADP as acceptor substrate for the NDPK activity present in the A549 cell-conditioned medium. In the absence of ebselen, the reaction displayed an apparent KM value of 18 μM UTP, and maximal ATP formation (6.8 μM/10 min) occurred with an apparent V max = 68 ± 6 pmoles/min. Ebselen dose-dependently decreased the V max of the reaction (Fig. 4a) while displaying a minor effect on the UTP K M value (Table 1). ADP concentration–effect relationships generated in the presence of 100 μM UTP indicated K M and V max values of 1.3 μM and 47 + 4 pmoles/min, respectively (Fig. 4b and Table 1). Ebselen reduced the V max (Fig. 4b) but had no effect on the ADP K M value (Table 1). The data suggest that ebselen behaves as a non-competitive inhibitor. Dixon Plot analysis [32] of the data indicated a K i value for ebselen-inhibited NDPK activity of 7.6 ± 3 μM. In the absence of exogenous UTP, some ATP formation was observed, which was likely mediated by adenyl kinase and represented approximately 5% of that attained in the presence of 100 μM UTP (compare Figs. 4c vs. 4b). In contrast to the robust inhibition of NDPK activity, ebselen (30 μM) had negligible effect on the adenyl kinase-mediated formation of ATP (Fig. 4c).
ATP formation as a function of substrate concentration. Effect of ebselen. Concentration–effect relationships for UTP- and ADP-dependent formation of ATP were established in A549 cell-conditioned medium. a UTP concentration vs. ATP formation was assessed in the presence of 20 μM ADP and the indicated concentration of ebselen. b ADP concentration vs. ATP formation in the presence of 100 μM UTP and the indicated concentration of ebselen. c Eebselen (30 μM) exhibited negligible effect on the ADP-dependent ATP formation in the absence of exogenous UTP. The data represent the mean (±SD) from one experiment performed in triplicate; similar results were obtained with three independent preparations of A549 cells. ATP values observed in the absence of UTP [as shown in (c)] were subtracted from the corresponding ADP/UTP data point in (a) and (b). All incubations were for 10 min at 37°C
Several ebselen derivatives have been recently used to assess the redox properties of selenium compounds [29]. Concentration–effect curves generated for a series of ebselen analogues indicated that the organotellurium EbTe2 reduced UTP-dependent formation of ATP in conditioned medium, but the inhibitory effect of EbTe2 on ATP formation was markedly weaker than that of ebselen (Fig. 5). Se-methylated (EbMe) and Se-benzylated (EbBz) derivatives of ebselen and the diselenide EbSe2 had negligible effect on ATP formation (Fig. 5). These results were consistent with the notion that structural modification in organoselenium compounds alter their redox activities [29].
Effect of ebselen derivatives on the UTP- and ADP-dependent formation of ATP. A549 cell-conditioned medium was incubated for 5 min at 37°C in the presence of 60 μM UTP, 20 μM ADP, and the indicated concentration of ebselen or ebselen derivative. The data represent the percent value (mean ± SD, n = 4) relative to the ATP formation observed in the obscene of inhibitor
Discussion
Our present study demonstrates that ebselen is a potent non-competitive inhibitor of NDPK. NDPK (also known as Nm23) is a housekeeping enzyme catalyzing the phosphorylation of NDPs, utilizing NTPs as terminal phosphate donor. Intracellular NDPK fulfills a crucial role in maintaining the high energy phosphate bond in ATP as part of the citric acid chain. NDPK also has been proposed to play a major role in maintaining a relative balance in the concentrations of cellular NTPs. In addition to its central role in cellular metabolism, NDPK is released from cells to catalyzed transphosphorylation reactions between extracellular nucleotides. Robust NDPK activity has been reported in conditioned medium from astrocytes, glioma, and airway epithelial cells, endothelial cells, osteoblasts, and keratinocytes [11, 31, 33–35].
Local transphosporylation complicates the task of accurately quantifying nucleotide concentrations at the cell surface upon release. For example, Buxton et al. [33] reported the involvement of bradykinin receptors in the release of ATP in coronary endothelium and also illustrated that although application of ADP resulted in an increase in ATP levels, this likely occurred as a consequence of phosphorylation of ADP by NDPK rather than stimulation of P2Y1 receptors. Bradykinin and phenylephrine promoted ATP release in Madin–Darby canine kidney, simian COS-7, and human embryonic kidney (HEK)-293 cells [36]. UTP also enhanced extracellular ATP accumulation in COS-7 and HEK-293 cells, but the effect of UTP probably reflected competitive inhibition of ATP hydrolysis and phosphorylation of endogenous ADP by NDPK rather than P2Y2 receptor-stimulated ATP release [36]. In a study designed to investigate the potential role of connexin hemichannels in ATP release, Cotrina et al. reported that stimulation of purinergic receptors with 100 μM UTP in HeLa cells, C6 glioma cells, and U373 glioblastoma cells resulted in a Ca2+-dependent increase of extracellular ATP concentrations. However, other Ca2+-mobilizing agents such as bradykinin, endothelin, and the calcium ionophore A2317 had little or no effect on extracellular ATP levels [37]. Because the likely contribution of NDPK in the phosphorylation of endogenous ADP was not examined, the significance of the ATP measurements in response to exogenous UTP remains unclear. Our present finding that ebselen inhibits NDPK activity provides a tool to assess the potential contribution of NDPK to nucleotide-promoted ATP release.
It has been previously reported that ebselen exhibits both glutathione peroxidase activity and antioxidant activity and that ebselen inhibits several redox-sensitive enzymes such as constitutive endothelial nitric oxide synthase, lipoxygenases, nicotinamide adenine dinucleotide phosphate oxidase, and other activities (Table 2 and [29, 38–43]). Furthermore, organoselenium compounds in the form of seleninic acids can couple with thiols on catalytically important cysteine residues (e.g., of phosphotyrosine phosphatase), resulting in enzyme inhibition [44]. It has been suggested that cysteine residues sensitive to redox conditions are involved in the functional regulation of NDPK [45]. Our data indicating that structural modifications affecting the redox properties of the organoselenium compound resulted in reduced NDPK inhibition (Fig. 5) are consistent with the notion that oxidative modifications regulate NDPK functions.
Two functionally active human NDPK isoenzymes have been characterized: NDPK-A (Nm23-H1) and NDPK-B (Nm23-H2) [46, 47]. In addition, four putative additional NDPK genes have been identified: DR-nm23, nm23-H4, nm23-H5, and nm23-H6, however, the presence of NDP kinase activity in these gene products has not been unambiguously demonstrated [48–51].
Recent studies with guinea pig endothelial cells and human breast carcinoma MDA-MB-435 cells have identified NDPK-A and NDPK-B as the major NDPK enzymes secreted from these cells, respectively [33, 52]. Whether NDPK-A, NDPK-B, or both are released from lung epithelial cells and what is the physiological role of such an activity remains to be elucidated. However, an important role of secreted NDPK may reside in regulating purinergic signaling, by affecting the extracellular concentrations of ATP, ADP, UTP, and UDP [31, 33, 53]. By regulating ATP levels locally, secreted NDPK-B supports angiogenesis, via endothelial cell P2Y receptor activation [52]. The mechanism of NDPK secretion is not understood. Based on the lack of secretion signal sequence of NDPK-A and NDPK-B, it has been proposed that NDPK may be secreted via non-classical export mechanisms similar to those involved in the release of fibroblast growth factors 1 and 2 [52].
Irrespective of the NDPK release mechanism, identification of potent and selective NDPK inhibitors would be relevant to studies of P2Y and P2X receptors and, potentially, may provide useful in elucidating the role of secreted NDPKs in angiogenesis and tumor development. Previously, cyclic AMP analogues were shown to inhibit NDPK activity, with IC50 values in the 100–500 μM range [54]. More recently, angiostatin (a proteolytic fragment of plasminogen), polyphenols, and adenosine 3′-phosphate 5′-phosphosulfate were shown to inhibit secreted NDPK-B, but only one of these compounds, ellagic acid, displayed inhibitory effect in the low micromolar concentration range (IC50 ∼ 10 μM [55]).
By illustrating that ebselen non-competitively inhibits extracellular lung epithelial cell NDPK activity with a <10 μM K i value, our present study suggest a valuable tool to assess the contribution of nucleotide release to lung epithelial cell functions. In addition, while ebselen has broad substrate selectivity, it may be a candidate structure for the development of highly potent and selective NDPK inhibitors useful to studies of the physiological consequences of extracellular NDPK activity.
References
Boucher RC (2003) Regulation of airway surface liquid volume by human airway epithelia. Pflugers Arch 445:495–498
Davis CW, Lazarowski E (2008) Coupling of airway ciliary activity and mucin secretion to mechanical stresses by purinergic signaling. Respir Physiol Neurobiol 163:208–213
Lazarowski ER, Boucher RC (2009) Purinergic receptors in airway epithelia. Curr Opin Pharmacol 9:262–267
Boucher RC (2002) An overview of the pathogenesis of cystic fibrosis lung disease. Adv Drug Deliv Rev 54:1359–1371
Morse DM, Smullen JL, Davis CW (2001) Differential effects of UTP, ATP, and adenosine on ciliary activity of human nasal epithelial cells. Am J Physiol Cell Physiol 280:C1485–C1497
Rooney SA (2001) Regulation of surfactant secretion. Comp Biochem Physiol A Mol Integr Physiol 129:233–243
Factor P, Mutlu GM, Chen L, Mohameed J, Akhmedov AT, Meng FJ, Jilling T, Lewis ER, Johnson MD, Xu A, Kass D, Martino JM, Bellmeyer A, Albazi JS, Emala C, Lee HT, Dobbs LG, Matalon S (2007) Adenosine regulation of alveolar fluid clearance. Proc Natl Acad Sci USA 104:4083–4088
Davis IC, Sullender WM, Hickman-Davis JM, Lindsey JR, Matalon S (2004) Nucleotide-mediated inhibition of alveolar fluid clearance in BALB/c mice after respiratory syncytial virus infection. Am J Physiol Lung Cell Mol Physiol 286:L112–L120
Blackburn MR, Lee CG, Young HW, Zhu Z, Chunn JL, Kang MJ, Banerjee SK, Elias JA (2003) Adenosine mediates IL-13-induced inflammation and remodeling in the lung and interacts in an IL-13-adenosine amplification pathway. J Clin Invest 112:332–344
Sun CX, Zhong H, Mohsenin A, Morschl E, Chunn JL, Molina JG, Belardinelli L, Zeng D, Blackburn MR (2006) Role of A2B adenosine receptor signaling in adenosine-dependent pulmonary inflammation and injury. J Clin Invest 116:2173–2182
Lazarowski ER, Boucher RC, Harden TK (2000) Constitutive release of ATP and evidence for major contribution of ecto-nucleotide pyrophosphatase and nucleoside diphosphokinase to extracellular nucleotide concentrations. J Biol Chem 275:31061–31068
Watt WC, Lazarowski ER, Boucher RC (1998) Cystic fibrosis transmembrane regulator-independent release of ATP—its implications for the regulation of P2Y(2) receptors in airway epithelia. J Biol Chem 273:14053–14058
Lazarowski ER, Tarran R, Grubb BR, van Heusden CA, Okada S, Boucher RC (2004) Nucleotide release provides a mechanism for airway surface liquid homeostasis. J Biol Chem 279:36855–36864
Picher M, Burch LH, Boucher RC (2004) Metabolism of P2 receptor agonists in human airways: Implications for mucociliary clearance and cystic fibrosis. J Biol Chem 279:20234–20241
Picher M, Burch LH, Hirsh AJ, Spychala J, Boucher RC (2003) Ecto 5′-nucleotidase and non-specific alkaline phosphatase: two AMP-hydrolyzing ectoenzymes with distinct roles in human airways. J Biol Chem 278:13468–13479
Donaldson SH, Picher M, Boucher RC (2002) Secreted and cell-associated adenylate kinase and nucleoside diphosphokinase contribute to extracellular nucleotide metabolism on human airway surfaces. Am J Respir Cell Mol Biol 26:209–215
Picher M, Boucher RC (2003) Human Airway Ecto-adenylate Kinase. A mechanism to propagate atp signaling on airway surfaces. J Biol Chem 278:11256–11264
Zuo P, Picher M, Okada SF, Lazarowski ER, Button B, Boucher RC, Elston TC (2008) Mathematical model of nucleotide regulation on airway epithelia. Implications for airway homeostasis. J Biol Chem 283:26805–26819
Kreda SM, Seminario-Vidal L, van Heusden CA, O’Neal W, Jones L, Boucher RC, Lazarowski ER (2010) Receptor-promoted exocytosis of airway epithelial mucin granules containing a spectrum of adenine nucleotides. J Physiol 588:2255–2267
Sesma JI, Esther CR Jr, Kreda SM, Jones L, O’Neal W, Nishihara S, Nicholas RA, Lazarowski ER (2009) ER/golgi nucelotide sugar transporters contribute to the cellular release of UDP-sugar signaling molecules. J Biol Chem 284:12572–12583
Lazarowski ER, Boucher RC, Harden TK (2003) Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol Pharmacol 64:785–795
Tatur S, Kreda S, Lazarowski E, Grygorczyk R (2008) Calcium-dependent release of adenosine and uridine nucleotides from A549 cells. Purinergic Signal 4:139–146
Donaldson SH, Lazarowski ER, Picher M, Knowles MR, Stutts MJ, Boucher RC (2000) Basal nucleotide levels, release, and metabolism in normal and cystic fibrosis airways. Mol Med 6:969–982
Huang PB, Lazarowski ER, Tarran R, Milgram SL, Boucher RC, Stutts MJ (2001) Compartmentalized autocrine signaling to cystic fibrosis transmembrane conductance regulator at the apical membrane of airway epithelial cells. Proc Natl Acad Sci USA 98:14120–14125
Okada SF, Nicholas RA, Kreda SM, Lazarowski ER, Boucher RC (2006) Physiological regulation of ATP release at the apical surface of human airway epithelia. J Biol Chem 281:22992–23002
Tarran R, Button B, Picher M, Paradiso AM, Ribeiro CM, Lazarowski ER, Zhang L, Collins PL, Pickles RJ, Fredburg JJ, Boucher RC (2005) Normal and cystic fbrosis airway surface liquid homeostasis: the effects of phasic shear stress and viral infections. J Biol Chem 280:35751–35759
Lazarowski ER, Paradiso AM, Watt WC, Harden TK, Boucher RC (1997) UDP activates a mucosal-restricted receptor on human nasal epithelial cells that is distinct from the P2Y(2) receptor. Proc Natl Acad Sci USA 94:2599–2603
Furstenau CR, Spier AP, Rucker B, Luisa BS, Battastini AM, Sarkis JJ (2004) The effect of ebselen on adenine nucleotide hydrolysis by platelets from adult rats. Chem Biol Interact 148:93–99
Mishra B, Priyadarsini KI, Mohan H, Mugesh G (2006) Horseradish peroxidase inhibition and antioxidant activity of ebselen and related organoselenium compounds. Bioorg Med Chem Lett 16:5334–5338
Seminario-Vidal L, Kreda S, Jones L, O’Neal W, Trejo J, Boucher RC, Lazarowski ER (2009) Thrombin promotes release of ATP from lung epithelial cells through coordinated activation of Rho- and Ca2+-dependent signaling pathways. J Biol Chem 284:20638–20648
Lazarowski ER, Homolya L, Boucher RC, Harden TK (1997) Identification of an ecto-nucleoside diphosphokinase and its contribution to interconversion of P2 receptor agonists. J Biol Chem 272:20402–20407
Segel IH (1993) Enzyme Kinetics. Behavior and analysis of rapid equilibirum and steady-state enzyme systems. Wiley, New York
Buxton IL, Kaiser RA, Oxhorn BC, Cheek DJ (2001) Evidence supporting the Nucleotide Axis Hypothesis: ATP release and metabolism by coronary endothelium. Am J Physiol Heart Circ Physiol 281:H1657–H1666
Buckley KA, Golding SL, Rice JM, Dillon JP, Gallagher JA (2003) Release and interconversion of P2 receptor agonists by human osteoblast-like cells. FASEB J 17:1401–1410
Burrell HE, Wlodarski B, Foster BJ, Buckley KA, Sharpe GR, Quayle JM, Simpson AW, Gallagher JA (2005) Human keratinocytes release ATP and utilize three mechanisms for nucleotide interconversion at the cell surface. J Biol Chem 280:29667–29676
Ostrom RS, Gregorian C, Insel PA (2000) Cellular release of and response to ATP as key determinants of the set-point of signal transduction pathways. J Biol Chem 275:11735–11739
Cotrina ML, Lin JH, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, Kang J, Naus CC, Nedergaard M (1998) Connexins regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci USA 95:15735–15740
Zembowicz A, Hatchett RJ, Radziszewski W, Gryglewski RJ (1993) Inhibition of endothelial nitric oxide synthase by ebselen. Prevention by thiols suggests the inactivation by ebselen of a critical thiol essential for the catalytic activity of nitric oxide synthase. J Pharmacol Exp Ther 267:1112–1118
Schewe C, Schewe T, Wendel A (1994) Strong inhibition of mammalian lipoxygenases by the antiinflammatory seleno-organic compound ebselen in the absence of glutathione. Biochem Pharmacol 48:65–74
Cotgreave IA, Duddy SK, Kass GE, Thompson D, Moldeus P (1989) Studies on the anti-inflammatory activity of ebselen. Ebselen interferes with granulocyte oxidative burst by dual inhibition of NADPH oxidase and protein kinase C? Biochem Pharmacol 38:649–656
Wetli HA, Buckett PD, Wessling-Resnick M (2006) Small-molecule screening identifies the selanazal drug ebselen as a potent inhibitor of DMT1-mediated iron uptake. Chem Biol 13:965–972
Soteropoulos P, Vaz T, Santangelo R, Paderu P, Huang DY, Tamas MJ, Perlin DS (2000) Molecular characterization of the plasma membrane H(+)-ATPase, an antifungal target in Cryptococcus neoformans. Antimicrob Agents Chemother 44:2349–2355
Chan G, Hardej D, Santoro M, Lau-Cam C, Billack B (2007) Evaluation of the antimicrobial activity of ebselen: role of the yeast plasma membrane H+-ATPase. J Biochem Mol Toxicol 21:252–264
Abdo M, Liu S, Zhou B, Walls CD, Wu L, Knapp S, Zhang ZY (2008) Seleninate in place of phosphate: irreversible inhibition of protein tyrosine phosphatases. J Am Chem Soc 130:13196–13197
Song EJ, Kim YS, Chung JY, Kim E, Chae SK, Lee KJ (2000) Oxidative modification of nucleoside diphosphate kinase and its identification by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Biochemistry 39:10090–10097
Rosengard AM, Krutzsch HC, Shearn A, Biggs JR, Barker E, Margulies IM, King CR, Liotta LA, Steeg PS (1989) Reduced Nm23/Awd protein in tumour metastasis and aberrant Drosophila development. Nature 342:177–180
Stahl JA, Leone A, Rosengard AM, Porter L, King CR, Steeg PS (1991) Identification of a second human nm23 gene, nm23-H2. Cancer Res 51:445–449
Venturelli D, Martinez R, Melotti P, Casella I, Peschle C, Cucco C, Spampinato G, Darzynkiewicz Z, Calabretta B (1995) Overexpression of DR-nm23, a protein encoded by a member of the nm23 gene family, inhibits granulocyte differentiation and induces apoptosis in 32Dc13 myeloid cells. Proc Natl Acad Sci USA 92:7435–7439
Milon L, Rousseau-Merck MF, Munier A, Erent M, Lascu I, Capeau J, Lacombe ML (1997) nm23-H4, a new member of the family of human nm23/nucleoside diphosphate kinase genes localised on chromosome 16p13. Hum Genet 99:550–557
Munier A, Feral C, Milon L, Pinon VP, Gyapay G, Capeau J, Guellaen G, Lacombe ML (1998) A new human nm23 homologue (nm23-H5) specifically expressed in testis germinal cells. FEBS Lett 434:289–294
Tsuiki H, Nitta M, Furuya A, Hanai N, Fujiwara T, Inagaki M, Kochi M, Ushio Y, Saya H, Nakamura H (1999) A novel human nucleoside diphosphate (NDP) kinase, Nm23-H6, localizes in mitochondria and affects cytokinesis. J Cell Biochem 76:254–269
Rumjahn SM, Javed MA, Wong N, Law WE, Buxton IL (2007) Purinergic regulation of angiogenesis by human breast carcinoma-secreted nucleoside diphosphate kinase. Br J Cancer 97:1372–1380
Lazarowski ER, Homolya L, Boucher RC, Harden TK (1997) Direct demonstration of mechanically induced release of cellular UTP and its implication for uridine nucleotide receptor activation. J Biol Chem 272:24348–24354
Anciaux K, VanDommelen K, Willems R, Roymans D, Slegers H (1997) Inhibition of nucleoside diphosphate kinase (NDPK/nm23) by cAMP analogues. FEBS Lett 400:75–79
Buxton IL (2008) Inhibition of Nm23H2 gene product (NDPK-B) by angiostatin, polyphenols and nucleoside analogs. Proc West Pharmacol Soc 51:30–34
Acknowledgments
We thank Dr. Scott Randell for providing primary cultures of HBE cells. We are indebted to Lisa Brown for editorial assistance with the manuscript. This work was supported, in whole or in part, by National Institutes of Health Grant P01-HL034322 (ERL, CH) and Cystic Fibrosis Foundation Grant CFF-SEMINA08FO (LS-V). GM was supported by the Department of Science and Technology (DST), India.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s11302-011-9230-2
Rights and permissions
About this article
Cite this article
Semianrio-Vidal, L., van Hesuden, C., Mugesh, G. et al. Ebselen is a potent non-competitive inhibitor of extracellular nucleoside diphosphokinase. Purinergic Signalling 6, 383–391 (2010). https://doi.org/10.1007/s11302-010-9203-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-010-9203-x