Abstract
Salinization is one of the main types causing land desertification in arid and semi-arid regions. Little is known about the impacts of salinization on the distribution and diversity of plant species, especially in semiarid inland saline habitats. We established a total of 40 sampling plots to determine plant community (floristic composition, species abundance, cover, frequency, and aboveground biomass) and soil characteristics (moisture, pH, electrical conductivity (EC), and the contents of Na+, K+, Ca2+, Mg2+, Cl−, SO42− and HCO3−). The TWINSPAN method was used to distinguish plant communities, by which three plant communities (I, II, III) were identified, namely Artemisia scoparia + Agropyron cristatum + Sophora alopecuroides (I), Kalidium gracile + Atriplex centralasiatica (II), and Salicornia europaea + Suaeda salsa (III). From I to III, the indices reflecting species diversity all decreased except for Pielou’s index of evenness, while the aboveground biomass and cover increased; the characteristic indices of soil moisture, EC, the contents of Na+, K+, Ca2+, Mg2+, Cl−, SO42− and total dissolved salts (TDS) significantly increased, while the Ca2+/Na+, K+/Na+ ratios and HCO3− content significantly decreased. Canonical correspondence analysis (CCA) showed that the most important environmental factors influencing species distribution were: soil moisture, SO42−, K+/Na+ and pH. Stepwise multiple-regression analysis indicated that species richness was correlated mainly with TDS and moisture. These results also suggested that vegetation can be used as a proxy indicating soil salt conditions, and their relationships can further provide important information for the improvement of salt-affected soil’s management and salt-tolerant species utilization in environmental restoration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Salinity affects more than 800 million hectares of land worldwide (FAO 2008), which accounts for approximately 10% of the world’s total land area (Schofield and Kirkby 2003). Soil salinity is continuously increasing in many parts of the world as a result of irrigation, high evaporation-to-rainfall ratios, and rising sea levels associated with global warming (Zhao et al. 2011). Excessive salinity in soil inhibits plant’s growth, reduces agricultural productivity, decreases species diversity and increases soil erosion (Szabolcs 1989; Parida and Das 2005). Many regions of the world are suffering from increasingly serious problem of soil salinization. Therefore, studies related to salinization control are particularly urgent and important to maintain sustainable development of ecological environment.
In arid and semi-arid inland regions, climate is one of the most important factors influencing salinity (Thomas and Middleton 1993). Various climatic factors, such as low rainfall, high temperature, intensive evaporation, are essential prerequisite for the formation of saline-alkali soils (Kasera and Mohammed 2010). Because of scarce and unevenly distributed precipitation during the growing season, soil salinity concentrations in these habitats, tend to be more variable than coastal marshes (Ungar 1979; Breckle and Wucherer 2012). Along the salt gradient, plant species presented the zonal distribution pattern, and the development of which, to a certain extent, is determined by the salt tolerance of dominant species in each zone (Breckle 1986; Bertness et al. 1992). For example, in the Central Asian saline deserts, enhalophytes with succulent stem and leaf play the major role in the zones close to the saline lakes or basins, where salinity is high; recretohlophytes with salt gland or bladders predominate in the middle part of the transect, where salinity is more variable; pseudohalophytes dominate on the less saline side, and finally on almost salt-free zones nonhalophytes dominate (Breckle 1986). In addition, with the increase of soil salinity, the species richness exhibited a downward trend (García et al. 1993; Greenberg et al. 2006). Hence, zonal distribution of plant community is a common feature in inland salt marsh environments (Rogel 2000; Carter and Ungar 2004; Piernik 2006).
Topography, precipitation and edaphic factors are all the main contributors to distribution and diversity of plant community (Hegazy et al. 2008). Edaphic properties are of primary importance factor that influence plant distribution and community structure (Hoveizeh 1997). These edaphic factors which affect community structure and distribution differ greatly across different ecosystems. In saline ecosystems, especially coastal salt marshes, soil salinity has been reported to be the principal factor influencing vegetation pattern (Chapman 1960; Adam 1990; Ortiz et al. 1995; Wanner 2009). In arid and semiarid desertified lands, water is the primary limiting resource for plant growth, especially during seed germination and seedling establishment stage, and poses a considerable influence on species composition, distribution, and diversity (James et al. 2005; Naz et al. 2010). Therefore, soil moisture and salinity play key roles in vegetation distribution in arid and semiarid saline lands.
Soil salinity implies the presence of chlorides, sulfates, nitrates, and bicarbonates of sodium, calcium, magnesium, and potassium in soil (Bui 2013). What salt ionic content in soil does determine which species can successfully compete in the corresponding zones (Adam 1990; Jafari et al. 2004). Aeluropus lagopoides, a highly saline tolerant grass, the distributional pattern of it was strongly influenced by soil Na+ and to some extent soil Ca2+ rather than soli salinity (Naz et al. 2010). Soil Cl− was the major determinant of the distributional of Haloxylon salicornicum in the Cholistan desert (Naz et al. 2010). Moreover, the ratios of cation (K+, Na+, Ca2+ and Mg2+) in soils are also critical to plant zonation in saline environments (Rogel et al. 2000). Piernik et al. (2005) found that the occurrence of the Elymus repens community was connected with Ca2+/Na+ ratio in the soil in the Kujawy region. Breckle and Wucherer (2012) found that Salicornia europaea and Suaeda maritime, belong to the family Chenopodiaceae, can be termed sodiophilic and their rhizosphere soil exhibit rather low K+/Na+ ratios; while grasses Puccinellia distans, Stipagrostis pennata and Eremosparton aphyllum selectively accumulate potassium and prefer to grow in the soils with high K+/Na+ ratio in salt desert area. In addition, soil salt ions also play a critical role in regulating species richness; for example, Ca2+ and Mg2+ are favorable for increasing species richness in a heathland (Roem et al. 2002). Salt constituent and concentration differ from one habit to another, and each habit supports one or more distinct plant species with their own characteristic (Kasera and Mohammed 2010). Thus, Investigations on salt ion composition and contents seem to be particularly important for the understanding of mechanism influencing species distribution and diversity.
In inland saline ecosystems, the importance of edaphic factors in structuring plant communities has long been noted in a number of studies throughout the world (Ungar 1974; Wilkoń-Michalska 1985; Burchill and Kenkel 1991; Cantero et al. 1998; Rogel et al. 2000; Piernik 2005; González-Alcaraz et al. 2014), but the study in China is still limited (Pan et al. 1998; Du et al. 2007; Yang et al. 2013). Saline lands widely distributed in semiarid inland areas with scant rainfall, strong wind, high temperature and evaporation, in northwestern China (Zhao et al. 2011; Minggagud and Yang 2013). These environment host salt and drought-resistant species and hence are important for biodiversity conservation (Rogel et al. 2000). However, salinization problem in the areas became serious due to unique geographical and climatic conditions and human activities, resulting in the loss in plant biomass, vegetation degradation and accelerating the process of desertification (Thomas and Middleton 1993; Xiong et al. 1996; Kasera and Mohammed 2010). Thus, studies involving soil–plant relationships are needed to guide vegetation restoration and preservation of these habitats. In this study, taking a typical semi-arid inland saline habitat in northwestern China for example, we aim to (1) identify and describe the major plant communities; (2) examine differences in plant community characteristics (cover, density, aboveground biomass and diversity), and soil properties (moisture and salinity) among plant community types; and (3) determine key soil properties influencing species distribution and richness. The results will contribute to our knowledge of species distribution patterns and vegetation-soil relationship around saline marsh in semiarid inland areas of China.
Methods
Site description
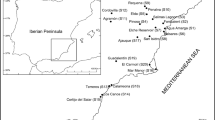
The study site was located at the southern tip of the Mu Us Sand Land (37°41′9″N, 107°32′17″E, 1259 m a.s.l.) in Yanchangbu town, Dingbian County, Shaanxi Province, northwestern China. The site lies in a transitional zone between the Loess Plateau with semi-arid climate and the Ordos Plateau with arid climate, being characterized by a temperate continental climate. The mean annual temperature is 7.9 °C. The average annual precipitation is 316 mm, falling primarily in spring and autumn, and the average amount of evaporation totals 2490 mm per year (Liu et al. 2014).
Topography in study area is dominated by sand dunes and salt marsh, and the dunes are sand source of local blowing sand weather in spring and autumn. Aeolian sandy soil and solonchak soil are the main soil types. In solonchak soil, water-soluble sulfate is the main component (Qiao 2005). The natural vegetation is mainly occupied by species of grasslands, shrubs, meadows, and desert steppe. In arid grasslands, the chief species are Stipa grandis, Agropyron cristatum, Artemisia frigida, and Artemisia scoparia; those in desert steppe are Caragana tibetica, Oxytropis aciphylla, Nitraria tangutorum, and Kalidium foliatum.
Experimental design
Field investigation and sampling were carried out in September 2015. The study site exhibited a difference in elevation of 15 m. The eleven 400 m long line transects, according to landforms and plants distribution, are radially set from the topographically lower part (border of salt marsh) to the upland, and each two adjacent transects are approximately 100 m apart at the lower part. The forty investigated plots are distributed along eleven line transects, with four plots on each of seven lines and three plots on each of four ones (Fig. 1). Plots were 20 × 20 m, and spaced at least 100 m apart. In each plot, five 2 × 2 m quadrats were established (one on the center and four on the four corners of square plots), totaling 200 quadrats. To minimize the ‘edge effect’, plots were located at least 5 m away from the edge of the salt marsh.
Floristic surveys, including vegetation composition, cover, species height, individual density, frequency, and aboveground biomass, were conducted in each quadrat. Data for five quadrats were averaged to describe each plot. For vegetation cover, it was visually estimated by experienced observers. The densities of individuals are obtained directly from counting the number of clusters for bunchgrasses and the individual number for species with distinct stems (Liu et al. 2007). Lastly, the aboveground parts of all species in each quadrat were harvested and oven-dried at 65 °C for 48 h to determine the aboveground biomass. We used the “Chinese Halophytes” (Zhao et al. 1999), and the Flora Republicae Popularis Sinicae from the Flora of China (www.efloras.org) to classify the species. The functional groups of species were based on the definitions of Bai et al. (2004).
Soil samples were collected with a soil corer from the center of each quadrat at a soil depth of 0–20 cm in parallel with the vegetation survey. Each sample was air-dried and crushed. Subsequently, we removed coarse fragments and passed the soils through a 1-mm sieve. Soil samples were analyzed for pH, electrical conductivity (EC) and soluble ion content (Na+, K+, Ca2+, Mg2+, Cl−, SO42− and HCO3−) with soluble extracts (1:5 soil to distilled water). There is no carbonate ion content detected in our study. The pH was obtained with a calibrated pH meter (PHS-4, Jiangsu Manufacturer of Electrical Analysis Instruments, Jiangying, China), EC with a portable conductivity meter (1481-61, Cole-Parmer Instrument Co., IL, USA), Na+ and K+ with an atomic absorption spectrophotometer (2655-00, Cole-Parmer Instrument Co., IL, USA), Ca2+ and Mg2+ by titration with EDTA, Cl− with 0.1 N AgNO3 using K2CrO4 indicator, SO42− by the nephelometric method, HCO3− with 0.01 N H2SO4 using phenolphthalein and bromophenol blue indicator (Bao 2000). The content of anions and cations are given in percentages (g 100−1 g dry soil). Total dissolved salts (TDS) were determined by summing up all cations and anions (Li et al. 2008).
Soil samples for moisture determination were collected with a soil corer from the center of each quadrat at depths of every 0.2 m from 0 to 1.0 m. Samples were dried at 105 °C for 48 h prior to analysis (Kirk et al. 2005).
Data analysis
The importance value (IV, %) of each semi-shrub and herb in a quadrat was calculated with the following formula (Zhang et al. 2005):
where RD is the relative density (the ratio of the number of a species to the number of all species within a quadrat, %); RF is the relative frequency (the ratio of the frequency of a species to the frequency of all species, %), and RC is the relative cover (the ratio of the cover of a species to the cover of all species, %) (Jiang et al. 2007).
We selected species richness (S), the Shannon–Wiener index (H), the Simpson index (D), and Pielou’s index of evenness (J sw ) to describe community diversity at plot level. They were calculated using the following equations:
where P i is the relative importance value of species i and S is the total number of species in the ith plot.
The relationships between species IVs and environmental factors were analyzed with Canonical correspondence analysis (CCA) after results of Detrended correspondence analysis (DCA) indicated a strong unimodal structure in the vegetation data (Jongman et al. 1995). The relative importance of each environmental variable in vegetation differentiation was assessed by the forward selection of the explanatory variables and the Monte Carlo Permutation Test (499 unrestrained permutations). CCA was performed using CANOCO version 4.5, and ordination plots were produced with CanoDraw (Ter Braak and Smilauer 2002).
Two-way indicator species analysis (TWINSPAN) (Hill 1979a, b) was applied to obtain the classification of plant communities in the study area. After performing One-way analysis of variance (ANOVA) analysis, the least significant difference (LSD) test for multiple comparisons were used to examine the differences in community characteristics, diversity indices, and environmental variables (soil moisture, EC, pH, the contents of Na+, K+, Ca2+, Mg2+, Cl−, SO42−, HCO3−, TDS, K+/Na+ and Ca2+/Na+ ratio) in different plant communities. Prior to ANOVA, the homogeneity of variances was tested. If necessary, data for aboveground biomass, and environmental variables (with the exception of pH and HCO3−) were transformed (log10). The cover was arcsine transformed. The D and J sw were square-root transformed. Differences obtained at the level of P < 0.05 were considered to be significant.
The relationships between species richness and environmental factors were analyzed by Stepwise Multiple Regression. In order to test the impact of key environmental variable on species richness, simple linear regression was performed. Species richness (number of species per sample plot) was considered as dependent variable and environmental factors as predictor variables in the regression analysis. Environmental variables were log-transformed (with the exception of pH and HCO3−) prior to analysis. In addition, variance inflation factor (VIF) was used to test the multicollinearity between the predictor variables (Neter et al. 1996). The selection of predictors was terminated when remaining predictors failed to improve the model at 0.05 level. All statistical analyses were conducted using SPSS 18.0 software package (SPSS Inc., Chicago, IL, USA).
Results
Species composition and classification
Forty-seven plant species have been identified in the study area, including 87.2% herbs (23.4% annual, 4.3% annual-biennial, and 59.6% perennial) and 12.7% shrubs (8.5% semi-shrub). These 47 species belong to 17 different families, with 66.0% of the species in the Gramineae, Chenopodiaceae, Compositae, and Leguminosae families.
TWINSPAN analysis resulted in three major plant community types (Fig. 2): type I, mainly Artemisia scoparia + Agropyron cristatum + Sophora alopecuroides, commonly accompanied by most annual or perennial herbs, such as Suaeda glauca, Cynanchum hancockianum, Thermopsis lanceolata, Limonium aureum, and Salsola collina; type II, mainly Kalidium gracile + Atriplex centralasiatica, and the companion species were Reaumuria songarica, Nitraria tangutorum and Suaeda glauca; type III, mainly Salicornia europaea + Suaeda salsa, and the companion species were Phragmites australis and semi-shrub Kalidium cuspidatum.
Dendrogram generated by TWINSPAN of plant communities in inland saline habitats of northwestern China. Forty plots were classified into three plant community types: I: Artemisia scoparia + Agropyron cristatum + Sophora alopecuroides; II: Kalidium gracile + Atriplex centralasiatica and III: Salicornia europaea + Suaeda salsa
Community characteristics and soil properties in each plant community
Table 1 showed the statistical results of community characteristic parameters in the three plant communities. Among the three major plant community types, plant cover and aboveground biomass in type I was significantly lower than that in types II and III, while plant density in type I was significantly higher than that in the other two (P < 0.05). Plant cover, density, and aboveground biomass did not exhibit significant differences between types II and III (P > 0.05) though they were a little higher in type III than in type II. Species diversity indices other than Pielou’s index of evenness significantly decreased from types I to III (P < 0.05), that is, type I had the largest values of Species richness (S), the Shannon–Wiener (H) and the Simpson’s index of diversity (D).
Soil moisture showed a significantly increasing trend at each layer from I to III (P < 0.001). With soil depth increasing, soil moisture in types I and II gradually increased, but it exhibited little change in type III (Fig. 3).
Soil salinity parameters, as EC, pH, salt ion contents, and ionic ratios, differed significantly among the three communities (Fig. 4). From I to III, EC (0.3–19.0 dS m−1), TDS (0.06–3.12%), and the contents of Na+, Cl− and Mg2+ significantly increased, whereas the K+/Na+ ratio (0.008–0.653) remarkably decreased (P < 0.05). In type I, the contents of K+, Ca2+, and SO42− were distinctly lower, while the pH value and the HCO3− content were higher than those in the other two types (P < 0.05), but these parameters did not exhibit significant differences between type II and III (P > 0.05). In type II, the contents of Na+, Cl− and TDS were distinctly lower, while the K+/Na+ and Ca2+/Na+ ratios were higher than those in type III (P < 0.05). The Ca2+/Na+ ratio was remarkably higher in types I and II than that in type III.
Box plots for the soil EC (a), pH (b), Na+ (c), K+ (d), Ca2+(e), Mg2+ (f), Cl− (g), SO42− (h), HCO3− (i), TDS (j), K+/Na+ (k) and Ca2+/Na+ (l). “TDS” means total dissolved salts. Solid line within the boxes indicates the median value. Hollow squares within the boxes indicate the mean value. The box includes data within the 25th and 75th percentiles; whisker lines refer to the 5th and 95th percentiles. Asterisk indicates outliers. Different lower-case letters represent significant differences across different plant community types (P < 0.05)
Relationships between soil properties and species distribution
CCA analysis revealed that the selected environmental variables all are important factors for species composition and distribution (Table 2). Because many factors were closely correlated with each other, conditional effects were analyzed in the study. Among the factors, soil moisture, SO42−, K+/Na+, and pH were the most important (Table 2); they together accounted for 64.2% of the total variance in species composition (moisture for the highest 30.9%, SO42− for 16.4%, K+/Na+ for 9.1%, and pH for the smallest 7.8%).
In Fig. 5, plots dominated by herbs were clustered at the negative extreme of axis 1, and showed the strongest correlation with pH and K+/Na+; plots dominated by Sa. europaea and Su. salsa were clustered along the positive end of axis 1, and were closely correlated with soil moisture. Along the first CCA ordination axis, Ph. australis and obligatory halophytes Sa. europaea and Su. salsa were found at the wettest sites. Ka. cuspidatum was related to high SO42− content in soil. Ar. scoparia, Ag. cristatum, So. alopecuroides and Cy. hancockianum reached their highest biomass in soils with a relatively high K+/Na+ ratio and pH.
Canonical Correspondence Analysis biplot with significant environmental factors (arrow) and sample plots represented by Arabic numbers (a) and species represented by the first two letters of genus and species name (b). For complete names of species, see Table S1. Rare species with one occurrence are omitted
Relationships between soil properties and species richness
Fig. 6 suggested close correlations of species richness with soil moisture and the contents of TDS. With the increase of soil moisture and TDS, the species richness significantly declined (P < 0.001). The results of Stepwise Regression indicated that species richness was mainly correlated with TDS and moisture (R2 = 0.794, P < 0.001). The models explained 79.4% of the variation in species richness (Table 3).
Discussion
Species with similar bio-ecological traits have similar responses to changes in their habitats (Gitay and Noble 1997). Therefore, species can be grouped into different “response types” on the basis of their response to the environment (Díaz and Cabido 2001). The results of the TWINSPAN classification of the 40 plots in this study showed that the three main community types differed in their responses to soil moisture and salinity.
In type I, a large proportion of the species are low herbs, mainly distributed in the periphery of the salt marsh where the soil moisture and salinity were the low. These dwarf herbs are tolerant of drought and mild saline soil (Du et al. 2007; Zhang et al. 2008; Breckle and Wucherer 2012), and grow well in this site partly because of their reproductive strategies (Brewer et al. 1998). Some species (e.g. Ar. scoparia) produce and disperse large numbers of seeds (Wang 2008), while some other species (e.g. So. alopecuroides and Thermopsis lanceolat) form dense stands, favoured by a strong system of rhizomes that facilitated soil exploration and nutrients absorption (Yu and Dong 1999; González-Alcaraz et al. 2014). In our study, the sites colonized by type I had the lowest soil moisture and salinity, but the highest species diversity. Similarly, the low-salinity and dry sites in a Mediterranean semiarid saline watershed exhibited higher species richness (González-Alcaraz et al. 2014). This was because less saline and drier sites represented less stressful environments for vegetation; favourable conditions are related to a greater number of species (García et al. 1993).
In type II, the dominant species are Ka. gracile and At. centralasiatica, adapting to moderate moisture and salinity (Du et al. 2007; Zhao et al. 2011). These two species together with companion species (such as Re. songarica, Ni. tangutorum and Su. glauca) all evolved special salt-resistant structures of succulent stem or leaf and salt gland for salt dilution or excretion, adapting the salinization environment (Zhao et al. 2002; Breckle and Wucherer 2012). Besides, aboveground biomass in type II and III was higher than that in type I (Table 1). These results were in agreement with other work indicating that aboveground biomass was significantly higher in the high and medium-salinity soils than in the non or low-salinity soils (Onkware 2000).
The type III dominated by Sa. europaea and Su. salsa is adjacent to the salt marsh. This habitat is characterized by abundant water and high salinity. These two annual succulent euhalophyte species, belong to the family Chenopodiaceae, are widely distributed and are to inhabit coastal and inland salt marshes (Bertness et al. 1992; Lv et al. 2012; Song and Wang 2015). Sa. europaea and Su. Salsa all can thrive with high concentration of NaCl mainly because of Na+ compartmentalization (Zhao et al. 2003; Ushakova et al. 2005; Lv et al. 2012). Moreover, they developed another adaptive strategy—seed heteromorphism, that is, they could also produce large quantities of seeds that are variable in size, enabling plants to tolerate this harsh and unpredictable environment (Rand 2000; Li et al. 2005). We also found type III had the highest cover and aboveground biomass among all plant communities. Dominant Species Sa. europaea and Su. Salsa grew better and gave higher yield in soils of lower marsh or intertidal zone than in inland saline soils of arid zones (Cooper 1982; Li et al. 2012). We attributed the higher productivity, which is associated with light, and water and nutrient availability, to a narrower ecological and evolutionary filter (e.g. salinity and water-logging) (García et al. 1993).
At local scale, topographic and edaphic conditions affect the colonization and persistence of plant species, and determine local plant community composition and distribution (Naz et al. 2010; Sciandrello et al. 2014). The critical factors influencing species composition are commonly not consistent across a region (Duan et al. 2010). Some studies concluded that, in arid salinized regions, soil moisture and salinity gradients were determined to be the decisive factors for vegetation pattern (Pan et al. 1998; Naz et al. 2010; Eliáš et al. 2013). In our study, the result of CCA analysis demonstrated that soil moisture was the principal factor influencing species distribution. This may be due to the fact that water is the limiting ecological factor controlling species distribution in arid and semiarid regions (Kasera and Mohammed 2010; Zhou et al. 2015). Eliáš et al. (2013) also found that the water regime overriding the salinity gradient played an important role in dry zones. In our study, the occurrence of Sa. europaea, Su. salsa and Ph. australis were closely correlation with moisture. Our results are in accordance with those of McGraw and Ungar (1981), who found that seed germination and seedling establishment of Sa. europaea need plentiful moisture in inland saline environment where sporadic precipitation occurs during the growing season, and those of Cui et al. (2008), who found that Su. salsa accompanied with Ph. australis always appear in the sites where water table depth is shallow.
Hydrogen ion concentration (pH) has been suggested as an important determinant of halophyte species distribution in a saline meadow near utah lake (Brotherson 1987) and inland saline and alkaline soils in Central Otago (Allen et al. 1997). Piernik et al. (2003, 2005) demonstrated that Cl−, K+, SO42− contents, EC, and pH were significantly important for halophilous species distribution in the inland habitats of central Poland. In our study, we also found some significant relationships between halophytic species distribution and SO42− content and pH, partially confirming the results of previous research. Research by Rogel et al. (2000) indicated that, in inland salt marshes in Spain, the cationic ratios K+/Na+ and Ca2+/Na+ explained the majority of the soil-vegetation relationships. Correspondingly, we also found some close relationship of halophytic species distribution pattern with the K+/Na+ ratio, particularly herbaceous plant species in typeI(e.g. Ar. scoparia and So. alopecuroides) only appeared in the sites with high K+/Na+ ratio. The highest K+/Na+ ratio were obtained in the steppe grass zones dominated by L. spartum in mediterranean salt marshes (Rogel et al. 2000). In addition, adequate potassium application enhanced salt tolerance of So. Alopecuroides, and contributed to its better growth in the salt-affected soil of desert steppe zone (Wei et al. 2015). Our study confirmed these results.
Multi-regression analysis is an effective approach for analyzing the relationships between species diversity and environmental factors (Härdtle et al. 2006, Li et al. 2009). We found that soil moisture and TDS, as the ecological and evolutionary filter, were two key factors controlling species richness. It was proved that there are negative correlations of species richness with soil moisture and salinity, these results confirmed the conclusions of former study by Shaltout et al. (1995) in the Mediterranean region and researches from around the world (García et al. 1993; Greenberg et al. 2006; Sharpe and Baldwin 2009). Further, it was disclosed in our study that species richness decreased as TDS increased, and there was no obvious correlation between cationic ratios and species richness. This may be due to other factors, such as oxygen concentration, nutrient limitation, various forms of biotic interaction, may also affect species richness (Williams 1998; Greiner et al. 2001; Härdtle et al. 2006), and these factors are not included in the present study.
In conclusion, soil moisture and salt ion composition are two major factors affecting species distribution and diversity. For details, soil moisture, SO42−, K+/Na+ ratio and pH were mainly responsible for vegetation community shift from arid grassland to salt marsh. Soil moisture and TDS remarkably affected species richness; and with the increase of soil moisture and TDS, the species richness declined. Plant species growing in semiarid inland saline habitats may provide useful information regarding the degree of salinization and consequent soil deterioration. Such information may be helpful for the more effective planning in practical reclamation of saline wastelands. How seasonal gradients of soil moisture and salinity exert effect on plant communities need to be further investigated.
References
Adam P (1990) Salt marsh ecology. Cambridge University Press, Cambridge
Allen RB, McIntosh PD, Wilson JB (1997) The distribution of plants in relation to pH and salinity on inland saline/alkaline soils in Central Otago, New Zealand. New Zeal J Bot 35:517–523. https://doi.org/10.1080/0028825X.1987.10410175
Bai YF, Han XG, Wu JG, Chen ZZ, Li LH (2004) Ecosystem stability and compensatory effects in the Inner Mongolia grassland. Nature 431:181–184. https://doi.org/10.1038/nature02850
Bao SD (2000) Soil and agricultural chemistry analysis. China Agriculture Press, Beijing (in Chinese)
Bertness MD, Gough L, Shumway SW (1992) Salt tolerances and the distribution of fugitive salt marsh plants. Ecology 73:1842–1851. https://doi.org/10.2307/1940035
Breckle SW (1986) Studies on halophytes from Iran and Afganistan. II. Ecology of halophytes along salt gradients. Proc R Soc Edinb B Bio Sci 89:203–215. https://doi.org/10.1017/S0269727000009039
Breckle SW, Wucherer W (2012) Halophytes and salt desertification in the Aralkum Area. In: Breckle SW, Wucherer W, Dimeyeva LA, Ogar NP (eds) Aralkum-a man-made desert. Springer, Berlin Heidelberg, pp 271–299
Brewer JS, Rand T, Levine JM, Bertness MD (1998) Biomass allocation, clonal dispersal, and competitive success in three salt marsh plants. Oikos 82:347–353. https://doi.org/10.2307/3546975
Brotherson JD (1987) Plant community zonation in response to soil gradients in a saline meadow near Utah Lake, Utah County, Utah. Great Basin Naturalist 47:322–333
Bui EN (2013) Soil salinity: a neglected factor in plant ecology and biogeography. J Arid Environ 92:14–25. https://doi.org/10.1016/j.jaridenv.2012.12.014
Burchill CA, Kenkel NC (1991) Vegetation-environment relationships of an inland boreal salt pan. Can J Bot 69:722–732. https://doi.org/10.1139/b91-098
Cantero JJ, Cisneros JM, Zobel M, Cantero A (1998) Environmental relationships of vegetation patterns in salt marshes of central Argentina. Folia Geobot 33:133–145. https://doi.org/10.1007/BF02913341
Carter CT, Ungar IA (2004) Relationships between seed germinability of Spergularia marina (Caryophyllaceae) and the formation of zonal communities in an inland salt marsh. Ann Bot 93:119–125. https://doi.org/10.1093/aob/mch018
Chapman VJ (1960) Salt marshes and salt deserts of the world. Leonard Hill Books Limited, London
Cooper A (1982) The effects of salinity and waterlogging on the growth and cation uptake of salt marsh plants. New Phytol 90:263–275. https://doi.org/10.1111/j.1469-8137.1982.tb03258.x
Cui BS, He Q, Zhao XS (2008) Ecological thresholds of Suaeda salsa to the environmental gradients of water table depth and soil salinity. Acta Ecol Sin 28:1408–1418. https://doi.org/10.1016/S1872-2032(08)60050-5
Díaz S, Cabido M (2001) Vive la différence: plant functional diversity matters to ecosystem processes. Trends Ecol Evol 16:646–655. https://doi.org/10.1016/S0169-5347(01)02283-2
Du QY, Li Z, Liu SR, Hy Liu (2007) Dynamics of species diversity and characteristics of halophytic plant communities around saline lakes in arid and semi-arid regions of Inner Mongolia. Biodivers Sci 15:271–281. https://doi.org/10.1360/biodiv.060214 (in Chinese)
Duan WJ, Ren H, Fu SL, Wang J, Zhang JP, Yang L, Huang CL (2010) Community comparison and determinant analysis of understory vegetation in six plantations in South China. Restor Ecol 18:206–214. https://doi.org/10.1111/j.1526-100X.2008.00444.x
Eliáš P, Sopotlieva D, Dítě D, Hájková P, Apostolova I, Senko D, Melečková Z, Hájek M (2013) Vegetation diversity of salt-rich grasslands in Southeast Europe. Appl Veg Sci 16:521–537. https://doi.org/10.1111/avsc.12017
FAO (2008) FAO land and plant nutrition management service. http://www.fao.org/ag/agl/agll/spush. Accessed 20 Nov 2016
García LV, Maranón T, Moreno A, Clemente L (1993) Above-ground biomass and species richness in a Mediterranean salt marsh. J Veg Sci 4:417–424. https://doi.org/10.2307/3235601
Gitay H, Noble IR (1997) What are functional types and how should we seek them. In: Shugart HH, Woodward FI (eds) Plant functional types: their relevance to ecosystem properties and global change. Cambridge University Press, Cambridge, pp 3–19
González-Alcaraz MN, Jiménez-Cárceles FJ, Álvarez Y, Álvarez-Rogel J (2014) Gradients of soil salinity and moisture, and plant distribution, in a Mediterranean semiarid saline watershed: a model of soil-plant relationships for contributing to the management. Catena 115:150–158. https://doi.org/10.1016/j.catena.2013.11.011
Greenberg R, Maldonado JE, Droege S, McDonald MV (2006) Tidal marshes: a global perspective on the evolution and conservation of their terrestrial vertebrates. Bioscience 56:675–685. https://doi.org/10.1641/0006-3568(2006)56[675:TMAGPO]2.0.CO;2
Greiner La Peyre MK, Grace JB, Hahn E, Mendelssohn IA (2001) The importiomassance of competition in regulating plant species abundance along a salinity gradient. Ecology 82:62–69. https://doi.org/10.1890/0012-9658(2001)082[0062:TIOCIR]2.0.CO;2
Härdtle W, Redecker B, Assmann T, Meyer H (2006) Vegetation responses to environmental conditions in floodplain grasslands: prerequisites for preserving plant species diversity. Basic Appl Ecol 7:280–288. https://doi.org/10.1016/j.baae.2005.09.003
Hegazy AK, Lovett-Doust J, Hammouda O, Gomaa NH (2008) Vegetation distribution along the altitudinal gradient in the northwestern Red Sea region. Community Ecol 8:151–162. https://doi.org/10.1556/ComEc.8.2007.2.2
Hill MO (1979a) DECORANA—a FORTRAN program from detrended correspondence analysis and reciprocal averaging. Cornell University, Ithaca
Hill MO (1979b) TWINSPAN—a FORTRAN program from arranging multivariate data in an order two-way table by Classification of the individuals and attributes. Cornell University, Ithaca
Hoveizeh H (1997) Study of the vegetation cover and ecological characteristics in saline habitats of Hoor-e-Shadegan. J Res Const 34:27–31
Jafari M, Chahouki MAZ, Tavili A, Azarnivand H, Amiri GZ (2004) Effective environmental factors in the distribution of vegetation types in Poshtkouh rangelands of Yazd Province (Iran). J Arid Environ 56:627–641. https://doi.org/10.1016/S0140-1963(03)00077-6
James JJ, Tiller RL, Richards JH (2005) Multiple resources limit plant growth and function in a saline-alkaline desert community. J Ecol 93:113–126. https://doi.org/10.1111/j.0022-0477.2004.00948.x
Jiang Y, Kang MY, Zhu Y, Xu GC (2007) Plant biodiversity patterns on Helan Mountain, China. Acta Oecol 32:125–133. https://doi.org/10.1016/j.actao.2006.12.003
Jongman RHG, Ter Braak CJF, van Tongeren OFR (1995) Data analysis in community and landscape ecology. Cambridge University Press, Cambridge
Kasera PK, Mohammed S (2010) Ecology of inland saline plants. In: Ramawat KG (ed) Desert Plants. Springer, Berlin Heidelberg, pp 299–320
Kirk JL, Klironomos JN, Lee H, Trevors JT (2005) The effects of perennial ryegrass and alfalfa on microbial abundance and diversity in petroleum contaminated soil. Environ Pollut 133:455–465. https://doi.org/10.1016/j.envpol.2004.06.002
Li WQ, Liu XJ, Khan MA, Yamaguchi S (2005) The effect of plant growth regulators, nitric oxide, nitrate, nitrite and light on the germination of dimorphic seeds of Suaeda salsa under saline conditions. J Plant Res 118:207–214. https://doi.org/10.1007/s10265-005-0212-8
Li WQ, Liu XJ, Khan MA, Gul B (2008) Relationship between soil characteristics and halophytic vegetation in coastal region of North China. Pak J Bot 40:1081–1090
Li XR, Tan HJ, He MZ, Wang XP, Li XJ (2009) Patterns of shrub species richness and abundance in relation to environmental factors on the Alxa Plateau: prerequisites for conserving shrub diversity in extreme arid desert regions. Sci China Ser D-Earth Sci 52:669–680. https://doi.org/10.1007/s11430-009-0054-7
Li X, Liu Y, Chen M, Song YP, Song J, Wang BS, Feng G (2012) Relationships between ion and chlorophyll accumulation in seeds and adaptation to saline environments in Suaeda salsa populations. Plant Biosyst 146:142–149. https://doi.org/10.1080/11263504.2012.727880
Liu ZM, Li XL, Yan QL, Wu JG (2007) Species richness and vegetation pattern in interdune lowlands of an active dune field in Inner Mongolia, China. Biol Conserv 140:29–39. https://doi.org/10.1016/j.biocon.2007.07.030
Liu KH, Ding XW, Wang HF, Zhang XM, Hozzein WN, Wadaan MAM, Lan A, Zhang B, Li WJ (2014) Eukaryotic microbial communities in hypersaline soils and sediments from the alkaline hypersaline Huama Lake as revealed by 454 pyrosequencing. Anton Leeuw 105:871–880. https://doi.org/10.1007/s10482-014-0141-4
Lv SL, Jiang P, Chen XY, Wang XC, Li YX (2012) Multiple compartmentalization of sodium conferred salt tolerance in Salicornia europaea. Plant Physiol Bioch 51:47–52. https://doi.org/10.1016/j.plaphy.2011.10.015
McGraw DC, Ungar IA (1981) Growth and survival of the halophyte Salicornia Europaera L. under saline field conditions. Ohio J Sci 81:109–113
Minggagud H, Yang J (2013) Wetland plant species diversity in sandy land of a semi-arid inland region of China. Plant Biosyst 147:25–32. https://doi.org/10.1080/11263504.2012.737865
Naz N, Hameed M, Sajid Aqeel Ahmad M, Ashraf M, Arshad M (2010) Is soil salinity one of the major determinants of community structure under arid environments? Community Ecol 11:84–90. https://doi.org/10.1556/ComEc.11.2010.1.12
Neter J, Kutner MH, Nachtsheim CJ, Wasserman W (1996) Applied linear statistical models. Irwin, Chicago
Onkware AO (2000) Effect of soil salinity on plant distribution and production at Loburu delta, Lake Bogoria National Reserve, Kenya. Austral Ecol 25:140–149. https://doi.org/10.1046/j.1442-9993.2000.01020.x
Ortiz R, Alvarez Rogel J, Alcaraz F (1995) Soil-vegetation relationships in two coastal salt marshes in southeastern Spain. Arid Soil Res Rehabil 9:481–493. https://doi.org/10.1080/15324989509385914
Pan DY, Bouchard A, Legendre P, Domon G (1998) Influence of edaphic factors on the spatial structure of inland halophytic communities: a case study in China. J Veg Sci 9:797–804. https://doi.org/10.2307/3237045
Parida AK, Das AB (2005) Salt tolerance and salinity effects on plants: a review. Ecotox Environ Safe 60:324–349. https://doi.org/10.1016/j.ecoenv.2004.06.010
Piernik A (2003) Inland halophilous vegetation as indicator of soil salinity. Basic Appl Ecol 4:525–536. https://doi.org/10.1078/1439-1791-00154
Piernik A (2005) Vegetation-environment relations on inland saline habitats in Central Poland. Phytocoenologia 35:19–38. https://doi.org/10.1127/0340-269X/2005/0035-0019
Piernik A (2006) Growth of three meadow species along a salinity gradient in an inland saline habitat: transplant experiment. Pak J Bot 54:117–125 (WOS: 000236073300009)
Qiao ZL (2005) The study on microbial ecology and actinomycete resource from saline-alkali soil in Shanxi province. Master’s thesis, Northwest agriculture and and forestry Universitiy (in Chinese)
Rand TA (2000) Seed dispersal, habitat suitability and the distribution of halophytes across a salt marsh tidal gradient. J Ecol 88:608–621. https://doi.org/10.1046/j.1365-2745.2000.00484.x
Roem WJ, Klees H, Berendse F (2002) Effects of nutrient addition and acidification on plant species diversity and seed germination in heathland. J Appl Ecol 39:937–948. https://doi.org/10.1046/j.1365-2664.2002.00768.x
Rogel JA, Ariza FA, Silla RO (2000) Soil salinity and moisture gradients and plant zonation in Mediterranean salt marshes of Southeast Spain. Wetlands 20:357–372. https://doi.org/10.1672/0277-5212 (2000)020[0357:SSAMGA]2.0.CO;2
Schofield RV, Kirkby MJ (2003) Application of salinization indicators and initial development of potential global soil salinization scenario under climatic change. Global Biogeochem Cy 17:1078. https://doi.org/10.1029/2002gb001935
Sciandrello S, Guglielmo A, Spampinato G (2014) Spatial patterns and floristic composition of plant communities in coastal salt marshes of southeastern Sicily (Italy). Acta Bot Gallica Bot Lett 161:99–109. https://doi.org/10.1080/12538078.2014.892026
Shaltout KH, El-Kady HF, Al-Sodany YM (1995) Vegetation analysis of the Mediterranean region of Nile Delta. Vegetatio 116:73–83. https://doi.org/10.1007/BF00045280
Sharpe PJ, Baldwin AH (2009) Patterns of wetland plant species richness across estuarine gradients of Chesapeake Bay. Wetlands 29:225–235. https://doi.org/10.1672/08-111.1
Song J, Wang BS (2015) Using euhalophytes to understand salt tolerance and to develop saline agriculture: Suaeda salsa as a promising model. Ann Bot 115:541–553. https://doi.org/10.1093/aob/mcu
Szabolcs I (1989) Salt-Affected Soils. CRC Press Inc., Boca Raton
Ter Braak CJF, Smilauer P (2002) CANOCO reference manual and CanoDraw for Windows user’s guide: Software for canonical community ordination (version 4.5). Microcomputer Power, Ithaca
Thomas DSG, Middleton NJ (1993) Salinization: new perspectives on a major desertification issue. J Arid Environ 24:95–105. https://doi.org/10.1006/jare.1993.1008
Ungar IA (1974) Inland halophytes of the United States. In: Reimold RJ, Queen WH (eds) Ecology of halophytes. Academic press INC, New York and London, pp 235–305
Ungar IA, Benner DK, McGraw DC (1979) The distribution and growth of Salicornia europaea on an inland salt pan. Ecology 60:329–336. https://doi.org/10.2307/1937662
Ushakova SA, Kovaleva NP, Gribovskaya IV, Dolgushev VA, Tikhomirova NA (2005) Effect of NaCl concentration on productivity and mineral composition of Salicornia europaea as a potential crop for utilization NaCl in LSS. Adv Space Res 36:1349–1353. https://doi.org/10.1016/j.asr.2004.09.017
Wang N (2008) Effects of lope-gully erosion environment on the propaguleand and seedling establishment-a case study in ZHIFANGGOU Ansai on the loess plateau. Master’s thesis, Northwest agriculture and and forestry Universitiy (in Chinese)
Wanner A (2009) Management, biodiversity and restoration potential of salt grassland vegetation of the Baltic Sea: Analyses along a complex ecological gradient. PhD thesis, University of Hamburg
Wei DD, Cheng D, Liu WB, Liu T, Yang XH, Zheng YH (2015) Adequate potassium application enhances salt tolerance of moderate-halophyte Sophora alopecuroides. Plant Soil Environ 61:364–370. https://doi.org/10.17221/232/2015-PSE
Wilkoń-Michalska J (1985) Structure and dynamics of the inland populations of Salicornia patula. In: Beeftink WG, Rozema J, Huiskes AHL (eds) Ecology of coastal vegetation. Springer, Netherlands, pp 145–154
Williams WD (1998) Salinity as a determinant of the structure of biological communities in salt lakes. Hydrobiologia 381:191–201. https://doi.org/10.1023/A:1003287826503
Xiong SY, Xiong ZX, Wang PW (1996) Soil salinity in the irrigated area of the Yellow River in Ningxia, China. Arid Land Res Manag 10:95–101. https://doi.org/10.1080/15324989609381423
Yang F, Wang ZC, Wang YH, An FH, Zhao CW (2013) The correlation between soil water salinity and plant community distribution under micro-topography in Songnen Plain. Acta Ecol Sin 33:6202–6208. https://doi.org/10.5846/stxb201306051346 (In Chinese)
Yu FH, Dong M (1999) Multi-scale distribution pattern of natural ramet population in the rhizomatous herb, Thermopsis lanceolata. Acta Bot Sin 41:1332–1338
Zhang J, Zhao H, Zhang T, Zhao X, Drake S (2005) Community succession along a chronosequence of vegetation restoration on sand dunes in Horqin Sandy Land. J Arid Environ 62:555–566. https://doi.org/10.1016/j.jaridenv.2005.01.016
Zhang KB, Li R, Yf Liu, Wang BT, Yang XH, Hou RP (2008) Spatial pattern of a plant community in a wetland ecosystem in a semi-arid region in northwestern China. Front For China 3:326–333. https://doi.org/10.1007/s11461-008-0048-1
Zhao KF, Li FZ (1999) Chinese halophytes. China Science Press, Beijing (In Chinese)
Zhao KF, Fan H, Ungar IA (2002) Survey of halophyte species in China. Plant Sci 163:491–498. https://doi.org/10.1016/S0168-9452(02)00160-7
Zhao KF, Fan H, Zhou S, Song J (2003) Study on the salt and drought tolerance of Suaeda salsa and Kalanchoe claigremontiana under iso-osmotic salt and water stress. Plant Sci 165:837–844. https://doi.org/10.1016/S0168-9452(03)00282-6
Zhao KF, Song J, Feng G, Zhao M, Liu JP (2011) Species, types, distribution, and economic potential of halophytes in China. Plant Soil 342:495–509. https://doi.org/10.1007/s11104-010-0470-7
Zhou H, Zhao WZ, Luo WC, Liu B (2015) Species diversity and vegetation distribution in nebkhas of Nitraria tangutorum in the Desert Steppes of China. Ecol Res 30:735–744. https://doi.org/10.1007/s11284-015-1277-z
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China subsidization project (Grant No. 41471435) and “Ecological restoration technology application in inland river basin of Silk road economic belt” project from sciences technology service network planning in Cold and arid regions environmental and engineering research institute, Chinese academy (Grant No. HHS-TSS-STS-1503). The authors are very grateful to the anonymous reviewers and editors for their critical review and comments which helped to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Zhang, Dm., Zhao, Wz. & Zhang, Gf. Soil moisture and salt ionic composition effects on species distribution and diversity in semiarid inland saline habitats, northwestern China. Ecol Res 33, 505–515 (2018). https://doi.org/10.1007/s11284-018-1570-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-018-1570-8