Abstract
Genetic variation for wood quality traits and diameter growth for radiata pine (Pinus radiata D. Don) at age 20/21 years was estimated from eight trials in Australia. The traits studied were wood density, acoustic time-of-flight (an indirect measure of stiffness) and diameter at breast height (DBH). Wood density and DBH exhibited significant additive genetic variation whereas non-additive effects were not significantly different from zero. Time of flight was also not significantly different from zero for both additive and non-additive effects, respectively. Average single-site heritability estimates (±SE) for wood density and DBH were 0.38 ± 0.10 and 0.16 ± 0.08, respectively. Pooled-site heritability estimates for wood density and DBH were 0.38 ± 0.10 and 0.08 ± 0.10, respectively. For density, there was little evidence of genotype-by-environment interaction (GEI) across the eight trials at the additive level (type B additive genetic correlation; r BADD = 0.73 ± 0.08) and type B genetic correlation for full-sib families (r BFS = 0.64 ± 0.08). In contrast, the type B additive genetic correlation for DBH was lower, (r BADD = 0.51 ± 0.14), suggesting evidence of GEI. However, type B genetic correlation for full-sib families was moderate (0.63 ± 0.11) for DBH, suggesting that there may be some stable full-sib families. On the basis of the results of this study, GEI should be considered in order to optimise deployment of improved germplasm in Australia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Forest tree breeding programmes have had a successful impact on increased productivity of plantation forestry around the globe (McKeand et al. 2006; Wu et al. 2007; Burdon et al. 2008). In Australia, two generations of radiata pine (Pinus radiata D. Don) breeding and selection have resulted in substantial economic benefits for the Australian forest industries (Matheson et al. 1986; Wu et al. 2008). For example, through genetic improvement and silviculture, the rotation length for sawn products was reduced from 40–45 to 25–30 years (Li and Wu 2005). Predicted genetic gains for second generation diameter growth were estimated to be 4% to 17% and would result in overall volume gains of more than 10% (White et al. 1999; Wu and Matheson 2005). As radiata pine breeding advances to third generation selections in Australia, there is an increasing need to include wood quality traits in the breeding programme. A large number of studies have been carried out to study variation and genetic control of various wood quality traits of radiata pine (Li and Wu 2005; Dungey et al. 2006; Gapare et al. 2006, 2007, 2008; Baltunis et al. 2007b; Matheson et al. 2008). However, genotype-by-environment interaction (GEI) for wood density or stiffness has not been assessed within Australian radiata pine breeding programmes. Such information is particularly useful as radiata pine breeding advances to third generation selections with inclusion of wood quality traits.
Wood stiffness, measured as its modulus of elasticity (MoE), is one of the radiata pine major breeding objective traits (Ivković et al. 2006) and is known to be under strong genetic control (Matheson et al. 1997; Dungey et al. 2006). However, direct measurement of the bending MoE (also known as the static MoE, usually performed on normalised specimens) requires destructive sampling and is expensive and time consuming. While a direct measure of bending stiffness is the most accurate, indirect measures that are far less destructive and expensive are the most desirable for breeding purposes. Recent work has shown that stiffness can be indirectly measured by either using mechanical and chemical properties of wood or using component wood quality traits. Instruments based on acoustic waves showed great promise for measuring stiffness of standing trees, logs (Carter et al. 2005; Wang et al. 2007; Auty and Achim 2008), small axial specimens from outer wood (Carter et al. 2005; Ilic 2003), for log segregation in radiata pine (Walker and Nakada 1999; Matheson et al. 2002) and juvenile standing trees in radiata pine (Matheson et al. 2008). Assuming the density of green wood is 1,000 kg/m3, the modulus of elasticity can be calculated approximately as V 2/106 in GPa, where V is the velocity of the stress wave in the tree (in metre per second). Time of flight (TOF) could be regarded itself as an indication of MoE. Longer flight times are associated with lower stiffness values. The standing tree TOF technique provides an acoustic wave velocity for the stem.
In forest tree improvement, the assessment of genotype-by-environment interaction (GEI) has been essential for formulating breeding and deployment strategies (White et al. 1993). The patterns of response across environments may involve change in the ranking of genotypes and/or alterations in scale (Lynch and Walsh 1998). When present at family or clonal levels, the former type of GEI is of particular concern to tree breeders since it determines critical decisions in developing optimal breeding strategies and realising genetic gains (e.g. Costa e Silva et al. 2006).
Genotype-by-environment interaction for radiata pine growth traits has been studied extensively (Cown and McConchie 1983; Shelbourne 1972; Shelbourne 1997; Matheson and Raymond 1984, 1986). For example, Matheson and Raymond (1984) studied GEI for 30 open-pollinated families from growth data collected in 11 sites, and they found the existence of interaction between families and sites. Similarly, Wu and Matheson (2005) investigated GEI in growth and stem characteristics at 10/11 years using the Australia-Wide Diallel (AWD) mating experiment for radiata pine. In addition to studying GEI, the AWD mating experiment allowed for estimating both realised and expected gains that could arise from both additive and non-additive genetic variation. The study also provided estimates of general combining ability (GCA) and specific combining ability (SCA) variances and covariances (Wu and Matheson 2004). Results from that analysis suggested that there was significant GEI for growth traits in radiata pine across the ten sites involved in the analysis. Two sites in New South Wales (NSW) contributed disproportionately to the interactions compared with the other sites. There were some considerable changes in rank between families growing in NSW compared with the same families growing elsewhere, particularly involving about ten parents (Wu and Matheson 2005).
There is little information on how genotypes, and more specifically, both additive and non-additive effects for radiata pine wood properties, interact with environments. The level of GEI would assist breeders and practical foresters in designing their effective breeding programmes by selecting genotypes with desirable wood traits that can be deployed in different environments. If GEI is deemed to be negligible, then selected genotypes could be used for plantations to produce uniform wood under different environmental conditions (e.g. Sykes et al. 2006). This would increase yield, improve product properties and lower processing costs (Zobel 1984).
Since trees are selected based on a composite index value rather than individual trait values, it is important to investigate if there would be GEI for the index values. Namkoong (1984) showed that when a value function (i.e. index) is affected by the environment (i.e. relative economic weights for traits are different in different environments), index of provenances may change ranking even in the absence of changes in component traits. However, even if both genotype and site linearly affect the phenotype for all traits, differences in the index in different environments can change ranking of genotypes for different planting sites. In other words, relative sizes of trait economic weights can influence the size and the sign of difference between genotypes in index values at different sites (Namkoong and Johnson 1987).
The purpose of this study was to determine the level of genetic variation of key wood properties in radiata pine across plantation estate in Australia and examine genotype-by-environment interaction. Specific objectives were (1) compare genetic variation in wood density, time of flight (surrogate for modulus of elasticity MoE) and diameter growth on eight test sites, (2) estimate genetic correlations between the same traits, (3) examine GEI of the same traits and (4) investigate the effects of GEI for composite selection index values.
Materials and methods
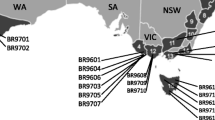
Details of the AWD mating programme are given in Wu and Matheson (2004). Briefly, the AWD project was conceived during the 1976 meeting of Research Working Group No 1 in Australia (Matheson 1976). The initiation of AWD was facilitated by the idea of sublines (Lindgren 1976) as a means of controlling inbreeding in breeding populations, and a similar mating plan by the North Carolina State University loblolly pine cooperative producing disconnected six-parent half-diallels as a means of producing their second generation material for selection and breeding (Griffin 1976). The numbers of families within each diallel set are presented in Table 1. Table 2 presents the details of trials in regard to site environmental conditions and experimental layout, while the eight trial locations are shown in Fig. 1. Diallels were chosen for inclusion in the analyses reported here to maximise overlap between sets of families planted each year and sites.
Locations of eight trial sites for Australia-wide radiata pine diallel mating progenies (Adapted from Wu and Matheson 2005)
This study used measures of stem diameter at breast height, time of flight in microsecond over one metre and whole-core density in kg m−3 from eight diallel trials of radiata pine (Table 3). Within the resources available for the project, it was not possible to measure every tree at all sites. Accordingly, approximately five trees from each of 100 families at each trial were measured (approximately 500 trees per trial). Two trials (RAD199 at Myrtleford (Victoria) and VRC60 in Gippsland (Victoria)) originally planted and included in the Wu and Matheson (2005) analyses no longer exist (see Fig. 1). A total of 4,152 trees were measured over all eight trials available in 2007.
The statistical analyses were done in several steps, beginning with univariate analyses, where variance components for each trait within each trial were estimated; followed by multivariate analyses, where variances and covariances between pairs of traits within trials were estimated. For DBH and density, estimates of GEI, expressed as type B genetic correlations (Burdon 1977), were obtained from multivariate analyses of variables measured in the two trials as different traits, e.g. wood density measurements in the two trials were considered as measurements of two different traits.
The following linear mixed model was used in the univariate analysis of the diallel trials:
where y is the vector of individual tree observations; b is the vector of fixed effects (overall mean and blocks); a is the vector of random additive effects of individual trees; s is the vector of random specific combining ability effects due to specific combinations of males and females; e is the vector of random residual deviations of individual trees; X, Z 1 and Z 2 are incidence matrices relating to the model effects. It was assumed that the random terms are jointly normal with moments:
and
where \( \oplus \) is the direct sum of matrices related to the random terms in the model; A is the additive genetic relationship matrix between trees and I is an identity matrix; \( \hat \sigma_a^2 \) is the additive genetic variance; \( \hat \sigma_s^2 \) is the variance due to SCA; \( \hat \sigma_e^2 \) is the residual variance.
A general linear mixed model was fitted to data to estimate variance components for combined sites.
where y is the vector of individual observations; b is the vector of fixed-effects parameters (overall mean, site and blocks within site); a is the vector of random effects parameters including additive effects, SCA, additive-by-site interaction and SCA-by-site interaction. The e is an unknown random error vector; X and Z are known design matrices for fixed and random effects, respectively.
For random terms, the first indication of their significance was given by the ratio of the variance components to their corresponding standard error. Terms for which this ratio was >2 were regarded as significant. Terms for which the ratio was <1 were regarded as not significant. For ratios between 1 and 2, the likelihood ratio test was applied (−2* (difference between log likelihoods including and excluding the term) ~χ 2; Gilmour et al. 2006).
Across-site analyses may be complicated by heterogeneous variance structures among sites if data from different sites are not standardised (e.g. Costa e Silva et al. 2000). In this study, the across-site heterogeneity of additive genetic and environmental variance estimates was not significant (based on Akaike Information Criterion—see Lynch and Walsh (1998)). As such, correction for heterogeneous variances was not needed.
Estimates of heritability were obtained for each trait at each site using the variance components from the univariate single-site analyses. Standard errors were also estimated after Gilmour et al. (2006).
where \( \hat h_b^2 \) is the individual-tree narrow-sense heritability for each trait at each trial, and \( \hat \sigma_{{p_i}}^2 \) is the phenotypic variance.
Variances are not independent of the scale and mean of the respective traits (Sokal and Rohlf 1995). Therefore, to relatively compare genetic variances of the different traits across tests, a parameter measuring the genetic coefficient of variation was calculated as:
where
- CV A :
-
coefficient of additive genetic variation
- σa :
-
square root additive genetic variance for a trait and
- \( \overline {\text{x}} \) :
-
population mean for the trait
The CV A expresses the genetic variance relative to the mean of the trait of interest and gives a standardised measure of the genetic variance relative to the mean of the trait. The higher the coefficient of additive genetic variation for a trait, the higher is its relative variation.
Genetic correlations between traits x and y were obtained from the estimated additive covariance and variance components (using the individual tree model) as:
where
- \( {\sigma_{{a_x}{a_y}}} \) :
-
additive genetic covariance component between traits x and y
- \( \sigma_{{a_x}}^2 \) :
-
additive genetic variance component for trait x
- \( \sigma_{{a_y}}^2 \) :
-
additive genetic variance component for trait y.
Standard errors were obtained by Taylor expansion within the ASReml programme.
One conceptual approach to GEI has been to consider the expression of a trait in two or more environments as a manifestation of two distinct traits. For example, different genes may be operative in one environment and not in another. This approach allows one to estimate genetic correlation between the two traits (i.e. the trait measured in two environments). This correlation has been termed a type B genetic correlation (r Bg; Burdon 1977). In order to measure the extent of genotype-by-environment interaction for each of the traits, between-site type B genetic correlations and their standard errors were estimated as
where \( {\hat r_{{B_{ADDITIVE}}}} \) is the type B genetic correlation of additive genetic effects across sites and all other terms as defined in Eq. 4. A value of \( {\hat r_{{B_{ADDITIVE}}}} \) close to one indicates little genotype-by-environment interaction, while a low \( {\hat r_{{B_{ADDITIVE}}}} \) indicates extensive genotype-by-environment interaction, and parental rankings may change across sites. An individual tree model was used for this analysis.
Single site analyses yield an estimate of heritability (h 2b ) which is biassed upward by the presence of GEI variance (Hodge and White 1992). In the analysis of single-site data, the interaction between additive genetic effects and environmental effects is confounded with additive genetic effects (Yamada 1962). The inability to separate additive-by-environment effects from the additive effects means that the estimated additive genetic variance and, consequently, the heritability are biassed upwards (Burdon 1977; Dickerson 1962; Comstock and Moll 1963). Unbiased estimates of heritability and type B genetic correlations for additive effects (rBADD) and type B genetic correlations for full sibs (rBFS; e.g. Baltunis et al. 2007a) were estimated from a pooled-site analysis.
Selection index-by-environment interaction
Greaves (1999) modelled a radiata pine production system for sawn timber and liner board products and reported the economic weights for different traits as change in profitability index (i.e. present value of profit as a percentage of present value costs) per unit trait change. The change in profitability index per unit trait change in mean annual increment (MAI) was 0.76%, and for wood density, it was 31%. Assuming that the weights are linear over a wide range of trait values, this would translate in approximately 1:1 weight proportion for unit change in volume (VOL; cubic metre per hectare) and DEN (kilogram per cubic metre). Apiolaza and Garrick (2000) reported breeding objectives for an integrated firm in Chile comprising a production forest, a sawmill and a pulpmill. Breeding objectives were derived for three management regimes: 1VOL + 2.4DEN for pulp, 1VOL + 1.1DEN for intermediate and 1VOL + 1.2DEN for intensive regime. In a more recent study, Ivković et al. (2006) derived economic weights for radiata pine integrated production systems in Australia. Growth, measured as MAI (cubic metre per hectare per year), received economic weight of Aus$416, and wood quality measured by MoE (GPa) received economic weight of Aus$977. Assuming that the percent increase in MoE would have approximately same value as wood density, this would translate to approximately 1:2 weight proportion for unit change in VOL (cubic metre per hectare) and DEN (kilogram per cubic metre).
We considered the range of relative economic weights for growth MAI or VOL and wood quality (DEN or MoE) reported in the literature (Greaves 1999; Apiolaza and Garrick 2000; Ivković et al. 2006). Based on the individual trait economic weights reported in the literature, we created three scenarios for index selection. Assuming that 1-cm change in DBH can result in change in average tree VOL of 0.05 to 0.15 m3 (Lewis et al. 1976, Table IV.1); the weights for VOL (cubic metre per hectare ) and DEN (kilogram per cubic metre) would indicate that 1-kg/m3 change in DEN can have between 1% and 15% of the economic value due to 1-cm change in DBH. Therefore, we created an index with a low (1%) weight on DEN (INDEXLD), an intermediate (7.5%) weight on DEN (INDEXMD) and a high (15%) weight on DEN relative to DBH (INDEXHD) as follows:
We then analysed the data by fitting Eq. 2 to investigate whether there would be GEI for composite selection index values.
Results
Trait means
The overall mean values for DBH, DEN and TOF for each site are presented in Table 3. DBH ranged from 23.9 cm at Bil133 in New South Wales to 31.48 cm at RS27B in Western Australia. DBH was lowest at two sites (Bil133 and Bil149) in New South Wales. Density values were highest at PT5455 in South Australia, followed by Bil133 and Bil149 in New South Wales, with density values ranging from 428 to 437 kg m−3. Lowest density values were recorded at RS27B and RS27A in Western Australia (363 and 374 kg m−3). As might be expected, TOF was the highest at the sites with lowest density. Average TOF over 1 m was lowest at PT5455 (305.72 μs m−1) and highest at VRC52 (385.21 μs m−1; Table 3). Genetic coefficient of variation (CV A) for DBH, DEN and TOF ranged from 5.4% to 9.7% for DBH, 3.1% to 5.7% for DEN and 0.1% to 5.0% for TOF. As might be expected, CV A for DBH and DEN matched expectation (Wu et al., 2008), i.e. more genetic variation in DBH growth compared to wood quality traits such as DEN and TOF (Table 3).
Single trait, individual site analyses
Estimates of variances and associated genetic parameters for DBH, DEN and TOF are presented in Table 3. We observed low heritability estimates for DBH, ranging from 0.09 ± 0.07 to 0.22 ± 0.11 across sites. Additive genetic variance for DEN was moderate to high, (and heritabilities low to high (0.19 ± 0.06 to 0.59 ± 0.14)) at all sites except Bil149 where the additive genetic variance was zero. Non-additive genetic variance was much higher at Bil149 than at other sites (Table 3). Residual variance was much the same at all sites except at VRC52 which had high residual variance.
For acoustic TOF, the additive genetic variance and heritability was insignificant at five of the eight sites (Table 3). Heritability for TOF at RS27A, RS27B and VRC52 was small (0.22 ± 0.11 to 0.25 ± 0.11). Non-additive genetic variance was much smaller than additive genetic variance at RS27A, RS27B, Bil149 and VRC52.
Trait–trait genetic correlations
Because of the general lack of significant additive genetic variation for TOF at five of the sites, it was not possible to estimate meaningful trait–trait genetic correlations between TOF and either DBH or DEN. As might be expected for radiata pine, genetic correlations between DBH and DEN were always negative and/or much the same or smaller than their standard errors. For example, at Bil133, the genetic correlation between DEN and DBH was −0.22 ± 0.23. Similarly, at PT5455, the genetic correlation between DEN and DBH was −0.09 ± 0.35.
Genotype-by-environment interactions
There were nine and 14 pair-wise estimates of type B additive genetic correlations for DBH and DEN, respectively (Table 4). In cases where additive genetic variance was not significant, no estimates of type B additive genetic correlations were estimated. For DBH, pair-wise type B additive genetic correlations ranged from 0 to 1. For example, DBH at one site in NSW (Bil133) had a correlation of zero with DBH in trials in SA (PT5455 and PT5459), suggesting evidence of GEI. These correlations indicate a large amount of variation due to families changing rank across sites. In contrast, DBH at sites other than Bil133 had type B additive genetic correlations ranging from 0.67 to 1.00, suggesting very little family-by-site interaction. DEN had type B additive genetic correlations ranging from 0.70 to 1.00, with an average of 0.73.
Type B genetic correlations for DBH and DEN due to both additive and non-additive effects are presented in Table 5. Type B genetic correlations for DBH due to full-sib family effects were relatively low (r BFS = 0.51 ± 0.14), suggesting evidence of GEI (Table 5). For DEN, type B genetic correlations due to full-sib family effects were moderate (r BFS = 0.64 ± 0.08), indicating a lack of GEI for non-additive genetic effects (Table 5).
Discussion
Trait means
Growth rates for radiata pine reported in this study are consistent with second rotation growth rates in radiata pine and other conifers (Dieters et al. 1995; Gapare et al. 2001; Burdon et al. 1992a, b; Li and Wu 2005; Johnson et al. 2008). It appears trees grew faster at RS27A and RS27B in Western Australia and VRC52 in Victoria. Previous assessments at age 11.5 years also showed that these trials had higher growth rates relative to other trials (Wu and Matheson 2002). The three sites were characterised by low elevation (93 to 120 m), high mean annual rainfall of 1,100 mm and were previously pasture sites, whereas other sites are second-rotation of radiata pine crop (see Table 2). DBH was lowest in two trials in NSW. These two sites are characterised by high mean annual rainfall (1,150 mm) but were on second-rotation of radiata pine crop sites. In this case, it appears growth was driven by soil fertility and temperature, rather than mean annual rainfall. In addition, the two sites in New South Wales experience cooler temperatures (mean maximum = 16oC; mean minimum = 7oC) and higher elevation (Table 2), which may limit growth potential of radiata pine. Another difference between the two regions was elevation. It appears radiata pine grows differently on ex-pasture and second-rotation sites. For possible expansion of radiata pine plantations, candidate areas would be low elevation sites with higher rainfall and warmer temperatures.
Density values were highest at PT5455 in South Australia, followed by Bil133 and Bil149 in New South Wales, with density values ranging from 428 to 437 kg m−3. However, an inspection of some of the cores sampled at PT5455 seemed to suggest that some of the trees had compression wood which would account for the higher density values observed. For example, some cores had densities as high as 600 kg m−3. A preliminary investigation suggested no family trends for compression wood—i.e. no specific families had compression wood (data not shown). It may be possible that trees growing at PT5455 have a relatively higher percentage of latewood density, hence, higher overall density. Lowest density values were recorded at RS27B and RS27A in Western Australia (363 and 374 kg m−3).
TOF could be regarded as an indication of modulus of elasticity. Longer flight times are associated with lower stiffness values (Matheson et al. 2008). Generally, faster growth rates are associated with lower density as evidenced by our results. As might be expected, TOF was the highest at the sites with lowest density. Average TOF over 1 m was lowest at PT5455 (305.72 μs m−1) and highest at VRC52 (385.21 μs m−1; Table 3). Several studies have shown that acoustic velocities measured using the time-of-flight method give a good indication of static MoE (Carter et al. 2005; Grabianowski et al. 2006; Auty and Achim 2008).
Single trait, individual site analyses
Heritability for DBH in radiata pine has been estimated in many studies in Australia and New Zealand. Most genetic studies have found low to moderate individual tree heritabilities for DBH ranging from 0.13 to 0.33 (Cown et al. 1992; Nyakuengama et al. 2000; Jayawickrama 2001; Wu et al. 2002; Wu et al. 2004). We observed low heritability estimates for DBH, ranging from 0.09 to 0.22 across eight sites. The average of these estimates (0.08 ± 0.02, (n = 8)) was slightly below the average for those derived from open-pollinated progeny trials of radiata pine (heritability ranged from 0.13 to 0.33; Cown et al. 1992; Matheson et al. 1994; Jayawickrama 2001; Wu et al. 2002; Kumar 2004; Li and Wu 2005). Compared to previous heritability estimates at age 11.5 years, there was a reduction in additive variance in the current estimates. For example, at age 11.5 years, heritability for DBH at Bil133 was 0.36 ± 0.08 (Wu and Matheson 2002), whereas at age 22, heritability for DBH was 0.17 ± 0.08. Possible reasons for the observed reduction in additive genetic variance may be that we only assessed a sub-set of the total number of families in each of the trials or the additive genetic variance decreased with increased competition among trees with age. Non-additive (SCA) effects for DBH were small in the current population.
Larger estimates of heritabilities for DEN compared with DBH were consistent with other research dealing with growth and wood quality traits in radiata pine and other conifers (e.g. Artwood et al. 2002; Kumar 2004; Li and Wu 2005; Baltunis et al. 2007b; Gapare et al. 2009). Similarly, heritabilities for DEN were lower in this study than have been reported elsewhere, averaging about 0.3–0.5. For example, a review of genetic parameters for radiata pine (Wu et al. 2008) showed heritability estimates for density to be generally above 0.55. Lack of additive variance for density at Bil149 was rather surprising, given that additive variance was significant at all other sites and also that wood density is generally under moderate to high levels of genetic control (Zobel and van Buijtenen 1989). In addition, SCA effects were highest and significant at Bil149 compared to other trials. If the SCA effects are real, then deployment of full-sib families could capture the non-additive effects.
Lack of additive genetic variance in the AWD trials using acoustic tools may be real or genetic diversity in the AWD full-sib crosses (each parent only mated with five other parents) may be low due to the relatively small mating numbers for each parent in full-sib crosses (Wu and Matheson 2005). Secondly, although tools such as IML hammer have been considered useful to assess acoustic stiffness on young standing trees, this may not be applicable to mature age trees. In a preliminary study on 20 year-old Douglas-fir in New Zealand, there was no evidence of additive genetic variance in trees measured with an acoustic tool (H. Dungey, personal communication).
One noteworthy observation from this study of mature trees (20/21 years) and a previous study of young trees (6/7 years) is that heritability for acoustic time of flight at young ages was much higher (0.54; Matheson et al. 2008) than mature age (average 0.12) in this study. Similarly, heritability for wood density at young ages was higher (0.65 of average of two sites (Baltunis et al. 2007b) than mature age (average 0.33) in this study. The trees measured at earlier ages almost certainly contained juvenile corewood only (Burdon et al. 2004). This may indicate that there was probably more variation in juvenile core wood than mature wood in the current study. Dungey et al. (2006) reported considerable variation and high heritability estimates in wood stiffness in corewood, but less variation and lower heritability estimates in outerwood of radiata pine.
The limited number of individuals per family might have contributed to genetic correlation estimates with high standard errors. However, literature suggests a negative genetic correlation between DEN and DBH in radiata pine and other species. Kumar (2004) reported a negative genetic correlation of −0.16 between DEN and DBH in juvenile core of radiata pine. More recently, Gapare et al. (2009) reported a negative genetic correlation of −0.55 between DEN and DBH in the juvenile core of radiata pine. Johnson and Gartner (2006) in coastal Douglas-fir also reported a negative genetic correlation between DBH and MoE (stiffness). Similarly, Artwood et al. (2002) reported a negative genetic correlation between volume and specific gravity in loblolly pine. Wu et al. (2008) reviewed estimates of genetic parameters including genetic correlations between density and growth in radiata pine and reported an average genetic correlation of −0.48.
Genotype-by-environment interactions
Type B genetic correlations for DBH ranged from 0 to 1, which suggests that a large amount of variation due to families changing rank across sites, but stable families could be identified. Previous analysis on GEI for growth reported in Wu and Matheson (2005) appeared to be confirmed. The two NSW sites (Bil133 and Bil149) appear to be rather different from most other sites. Further examination of GEI for DBH growth revealed that two trials (Bil133 and Bil149) in New South Wales formed a group within which there was no significant GEI. Existence of GEI for DBH has been reported in other radiata pine studies (Matheson and Raymond 1984; Raymond and Hensen 2009). Similarly, large GEI for growth traits has also been reported in other conifers (McKeand et al. 1990; Hodge and Dvorak 1999; Sierra-Lucero 1999; McKeand et al. 2006).
Type B genetic correlations for DEN were typically high. This lack of GEI effect has been commonly reported in wood density studies in fast growing conifers (Jett et al. 1991; Zobel and van Buijtenen 1989; Hodge and Purnell 1993). In general, wood properties for radiata pine exhibit little interaction with the multiple environments. This lack of GEI is ideal for large breeding programmes such as the radiata pine breeding programme which span many differing environments without the need to define multiple breeding or deployment units (e.g. Artwood et al. 2002).
The available diallel data allowed an assessment of GEI at half-sib and full-sib family levels. Depending on trait, full-sib families are expected to display a higher degree of interaction with the environment than half-sib families because both additive and non-additive effects may contribute to GEI (e.g. McKeand et al. 2008). Furthermore, there is less genetic variance within each family (i.e. a lower level of buffering to environmental variations) and more of the genetic variance exists among families (Falconer and Mackay 1996). Interestingly, our results (Table 5) for GEI effects due to half-sib families or full-sib families were relatively the same. This suggests that some full-sib families may be stable for DBH growth across radiata pine plantation estate in Australia. Using a similar analysis, McKeand et al. (2006) investigated stability of loblolly pine full-sib families across different sites and reported lack of GEI for both GCA (r BGCA = 0.77) and SCA (r BSCA = 0.69) effects for height.
It is interesting to note that PT5455 in South Australia had relatively high density and faster growth which is a rather unusual combination. Perhaps this was a result of a few families reacting more than others. However, the type B genetic correlations for DEN among sites suggested little GEI, but the standard errors of the estimates were high. These results confirm that wood quality traits are known to be stable across a range of sites (Zobel and van Buijtenen 1989). Burdon et al. (1992b) also observed minimal family-by-site interaction for wood density in New Zealand. However, there is always need to ascertain whether any significant GEI has biological importance or not, since most measures of GEI are population statistics. Even when GEI is low in a population, there can be individual families that display unpredictable reactions to specific sites.
Selection-index-by-environment interaction
Generally, there was evidence of GEI for the composite index values under a range of economic weights (Table 5). For example, type B additive genetic correlation (r BADD) estimates for scenarios with 1% and 7.5% relative economic weights on DEN (Table 5) were more similar to the estimate obtained for DBH (r BADD = 0.51). This suggested that GEI for the composite index with low weight on DEN is more influenced by DBH. However, as the relative economic weight on DEN increased to 15%, the type B additive genetic correlation estimate increased to r BADD = 0.68 and was more similar to that of DEN as a single trait. Similarly, type B genetic correlation for full-sibs with low and intermediate weight on DEN were high r BFS = 0.67 and r BFS = 0.64, respectively, which was similar to that of DBH. These results suggest that DBH influenced GEI for the composite index at low weight on DEN, but as its economic weight on DEN increased, the influence of DEN also increased.
Implications for radiata pine breeding and deployment in Australia
Different GEI patterns among DBH and DEN are a challenge for tree breeders as well as the observed negative genetic correlation between DBH and DEN. Examination of GEI showed that there are regional patterns for GEI in Australia which would have to be taken into account in order to optimise deployment of improved germplasm. These results give strong support to regionalization of radiata pine breeding into two main regions—the high-elevation NSW and all-non-NSW sites of Victoria, South Australia and Western Australia. Similar conclusions were reported by Wu and Matheson (2005) for DBH when the AWD trials were around 11 years. However, mere significance of GEI is not sufficient evidence upon which to subdivide a breeding programme. It is possible that the advantages of a larger population within which to carry out selection will outweigh some of the effects of interactions so that a single programme would be more efficient.
The implications for deployment of both half-sib and full-sib families are evident. Full-sib families will be just as stable as half-sib families for DBH growth within defined geographic regions. With a mixed mating structure being used to generate full- and half-sib families (White et al. 1999) for operational deployment, forest managers should be able to plant well tested crosses with confidence in their performance across the radiata pine plantation estate.
Although the results gave strong support to a relatively homogenous genetic behaviour of DEN across radiata pine plantation estate, DBH appeared to be influencing GEI for the composite trait under a range of economic weights. GEI was found for both DBH and the selection index across radiata pine plantation estate in Australia. However, as the economic weight on DEN increased, the influence of DEN also increased and GEI decreased. However, index weights vary not only with economic weights but also with changes in variances and covariances, and a selection index is a dynamic entity that may differ across populations and breeding programmes. Selection index is an economic construct for a particular production system, rather than a biological trait, and genetic control of the selection index is of more practical interest to breeders.
Long-term breeding strategies would also require an understanding of how index selection affects genetic correlations, particularly for adverse ones such as DEN and DBH growth. Restricted selection index that restricts changes in one trait while maximising the response in another trait may be a viable option for genetic improvement of two negatively correlated traits. However, in the long-term, simultaneously purging of the adverse genetic correlation (by selecting “correlation breakers”) and optimising index selection may be the best selection strategy in multiple-trait selection breeding programmes with adverse genetic correlations (e.g. Gapare et al. 2009).
Conclusions
-
1
Better growth rates in radiata pine at 20/21 years were observed in Victoria, South Australia and Western Australia than in high elevation sites of New South Wales. Similar conclusions were reported by Wu and Matheson (2005) for DBH when the AWD trials were around 11 years.
-
2
Additive effects were generally significant for DBH growth and DEN, but SCA was insignificant for both traits.
-
3
We were unable to detect any significant additive variance for acoustic time-of-flight in five of the eight trials assessed. We conclude that acoustic tools may not be ideal for assessing mature trees (20/21 years).
-
4
As expected, there was a general tendency towards a negative genetic correlation between DBH and DEN at all sites.
-
5
Type B genetic correlation for additive effects for DBH was moderately low, suggesting evidence of GEI, whereas type B genetic correlation for full-sib families was higher indicating, that stable full-sib families exist.
-
6
Type B genetic correlations for DEN were high, indicating a lack of GEI for both additive and non-additive genetic effects for DEN.
-
7
DBH influenced GEI for the composite index at low weight on DEN, but as its economic weight on DEN increased, the influence of DEN also increased and so did the type B genetic correlation for the index value.
References
Apiolaza LA, Garrick DJ (2000) Breeding objectives for three silvicultural regimes of radiata pine. Can J For Res 31:654–662
Artwood RA, White TL, Huber DA (2002) Genetic parameters and gains for growth and wood properties in Florida source loblolly pine in the southeastern United States. Can J For Res 32:1025–1038
Auty D, Achim A (2008) The relationship between standing tree acoustic assessment and timber quality in Scots pine and the practical implications for assessing timber quality from naturally regenerated stands. Forestry 81:475–487
Baltunis BS, Huber DA, White TL, Goldfarb B, Stelzer HE (2007a) Genetic gain from selection for rooting ability and early growth in vegetatively propagated clones of loblolly pine. Tree Genet and Genom 3:227–238
Baltunis BS, Wu HX, Powell BM (2007b) Inheritance of density, microfibril angle, and modulus of elasticity in juvenile wood of Pinus radiata. Can J For Res 37:2164–2174
Burdon RD (1977) Genetic correlation as a concept for studying genotype-environment interaction in forest tree breeding. Silvae Genet 26:168–175
Burdon RD, Bannister MH, Low CB (1992a) Genetic survey of Pinus radiata. 2. Population comparisons for growth rate, disease resistance and morphology. N Z J For Sci 22:138–159
Burdon RD, Bannister MH, Low CB (1992b) Genetic survey of Pinus radiata. 5. Between trait and age-age genetic correlations for growth rate, morphology, and disease resistance. N Z J For Sci 22:211–227
Burdon RD, Kibblewhite RP, Walker JCF, Megraw RA, Evans R, Cown DJ (2004) Juvenile Versus Mature Wood: A New Concept, Orthogonal to Corewood Versus Outerwood, with Special Reference to Pinus radiata and P. taeda. For Sci 50:399–415
Burdon RD, Carson MJ, Shelbourne CJA (2008) Achievement in forest tree improvement in Australia and New Zealand 10. Pinus radiata in New Zealand. Australian Forestry 71:263–279
Carter PS, Chauhan S, Walker L (2005) Sorting logs and lumber for stiffness using Director HM200. Wood and Fiber Science 38:49–54
Comstock RE, Moll RH (1963) Genotype-environment interactions. In: Hanson RE, Robinson HF (eds) Statistical genetics and plant breeding, 982. NAS–NRC Publ., Washington D.C., pp 53–93
Costa e Silva J, Borralho NMG, Wellendorf H (2000) Genetic parameter estimates for diameter growth, pilodyn penetration and spiral grain in Picea abies (L.) Karst. Silvae Genet 49:29–36
Costa e Silva J, Potts BM, Dutkowski GW (2006) Genotype by environment interaction for growth of Eucalyptus globulus in Australia. Tree Genet and Genom 2:61–75
Cown DJ, McConchie DL (1983) Radiata pine wood properties survey (1977–82). New Zealand Forest Service, FRI Bull. No. 50. The Government Printer, Wellington, p 50
Cown DJ, Young GD, Burdon RD (1992) Variation in wood characteristics of 20-year-old half-sib families of Pinus radiata. N Z J For Sci 22:63–76
Dickerson GE (1962) Implications of genetic-environment interactions in animal breeding. Animal Production 4:47–64
Dieters MJ, White TL, Hodge GR (1995) Genetic parameter estimates for volume from full-sib tests of slash pine (Pinus elliottii). Can J For Res 25:1397–1408
Dungey HS, Matheson AC, Kain D, Evans R (2006) Genetics of wood stiffness and its component traits in Pinus radiata. Can J For Res 36:1165–1178
Falconer DS, Mackay TFC (1996) Introduction to quantitative genetics, 4th edn. Longman Scientific and Technical, London 464 pp
Gapare WJ, Hodge GR, Dvorak DS (2001) Genetic parameters and provenance variation of Pinus maximinoi in Brazil, Colombia and South Africa. Forest Genetics 8:159–170
Gapare WJ, Wu HX, Abarquez A (2006) Genetic control in the time of transition from juvenile wood to mature wood in Pinus radiata D. Don. Ann For Sci 63:871–878
Gapare WJ, Hathorn AD, Kain D, Matheson AC, Wu HX (2007) Inheritance of spiral grain in the juvenile core of Pinus radiata D. Don. Can J For Res 37:116–127
Gapare WJ, Ivković M, Powell MB, McRae TA, Wu HX (2008) Genetics of shrinkage in juvenile trees of Pinus radiata D. Don from two test sites in Australia. Silvae Genet 57:145–151
Gapare WJ, Baltunis BS, Ivković M, Wu HX (2009) Genetic correlations among juvenile wood quality and growth traits and implications for selection strategy in Pinus radiata D. Don. Ann For Sci 66:606
Gilmour AR, Gogel BJ, Cullis BR, Thomson R (2006) ASReml User Guide release 2.0. VSN-International Ltd, Hemel Hempstead
Grabianowski M, Manley B, Walker JCF (2006) Acoustic measurements on standing trees, logs and green lumber. Wood Sci Tech 40:205–216
Greaves BL (1999) The value of tree improvement: a case study in radiata pine grown for structural timber and liner-board. In: Nepveu G (ed) Proceedings of the third workshop IUFRO S5.01.04 “Connection between silviculture and wood quality through modeling approaches and simulation software”, La Londe-Les Maures, France, September 5–12, 1999
Griffin AR (1976) Mating designs for P. radiata breeding programmesx. In: K.R. Sheperd (ed) Proc RWG-1 Fifth meeting of representatives, Canberra, ACT and Tumut, NSW 16–20 August 1976.CSIRO Division of Forest Research, Canberra
Hodge GR, Dvorak WS (1999) Genetic parameters and provenance variation of Pinus tecunumannii in 78 international trials. For Genetics 6:157–180
Hodge GR, White TM (1992) Genetic parameter estimates for growth traits at different ages in slash pine and some implications for breeding. Silvae Genetica 41:252–262
Hodge GR, Purnell RC (1993) Genetic parameter estimates for wood density, transition age, and radial growth in slash pine. Can J For Res 23:1881–1891
Ilic J (2003) Dynamic MOE of 55 species using small wood beams. Holz als Roh- und Werkstoff 61:167–172
Ivković M, Wu HX, McRae TA Powell MB (2006) Developing breeding objective for Pinus Radiata pine structural wood production I: bio economic model and economic weights. Can J For Res 36:2920–2931
Jayawickrama K (2001) Genetic parameter estimates for radiata pine in New Zealand and New South Wales: a synthesis of results. Silvae Genet 50:45–53
Jett JB, McKeand S, Weir RJ (1991) Stability of juvenile wood specific gravity of loblolly pine in diverse geographic areas. Can J For Res 21:1080–1085
Johnson IG, Cotterill IM, Raymond CA, Hensen M (2008) Half a century of radiata tree improvement in NSW. N Z J For 52:7–13
Johnson GR, Gartner B (2006) Genetic variation in basic density and modulus of elasticity of coastal Douglas-fir. Tree Genet and Genom 3:25–33
Kumar S (2004) Genetic parameter estimates for wood stiffness, strength, internal checking and resin bleeding for radiata pine. Can J For Res 34:2601–2610
Lewis NB, Keeves A, Leech JW (1976) Yield Regulation in South Australian Pinus radiata Plantations. Woods and Forests Department of South Australia, Adelaide
Li L, Wu HX (2005) Efficiency of early selection for rotation-aged rowth and wood density traits in Pinus radiata. Can J For Res 35:2019–2029
Lindgren D (1976) Inbreeding and coancestry. Proc IUFRO Joint Meeting of Working parties on Advanced Generation Breeding. Bordeaux 1976. INRA Bordeaux, pp 49-69
Lynch M, Walsh B (1998) Genetics and Analysis of Quantitative Traits. Sinauer Associates Inc., Sunderland
Matheson AC (1976) Report on Joint meeting of IUFRO Working Parties on advanced generation breeding, Bordeaux, France June 14–18 1976. In: K.R. Shepherd (ed)Proc RWG-1 Fifth meeting of representatives, Canberra, ACT and Tumut, NSW 16-20 August 1976.CSIRO Division of Forest Research, Canberra, pp 82–86
Matheson AC, Raymond CA (1984) The impact of genotype by environment interactions of Australian Pinus radiata breeding programs. Aust For Res 14:11–25
Matheson AC, Raymond CE (1986) A review of provenance x environment interaction: its practical importance and use with particular reference to the tropics. Commonwealth For Rev 65:283–302
Matheson AC, Eldridge KG, Brown AG, Spencer DJ (1986) Wood volume gains from first-generation radiata pine seed orchards. CSIRO Division of Forest Research Report Number 4
Matheson AC, Spencer DJ, Magnussen D (1994) Optimum age for selection in Pinus radiata basal area under bark for age: age correlations. Silvae Genet 43:352–357
Matheson AC, Spencer DJ, Nyakuengama GJ, Yang JL, Evans R (1997) Breeding for wood properties in radiata pine. In: Burdon RD, Moore JM (ed) 1997: “IUFRO ’97 Genetics of Radiata Pine”. Proceedings of NZFRI-IUFRO Conference 1–4 December and Workshop 5 December, Rotorua, New Zealand. FRI Bulletin No 203. pp 169–179
Matheson AC, Dickson RL, Spencer DJ, Joe B, Ilic J (2002) Acoustic segregation of Pinus radiata logs according to stiffness. Ann For Sci 59:471–477
Matheson AC, Gapare W, Ilic J, Wu HX (2008) Inheritance and genetic gain in wood stiffness in radiata pine assessed acoustically in young standing trees. Silvae Genet 57:56–64
McKeand S, Li B, Hatcher AV, Weir RJ (1990) Stability parameter estimates for stem volume for loblolly pine families growing in different regions in the southeastern USA. For Sci 36:10–17
McKeand S, Jokela E, Huber DA, Byram T, Allen L, Li B, Mullin T (2006) Performance of improved genotypes of loblolly pine across different soils, climates and silvicultural input. For Ecol Manag 227:178–184
McKeand S, Li B, Grissom JE, Isik F, Jayawickrama KJS (2008) Genetic parameter estimates for growth traits from diallel tests of Loblolly pine throughout the Southeastern United States. Silvae Genet 57:101–109
Namkoong G (1984) The influence of composite traits on genotype by environment relations. Theor Appl Genet 70:315–317
Namkoong G, Johnson JA (1987) Influence of value function on genotype by environment relations. Silvae Genet 36:92–94
Nyakuengama JG, Matheson AC, Evans R, Spencer DJ, Vinden P (2000) Genetics of heartwood formation and moisture status in Pinus radiata. Appita 53:30–35
Raymond CA, Hensen M (2009) Genotype by environment interaction in Pinus radiata within NSW. In: Proceedings, Talking Care of Business—Australian Forest Genetics Conference April 20–22, 2009, Perth, Western Australia
Shelbourne CJA (1972) Genotype-environment interaction: its study and its implications in forest tree improvement. IUFRO Genet.–SABRAO Joint Sympo., Tokyo, October 1972
Shelbourne CJA (1997) Genetics of adding value to the end-products of radiata pine. In: Burdon RD, Moore JM (eds) IUFRO ‘97 genetics of radiata pine, PROC. of NZ FRI/IUFRO conference 1–4 Dec. and workshop 5 Dec., Rotorua, New Zealand. New Zealand Forest Research Institute FRI Bull. No. 203, pp 129–141
Sierra-Lucero V (1999). Genetic parameter estimates of loblolly pine grown in the lower coastal plain of the southeastern United States. Masters Thesis, School of Forest Resources and Conservation, University of Florida, Gainesville
Sokal RR, Rohlf FJ (1995) Biometry, 3rd edn. W.H. Freeman, New York 887
Sykes R, Li B, Isik F, Kadla J, Chang H (2006) Genetic variation and genotype by environment interactions of juvenile wood chemical properties in Pinus taeda L. Ann For Sci 63:897–904
Walker JFC, Nakada R (1999) Understanding corewood in some conifers: a selective review on stiffness and acoustics. Inter For Rev 1:251–259
Wang X, Carter P, Ross RJ (2007) Acoustic evaluation of wood quality in standing trees. Part 1: acoustic wave behaviour. Wood and Fiber Sci 39:28–38
White TL, Hodge GR, Powell GL (1993) Advanced-generation breeding strategy for slash pine in the southeastern United States. Silvae Genet 42:6
White TL, Matheson AC, Cotterill PP, Johnson RG, Rout AF, Boomsma DB (1999) A nucleus breeding plan for radiata pine in Australia. Silvae Genet 48:122–133
Wu HX, Matheson, AC (2002) Quantitative genetics of growth and form traits in radiata pine. CSIRO Forestry and Forest Products Technical Report No. 138 and Southern Tree Breeding Association Technical Report TR2002-01. p 133
Wu HX, Matheson AC (2004) General and specific combining ability from partial diallels of radiata pine: implications for utility of SCA in breeding and deployment populations. Theo App Genet 108:1503–1512
Wu HX, Matheson AC (2005) Genotype by environment interaction in an Australia-wide radiata pine diallel mating experiment: implications for regionalized breeding. For Sci 51:29–40
Wu HX, Matheson AC, Abarquez A (2002) Inbreeding in Pinus radiata. IV: the effect of inbreeding on wood density. Ann For Sci 59:557–562
Wu HX, Yang JL, McRae TA, Powell MB (2004) Breeding for wood quality and profits with radiata pine, 1: MOE prediction and genetic correlation between early growth, density, microfibril angle and rotation-age MOE. In: Proceedings of Wood Quality 2004: Practical tools & new technologies to improve segregation of logs and lumber for processing, Albury
Wu HX, Eldridge KG, Matheson AC, Powell MB, McRae TA (2007) Achievement in forest tree improvement in Australia and New Zealand 8. Successful introduction and breeding of radiata pine to Australia. Australian Forestry 70:215–225
Wu HX, Ivković M, Gapare WJ, Matheson AC, Baltunis BS, Powell MB, McRae TA (2008) Breeding for wood quality and profit in Pinus radiata: a review of genetic parameter estimates and implications for breeding and deployment. N Z J For Sci 38:56–87
Yamada Y (1962) Genotype by environment interaction and genetic correlation of the same trait under different environments. Jap J Genetics 37:498–509
Zobel B (1984) The changing quality of the world wood supply. Wood Sci and Technol 18:1–17
Zobel BJ, van Buijtenen JP (1989) Wood Variation: it's Causes and Control. Springer-Verlag, Berlin, p 363
Acknowledgements
In addition to the acknowledgements given in Wu and Matheson (2005) for the crossing, planting and maintenance of the Australia-Wide diallels, we wish to thank FWPA, STBA, Forestry NSW, Forest Products Commission, WA and CSIRO for funding the project. Special thanks to David Spencer for the excellent field work planning and coordination. We also thank Drs. Chris Harwood and Gregory Rebetzke for helpful comments on an earlier draft of this manuscript and external reviewers for their comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Davis
Rights and permissions
About this article
Cite this article
Gapare, W.J., Ivković, M., Baltunis, B.S. et al. Genetic stability of wood density and diameter in Pinus radiata D. Don plantation estate across Australia. Tree Genetics & Genomes 6, 113–125 (2010). https://doi.org/10.1007/s11295-009-0233-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11295-009-0233-x