Abstract
To explain why the composition of evergreen and deciduous forests changes along air temperature gradients, we measured several traits of single leaves from temperate deciduous and evergreen broadleaf trees with simultaneous and successive leaf emergence growing at different altitudes in the field. The parameters included seasonal net photosynthetic rate, longevity, mass per area, nitrogen content, and photosynthetic nitrogen-use efficiency. With decreasing altitude, the leaf longevity of deciduous broadleaf trees increased, whereas the maximum net photosynthetic rate decreased. In contrast, leaf longevity of evergreen broadleaf trees decreased, whereas the minimum net photosynthetic rate in winter increased. Along the air temperature gradient, the annual production of deciduous trees with simultaneous leaf emergence may be constant, because the integrated lifetime net photosynthetic rate (ILNPR) of a single leaf changed little. In comparison, deciduous trees with successive leaf emergence may show enhanced annual production with increasing air temperature, by increasing the total leaf number per branch and tree under an extended growing season. Temperate evergreen broadleaf tree species may also show increased annual production with increasing air temperature by sufficiently raising the number of the first-year leaves to the total leaves of branch and tree, which is accelerated by raising the integrated first-year net photosynthetic rate of the single leaf, despite little change in the ILNPR. With increasing air temperature from cool-temperate to warm-temperate zones, evergreen broadleaf tree species gain an advantage of the annual production over deciduous broadleaf tree species with simultaneous leaf emergence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The composition of evergreen and deciduous forests changes along air temperature gradients. Late successional vegetation comprises evergreen broadleaf forests in the warm-temperate zone, and deciduous broadleaf forests in the cool-temperate zone, and evergreen conifer forests in the subarctic zone (Walter 1973). Evergreenness increases at low and high latitudes, and deciduousness increases at middle latitude (Chabot and Hicks 1982). The altitudinal distribution of percentages of evergreenness and deciduousness at mid-latitudes also reflects the latitudinal distribution pattern (Kikuzawa 1996). Chabot and Hicks (1982) stated that it was difficult to explain bimodal peaks in evergreenness. Kikuzawa (1991) explained the changing pattern in tree type on the basis of a cost–benefit model that described the leaf replacement required for maximum plant gain. The relative merits of evergreen versus deciduous strategies, in terms of photosynthetic gain, change along a latitudinal gradient, according to the cost–benefit model (Kikuzawa 1991). On the basis of the theory that the leaf longevity is determined for maximizing the gain of a plant, it is necessary to measure actual temperature responses of evergreen and deciduous trees in the field, in terms of photosynthetic activity and annual production, to understand how these two tree types respond to different geographical distributions and global warming.
In addition to evergreenness and deciduousness, trees have different leafing phenologies; leaf emergence can be simultaneous or successive (Kikuzawa 1988). In trees with simultaneous leaf emergence, most leaves unfold and senesce at the same time, while leaf number per branch is independent of the length of the growing season, and does not differ much with air temperature. In comparison, in trees with successive leaf emergence, leaves unfold and senesce successively, while leaf number per branch is dependent on the length of the growing season and may change with air temperature. If the leaf number per the branch and tree changes with different air temperatures, the annual production of a branch and tree may change with different air temperatures. In trees with successive leaf emergence, net photosynthetic rate, and longevity are also important for the first leaves that emerge, and for successively emerging leaves. Simultaneous and successive leafing patterns have accordingly attracted considerable attention in an effort to understand the annual production of trees.
How is it possible to measure annual production of a single leaf, branch, and tree? In this study, we calculated the lifetime carbon gain of a single leaf (Mediavilla and Escudero 2003) based on the instantaneous maximum net photosynthetic rate of a single leaf throughout its lifespan. To determine the relationship between instantaneous maximum net photosynthetic rate and daily gain of a leaf, Kikuzawa et al. (2004) considered that daily gain of a single leaf could be estimated from mean labor time under the maximum day photosynthetic rate of the leaf. If the mean labor time is constant throughout the leaf lifespan, it is possible that the lifetime carbon gain of the leaf is proportional to the maximum day photosynthetic rate. Photosynthetic rate and leaf longevity are important for determining the lifetime carbon gain of a leaf. A negative correlation between leaf longevity and leaf photosynthetic rate has been reported for a variety of plants (Koike 1990; Reich et al. 1991, 1992; Gower et al. 1993; Hikosaka and Hirose 2000). Furthermore, the regression line inclination between maximum net photosynthetic rate during the leaf lifespan and leaf longevity in a log–log graph is not −1 (inverse proportion) but close to −0.65 among species (Reich et al. 1991, 1992). This means that the lifetime carbon gains of a single leaf is not constant among species. In contrast, Mediavilla and Escudero (2003) considered that lifetime carbon gain of a leaf was nearly constant among species, based on the integration of the average net photosynthetic rate over the leaf lifetime, rather than the instantaneous photosynthetic rate. Kikuzawa and Lechowitz (2006, 2011) found that the lifetime carbon gain of leaves was generally constant across species. However, it has not been fully ascertained that the lifetime carbon gain of leaves is constant within the same species. Moreover, responses of the lifetime carbon gain of a leave to changing air temperature and the effects of different leafing patterns on the lifetime carbon gain of leaves have not been reported.
To elucidate the annual production of a single leaf, we calculated the integrated lifetime net photosynthetic rate (ILNPR) from the maximum day net photosynthetic rate measured frequently throughout the leaf lifespan. In deciduous trees, where leaf longevity is shorter than a year, the ILNPR of a single leaf is simply annual production of the leaf. In evergreen trees, where leaf longevity is greater than a year, the ILNPR of the leaf is more than the annual production of the leaf. We consider the integrated first-year net photosynthetic rate (IFYNPR) of the leaf, that is, the integrated net photosynthetic rate of the leaf during only the first year from the ILNPR. We consider also the number percentage the first-year leaves to total leaves (NPFYL) of the branch and tree. If the NPFYL of a branch and tree is different across different altitudes within the same evergreen species, the annual production of the branch and tree may change across the different altitudes. In the current study, we compared annual production between deciduous and evergreen broadleaf trees, using the ILNPR, IFYNPR, and NPFYL to characterize the annual production of branches and trees. We measured several leaf traits, including phenology, area, leaf mass per area (LMA), longevity, nitrogen content, and leaf photosynthetic nitrogen-use efficiency (PNUE) to explain differences in the photosynthetic responses among the same and different species.
There are several ways to estimate how trees respond to different air temperatures. One approach is to project tree response by growing at different air temperatures using experimental temperature-regulated chambers. Such experiments have reported positive (Lewis et al. 1999) and negative (Wang et al. 1995; Bruhn et al. 2000) effects of increased temperature on net photosynthetic rate. However, it is difficult to exactly reproduce the natural climate in a growth chamber, particularly for an entire year, to determine the effects of temperature on the annual production of trees. A plant cultivated under a temperature environment that is different from its native habitat inevitably acclimatizes to the new temperature environment (Berry and Björkman 1980; Ruel and Ayres 1996; Wang et al. 1996; Grunderson et al. 2000) or to an elevated temperature environment (Teskey and Will 1999). Thus, to avoid acclimatization, it is preferable to use native trees in the field that have already adapted to their climate. In this study, we measure the photosynthetic activity and annual production of trees of the same species at natural sites under different air temperature conditions to elucidate how trees respond to changing air temperature, following the methods used by Nomoto (1964) and Kusumoto (1978).
Although trees have responded to global warming in the past, the rate of change predicted in the 21st century is likely to be unprecedented. However, few experiments have shown the effects of temperature for this scenario for trees and forests (Saxe et al. 2001). To elucidate responses of trees and forests to global warming, knowledge about the responses of the lifetime carbon gain of leaves and annual production of branches and trees to changing air temperature is useful. Differences in deciduousness and evergreenness, and simultaneous and successive leafing patterns, of trees are key traits in determining responses to global warming.
In this study, we measure the maximum net photosynthetic rate of single leaves through a continuous 1-year period, to calculate the lifetime carbon gain of leaves from deciduous and evergreen trees at different altitudes and subjected to different air temperatures. We compare the lifetime carbon gain of leaves among the same and different species to elucidate how trees respond to different air temperature environments. We clarify the effects of different leafing phenology on the lifetime carbon gain of branches and trees. We also determine the relationships of several leaf traits, including phenology, area, LMA, longevity, nitrogen content, and leaf PNUE, with the responses of the lifetime carbon gain of leaves to different air temperatures. On the basis of the results obtained, we reveal the temperature-dependent annual production of branches and trees of different tree types with evergreenness and deciduousness, and leafing emergence. Finally, we discuss the effects of global warming on the annual production and geographical distribution of the two tree types.
Materials and methods
Study sites
We compare deciduous broadleaf tree species at a cool-temperate mountain zone and a warm-temperate level land site in western Japan. We established the cool-temperate study site at Jizo Pass [ca. 700 m above sea level (a.s.l.), N35°20′47″, E135°45′50″] within the Asiu Forest Research Station, Field Science Education and Research Center, Kyoto University, Japan. The dominant species at this site was Japanese beech (Fagus crenata Blume). The warm-temperate study site was established at the Center for Ecological Research (CER), Kyoto University, Japan (ca. 150 m a.s.l., N34°58′15″, E135°57′31″). This site was an early successional warm-temperate forest dominated by Quercus serrata Murray. We estimated monthly mean temperatures for Jizo Pass and the CER Forest (Fig. 1). Temperatures for Jizo Pass were based on temperature data from 1971 to 2000 at the Asiu Forest Research Station office (356 m a.s.l.), using a lapse rate of 0.6 °C per 100 m altitude. The CER Forest temperatures were calculated using temperature data for Ohtsu from 1979 to 2000 from the Japan Meteorological Agency by subtracting 1 °C from each monthly mean temperature, because the measured monthly mean temperature of the CER Forest from February 2006 to December 2010 was an average of 1 °C lower. The annual average temperature and the warmth index (Yim and Kira 1975) at Jizo Pass and the CER Forest are 9.6 and 78.8 °C, and 15.8 and 139.2 °C, respectively. Jizo Pass is located in the deciduous broadleaf forest zone in the cool-temperate zone, whereas the CER Forest is situated in the evergreen broadleaf forest zone in the warm-temperate zone. A warmth index of 85 demarcated the border between the cool-temperate and warm-temperate zones (Yim and Kira 1975).
a Monthly mean air temperature at Jizo Pass (unfilled circles) and the CER Forest (solid black circles). b Monthly mean air temperature on the east slope on Yakushima Island, calculated from Eguchi (2006), at 5, 200, 400, 600, 800, 1,000, and 1,200 m a.s.l., from the lowest to the highest curves
To compare evergreen broadleaf tree species along an air temperature gradient from the coast to 1,200 m a.s.l., we established a study site on Yakushima Island, approximately 24 km south of Kyushu Island, Japan. Yakushima Island is 504.88 km2 in size, with mountains exceeding 1,800 m a.s.l.; thus, it is possible to pass from low-altitude sites near the coast to high-altitude mountain sites in a short distance and time span. The study sites were set up at every 200 m rise in altitude on the east slope of the island along Anbo Forestry Road, ranging from 5 m a.s.l. near Anbo Fishing Port to 1,200 m a.s.l. near a plot of Kigensugi that is a big tree of Cryptomeria japonica (L.f.) D. Don tree. The study sites at 5, 200, 400, 600, 800, 1,000, and 1,200 m a.s.l. were located at N30°18′42″ and E130°39′22″, N30°18′23″ and E130°37′45″, N30°18′57″ and E130°37′20″, N30°18′57″ and E130°36′24″, N30°18′39″ and E130°35′16″, N30°18′20″ and E130°34′33″, and N30°18′11″ and E130°32′59″, respectively. Figure 1b shows the estimated monthly mean air temperatures at each study site based on temperature data that were measured on the east slope of Yakushima Island from September 1998 to August 1999 (Eguchi 2006). The annual mean air temperatures of the study sites at 5, 200, 400, 600, 800, 1,000, and 1,200 m a.s.l. were 19.4, 17.9, 16.7, 15.3, 13.8, 11.8, and 11.2 °C, respectively. The warmth indexes of the study sites at 0, 200, 400, 600, 800, 1,000, and 1,200 m a.s.l. were 172.3, 154.0, 140.0, 125.9, 105.3, 87.8, and 80.1, respectively (Yim and Kira 1975). According to Yim and Kira (1975), only the highest study site (1,200 m a.s.l.) was located in the deciduous broadleaf forest zone of the cool-temperate zone. Eguchi (2006) measured temperatures at 36, 175, 620, 1,010, and 1,360 m a.s.l.; thus, the temperatures for each study site were calculated based on the linear change in temperature between the measured heights. On the basis of the warmth index, Jizo Pass and the CER Forest correspond to 1,200 and 400 m a.s.l. on Yakushima Island, respectively.
All study sites were on flat ground or gentle slopes less than 10° at Jizo Pass and the CER Forest and on Yakushima Island to minimize differences in soil moisture among sites.
Study species
We selected three species that were common at both Jizo Pass and the CER Forest (Castanea crenata Sieb. et Zucc., Q. serrata, and Rhus tricocarpa Miq.) for comparison of photosynthetic activities and leaf traits at different altitudes. At Jizo Pass, we selected Betula grossa Sieb. et Zucc. to compare the leaves of long and short shoots. We selected C. crenata at Jizo Pass and the CER Forest and Alnus sieboldiana Matsumura at the CER Forest, as species producing many leaves successively throughout the season. On Yakushima Island, we selected four evergreen broadleaf species, Castanopsis cuspidata (Thunb. ex Murray) Schottky var. sieboldii Nakai, Distylium racemosum Sieb. et Zucc., Litsea acuminata (Bl.) Kurata, and Quercus salicina Blume, and one deciduous broadleaf species, Stewartia monadelpha Sieb. et Zucc., because they are dominant and have wide altitudinal distributions. In addition, these evergreen broadleaf species all exhibit simultaneous leaf emergence, and are late successional components of the warm-temperate zone evergreen broadleaf forest on Yakushima Island. These trees are distributed from the coast to ca. 1,000 m a.s.l.; the evergreen conifers Cryptomeria japonica (L. fil.) D. Don, Tsuga sieboldii Carriêre and Abies firma Sieb. et Zucc. become dominant in the forest above ca. 900 m a.s.l. (Suzuki 1997). S. monadelpha is a main component of the late successional Fagus crenata Blume forest in the cool-temperate zone of southwestern Japan.
Photosynthesis
In 1999, we measured the net photosynthetic rate every week from leafing to shedding at Jizo Pass and the CER Forest. On Yakushima Island, we measured net photosynthetic rate twice a month from October 2000 to October 2001 for the evergreen broadleaf species and from October 2000 to December 2001 for the deciduous broadleaf species. For each species, we selected three trees growing in a well-lit habitat in each site. For species with simple leaves, we measured the first leafing leaves from different current branches for each tree throughout the year. For R. tricocarpa, which has compound leaves, we used the third leaflet from the base of the first compound leaf. For evergreen species, in addition to the first leafing leaf of the current branch in the measured year, we measured 1-year-old and 2-year-old first leaves, if present, of old branches that had sprouted in the two preceding years, once every 2 months. For B. grossa at Jizo Pass, we measured the first and the sixth leaves of the long shoot in addition to the first leaf of the short shoot. In successive leafing species (A. sieboldiana at the CER Forest and C. crenata at both Jizo Pass and the CER Forest), we measured the first, 15th, 30th, and 45th leaves of the same long shoot.
The net photosynthetic rate was measured with a portable CO2 and H2O gas analyzer [LCA-4, Analytical Development Company, Ltd. (ADC), UK] using natural air at a height of 2 m in direct sunlight from 0800 to 1100 hours. If the measuring point was located near a forestry road, we stopped the measurement when vehicles were passing, to avoid the effect of exhaust fumes. CO2 concentration ranged from 350 to 420 ppm. More than 1,000 μmol m−2 s−1 photosynthetic active radiation was used to obtain the light-saturated net photosynthetic rate. The leaf-chamber temperature ranged from 39 °C in summer to 15 °C in winter. After photosynthetic measurement, we removed the leaf and measured its area and dry weight in the laboratory. Leaf area was measured using an area meter (LI-3100C, LI-Cor Environmental, Nebraska, USA), and dry weight was determined after drying at 75 °C for 2 days. The net photosynthetic rate per leaf mass was more correlated with the leaf longevity than that per leaf area (Reich et al. 1991, 1992), and we consider the net photosynthetic rate per leaf mass to be more related to the ratio of cost to benefit of the leaf. Therefore, we mainly used leaf mass to calculate the net photosynthetic rate.
Leaf longevity
For deciduous broadleaf species, we monitored leaves to determine leaf longevity, i.e., the number of days between leafing and shedding (Kikuzawa 1983). We used five trees per species growing in well-lit habitat in each study site. For each tree, we measured leaf length and the loss of green color of the first leaf on three marked branches every week in Jizo Pass and the CER Forest in 1999 and twice a month on Yakushima Island. The completion of leafing was defined as when a leaf stopped elongating, while the completion of shedding was defined as when the majority of a leaf’s area was no longer green. We calculated the average number of days from leafing to shedding for 15 leaves per species.
For the evergreen broadleaf species, leaf longevity was estimated based on a survival curve generated from the distribution of the number of leaves of different ages. For each species, with the exception of L. acuminata, at each study site, we counted the number of leaves of each age in the top 20 branches, just after the completion of new leafing. We trimmed the 20 branches off three to five marked canopy trees. Because L. acuminata had few branches, we used only the top ten branches. We counted the leaves every month for a year. We estimated the relative number of 1-, 2-, and 3-year-old leaves based on the number of the 1-year leaves, because the 1-year leaves did not fall before the next leafing. In Q. salicina, which often regrows new leaves on a regrown branch approximately 1 month after spring branch growth and leafing, we counted the number of leaves on the regrown branch that expanded on a top of spring branch separately from the spring leaves when each branch had regrown. We calculated regrowth percentage of the leaves on the basis of 60 new spring branches at each altitude to estimate the average leaf longevity, because regrown leaves had shorter longevity than spring leaves. At each leaf count, any decrease in the number of leaves of each age group was treated as leaves that had fallen since the previous count. Thus, we were able to estimate the average leaf longevity per branch of each species at each study site. However, we did not follow the same branch over time and worked under the assumption that, within a species and site, all upper branches had the same leaf longevity. To consider integrated yearly net photosynthetic rate per branch and tree of the evergreen species on Yakushima Island, we calculated the percentage of first-year leaves to total leaves (NPFYL) after new leaf sprouting in May or June in 2001, based on the number of year-old leaves on branches that were counted. The NPFYL increases with shorter leaf longevity within the same species.
Integrated net photosynthetic rate
We measured the net photosynthetic rate one to four times every month for each tree. We calculated ILNPR and IFYNPR of a single leaf, to determine its lifetime and yearly carbon gain, respectively, using the average of three trees at each height for each species. The linear change in net photosynthetic rate was estimated between measured net photosynthetic rates at each time point. Hence, the ILNPR is the product of the average net photosynthetic rate of a single leaf throughout its lifetime and leaf longevity. The IFYNPR is the product of the average net photosynthetic rate of the first year of the single leaf during 1 complete year from leafing. In deciduous trees with leaf longevity of less than 1 year, the IFYNPR of a single leaf does not differ from the ILNPR. In evergreen trees with leaf longevity longer than 1 year, the IFYNPR is smaller than ILNPR.
Leaf area and LMA
For the deciduous broadleaf tree species, we collected two leaves from the base of three branches per tree in mid-June. Five trees were sampled per species at each study site, amounting to a total 30 leaves per species per study site. For the evergreen broadleaf tree species, we collected the two most distal leaves from 10–20 branches of each species at each study site in mid-August. Hence, we measured a total of 20–40 leaves for each species in each study site. The methods of the area and dry weight measurements were the same as the photosynthesis measurement. We calculated LMA from area and dry weight of each leaf.
Nitrogen content
To measure leaf nitrogen content, we used the leaves that had the highest net photosynthetic rate in June or July of 2000 at Jizo Pass and the CER Forest, and in 2001 on Yakushima Island. We obtained samples of approximately 1 cm2 from the center half of the leaf blades, avoiding the midrib. After reducing the sample to powder, the nitrogen content of the leaf was measured using a CHN analyzer (2400II, Perkin Elmer, MA, USA). After measuring nitrogen content, we calculated PNUE. The PNUE was the mean photosynthetic capacity per unit leaf nitrogen for three trees at each measurement.
Statistical analysis
We used multiple comparison by Steel–Dwass test for mean comparison.
Results
Seasonal change in net photosynthetic rate
Seasonal changes in the net photosynthetic rates of C. crenata, Q. serrata, and R. trichocarpa at Jizo Pass and the CER Forest are shown in Fig. 2. For all three of these deciduous broadleaf tree species, the maximum net photosynthetic rate during the summer was lower at the lower altitude CER Forest than at the higher altitude Jizo Pass.
On Yakushima Island, the maximum net photosynthesis of the deciduous broadleaf tree species S. monadelpha decreased with decreasing altitude during summer (Fig. 3), similar to the deciduous broadleaf tree species at Jizo Pass and the CER Forest. In the four evergreen broadleaf tree species (C. cuspidata var. sieboldii, D. racemosum, L. acuminata, and Q. salicina), although the maximum net photosynthetic rate showed little difference with altitude during summer for all species, the minimum net photosynthetic rate increased with decreasing altitude during winter for all species (Fig. 4).
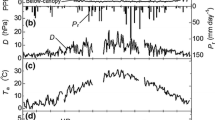
Seasonal changes in the net photosynthetic rate of a new leaf for a year for a C. cuspidata var. sieboldii, b D. racemosum. c L. acuminata, and d Q. salicina on Yakushima Island. Symbols that are not connected on the left show a new leafing leaf. Solid black circle 200 m a.s.l., solid black inverted triangle 400 m a.s.l., solid black triangle 600 m a.s.l., unfilled circle 1,000 m a.s.l.
Among leaves from short and long shoots of B. grossa, the maximum net photosynthetic rate was higher for leaves on long shoots than for leaves on short shoots (Fig. 5).
Seasonal changes in the net photosynthetic rate of a A. sieboldiana at the CER Forest, b B. grossa at Jizo Pass, c C. crenata at the CER Forest, and d C. crenata at Jizo Pass. In a, c, and d, circles the first leaf, triangles the 15th leaf, inverted triangles the 30th leaf, squares the 45th leaf. In b outlined circles the first leaf on the short shoot, solid black triangles the first leaf on the long shoot, solid black inverted triangles the sixth leaf on the long shoot
Leaf traits
Comparison of Jizo Pass and the CER Forest indicated that C. crenata, Q. serrata, and R. trichocarpa showed the same trends in photosynthetic activities and leaf traits (Table 1). However, leaf longevity and LMA were lower, and leaf area and nitrogen content were greater at Jizo Pass (higher altitude) compared to the CER Forest (lower altitude).
On Yakushima Island, leaf longevity in the deciduous species (S. monadelpha) increased and the PNUE decreased with decreasing altitude; however, LMA and leaf nitrogen content did not change with altitude (Table 2). In all four evergreen broadleaf tree species (C. cuspidata var. sieboldii, D. racemosum, L. acuminata, and Q. salicina), the leaf longevity decreased and NPFYL increased with decreasing altitude (Table 2). The leaf area of three evergreen broadleaf tree species (C. cuspidata var. sieboldii, D. racemosum, and L. acuminata) tended to increase as altitude decreased.
Among leaves from short and long shoots of B. grossa, the leaf longevity was greater in short versus long shoots (Table 3).
Integrated net photosynthetic rate
Within the same species, there was little difference in the ILNPR of a single leaf between Jizo Pass and the CER Forest (Table 1), or at different altitudes on Yakushima Island. For the evergreen species of Yakushima Island, the IFYNPR increased with decreasing altitude.
Among species, the ILNPR of C. crenata were lower than those of Q. serrata and R. trichocarpa at Jizo Pass and the CER Forest (Table 1). The ILNPR for S. monadelpha was higher compared to deciduous species at Jizo Pass and the CER Forest (Tables 1, 2), or for evergreen species on Yakushima Island (Table 2).
Among leaves from short and long shoots of B. grossa, the ILNPR declined with leafing order (i.e., the short shoot leaf, the first long shoot leaf, and the fifth long shoot leaf; Table 3). The ILNPR decreased with leafing order for the long shoot leaves of the successive-leaf-emergence species A. sieboldiana and C. crenata, (Table 3).
Discussion
Leaf traits in relation to photosynthetic activity
Leaf traits change in response to differences in environmental air temperature. The length of the growing season shortened in a cold climate, leaf longevity decreased, while LMA and leaf nitrogen content increased in deciduous broadleaf shrubs and trees. In comparison, leaf longevity, LMA, and leaf nitrogen content increased in evergreen shrubs (Kudo 1992, 1995, 1996). Similar to that reported by Kudo (1992, 1996), in the deciduous broadleaf tree species studied here, we found an increase in leaf longevity and LMA, and a decrease in leaf nitrogen content with decreasing altitude, except for S. monadelpha. S. monadelpha showed a tendency of decreasing PNUE with decreasing altitude. The increase in leaf longevity with decreasing altitude is associated with a decline in maximum net photosynthetic rate with decreasing altitude among the same species, as shown for different species by Reich et al. (1992); however, there was no difference in ILNPR. The decrease in leaf nitrogen content (Field and Mooney 1986) and PNUE also contribute to this decline. LMA differed for C. crenata, Q. serrata, and R. trichocarpa between the Jizo Pass and the CER Forest. This difference reduced the difference in the maximum net photosynthetic rate per area between the Jizo Pass and the CER Forest. In comparison, LMA did not vary at different altitudes for S. monadelpha. The tendency for the maximum net photosynthetic rate to increase with altitude was maintained when the net photosynthetic rate was compared to that based on leaf area. The former trees represent early successional species, while the latter is a late successional species.
In the evergreen broadleaf tree species, leaf longevity increased with increasing altitude, as also found by Kudo (1995) for evergreen shrubs; however, there was little variation in LMA and leaf nitrogen content with changing altitude. On Mt. Kinabalu, PNUE decreased with increasing altitude in evergreen broadleaf tree species (Hikosaka et al. 2002). On Yakushima Island, there was little change in PNUE with altitude in evergreen species. The small change in LMA, leaf nitrogen content, and PNUE with altitude may be associated with the small variation in maximum net photosynthetic rate with altitude. Our results indicating that leaf area increased with decreasing altitude support the trend of reduced leaf size toward the upper zones in evergreen broadleaf woody flora of subtropical mountains (Tang and Ohsawa 1999). On Yakushima Island (Table 2), leaf area declined with increasing leaf longevity and there was little change in LMA, except for Q. salicina.
Response of annual production to air temperature change
In deciduous broadleaf tree species at Jizo Pass, the CER forest, and Yakushima Island, the maximum net photosynthetic rate decreased with decreasing altitude, despite increased leaf longevity. In evergreen broadleaf tree species, the minimum net photosynthetic rate during winter increased with decreasing altitude, while leaf longevity decreased at Yakushima Island. The ILNPR of a single leaf was very similar for all first emerging leaves, regardless of altitude in both deciduous and evergreen tree species (Tables 1, 2). This means that the lifetime carbon gain of a leaf is nearly constant within the same species. It has previously been reported that the lifetime carbon gain of leaves is generally constant across species (Kikuzawa and Lechowitz 2006; Selaya and Anten 2010), although intraspecific differences were not compared. Our results show that the lifetime carbon gain of leaves was not relatively constant across species when compared with intraspecific differences. The result that the lifetime carbon gain of the leaf within the same species was nearly constant across different air temperature environments in the current study was well-grounded, because our results were specifically focused on the first emerging leaf, frequent measurement during the lifetime, and simultaneous intra-specific comparisons.
The type of leaf emergence affects the annual production of the deciduous trees, because the annual production of a branch and tree changes if the total leaf number changes. In flush-type (simultaneous) emergence, all leaves emerge and die simultaneously each year (Kikuzawa 1988), independent of leaf longevity and altitude. Because the ILNPR and IFYNPR remain similar at different altitudes, this type of leaf emergence might have a limited influence on the annual production of the leaf, branch, and tree. Late successional species, such as F. crenata in the Japanese cool-temperate zone and Larix gmelinii (Rupr.) ex Maxim in the subarctic zone of East Siberia, show simultaneous leaf emergence. In comparison, in succeeding-type (successive) emergence, total leaf number per branch and per tree was greater annually at the CER Forest (with a relatively long growing season) compared to Jizo Pass (with a relatively short growing season; Fig. 5c, d). Then, its annual production of the branch and tree might increase with increasing air temperature, even though the ILNPR and IFYNPR of the leaf remained similar at different altitudes, as in C. crenata (Table 3). This succeeding type occurs in the early successional forest like C. crenata and A. sieboldiana.
In evergreen broadleaf tree species, the NPFYL and IFYNPR of a leaf increased with decreasing altitude within the same species; however, ILNPR of the leaf remained nearly constant (Table 2). On condition that the ILNPR of the leaf is constant across different altitudes, the effects of the increase in the NPFYL of a branch with decreasing altitude depend on the leaf mass of the branch. If the total leaf mass of branch with different ages after new leafing is constant across different altitudes, the first-year leaf mass of the branch and resultant annual production of the branch and tree increases with decreasing altitude, corresponding to the increase in the NPFYL. If the first year leaf mass is constant across different altitudes despite differences in NPFYL, the total leaf mass of branch with different ages decreased and the annual production of the branch and tree does not increase with decreasing altitude. However, it has not been resolved whether the total leaf mass of the branch and tree after new leafing is constant with decreasing altitude. Among different tree species, leaf mass is positively correlated with leaf longevity (Kikuzawa and Lechowitz 2011). This means that leaf mass is not constant with changing leaf longevity; however, it is not known whether leaf mass per branch and tree is constant with decreasing altitude within the same species. Previous studies has shown that leaf longevity is positively correlated with the LMA among different species (Koike 1988; Reich et al. 1991); however, our results showed little differences in LMA with decreasing altitude, despite a decrease in the leaf longevity (Table 2). As each plant community might have optimal leaf area index (Monsi and Saeki 1953), in addition to the constant LMA with decreasing altitude, it may be reasonable that, within the same evergreen species, the total leaf mass of a branch and tree is constant with decreasing altitude. Therefore, we may conclude that an increase in the NPFYL of a branch and tree with decreasing altitude caused the annual production to increase with decreasing altitude for each tree species. On the premise that the NPFYL increases, an increase in the IFYNPR promotes an increase in the annual production of the branch and tree with decreasing altitude. In evergreen tree species, even in the flush leafing type, the annual production of the branch and tree might increase with increasing air temperature. This hypothesis means that the total leaf number on the branch and tree increased annually with decreasing altitude in the evergreen trees, similar to deciduous trees with successive leaf emergence, if area of a leaf does not change with decreasing altitude.
On the basis of these results, we may predict how the annual production of evergreen and deciduous trees changes in response to global warming in a temperate climate. Global warming has been shown to result in an extended growing season (Menzel and Fabian 1999; Walther 2002; Walther et al. 2002). Generally, the late successional deciduous broadleaf forest in the cool-temperate zone and the late successional deciduous conifer forest in the subarctic zone undergo little change in annual production with increasing air temperature, due to the simultaneous leafing. However, early successional forest in temperate zones, where successive leafing is dominant, might increase annual production with increasing air temperature, by increasing the total leaf number per branch and tree in a year. In contrast, both late and early successional evergreen broadleaf forests in temperate zones might increase annual production with increasing air temperature, because warmer temperatures shorten leaf longevity and increase NPFYL. Elevated temperature has been reported to enhance growth in deciduous trees more than in evergreen trees (Welp et al. 2007; Way and Oren 2010); however, these studies did not distinguish between simultaneous and successive leaf emergences. Mediavilla and Escudero (2003) compared the lifetime carbon gain of leaves between different altitudes and reported higher lifetime carbon gain of leaves at higher altitude. The authors compared different species of the same genus; however, in the current study, we compared the same species across different altitudes. Therefore, it is difficult to compare the results of the two studies.
Differences in distribution of deciduous and evergreen forests
In Japan, deciduous and evergreen broadleaf forests are distributed in cool- and warm-temperate zones, respectively. This may be explained by the different responses of annual production to air temperature change. The late successional species in the cool-temperate zone, Fagus crenata, is characterized as deciduous with simultaneous leafing; there are few changes in annual production with air temperature change. In contrast, late successional species in the warm-temperate zone are evergreen broadleaf species, which show changes in annual production with air temperature change. Hence, change in late successional species from evergreen to deciduous along an air temperature gradient from warm to cool may be explained. For instance, the annual production of evergreen trees decreases, whereas that of deciduous trees changes little in response to a reduction in annual mean air temperature. Our hypothesis that a tree with higher annual production dominates a tree with lower annual production might be verified by this change from the dominance of evergreen trees in the warm-temperate zone to the dominance of deciduous trees in the cool-temperate zone, accompanied by different changes in the annual production between deciduous and evergreen trees.
Throughout the world, there are areas where this change from evergreen to deciduous forests does not occur along a decreasing air temperature gradient. It is well known that deciduous broadleaf forests do not occur in the mountains of the tropical zone or in the west, from Yunnan in China to the Himalayas in the Sino-Japanese geographic region. On Yakushima Island, evergreen conifer–broadleaf mixed forests are distributed in the cool-temperate zone in place of deciduous broadleaf forests (Aiba et al. 2007). In the subarctic coniferous forest zone of Eurasia, deciduous coniferous forest appears as late successional forest only in East Siberia. When the warmth index (Yim and Kira 1975), i.e., integrated air temperature over a growth threshold during the growing season, is the same, different patterns of annual change in air temperature occur (Fig. 6). Both a long, warm growing season, with a narrow annual air temperature range and a short, hot growing season, with a wide annual air temperature range, may occur. The former is advantageous for evergreen trees that may gain a longer growing season over a year despite a low net photosynthetic rate. In comparison, the latter is advantageous for deciduous trees that may show high net photosynthetic rate within a short growing season. The effects of this annual air temperature pattern (average and amplitude) on the distribution of deciduous and evergreen forests have been shown by a mathematical cost–benefit model (Takada et al. 2006). From yearly patterns of mean monthly air temperature, it is clear that tropical mountains have a very narrow annual air temperature range and lack the deciduous broadleaf forests (Ohsawa 1990). In the Sino-Japanese geographic region, the deciduous broadleaf forest zone is found in a relatively narrow annual air temperature range, e.g., Kathmandu (Nepal) and Kunming (China) (Fig. 7a). On Yakushima Island, too, the annual air temperature range is relatively narrow compared to Karuizawa and Hakodate (Japan) (Fig. 7a). In the subarctic coniferous forest zone of Eurasia, the deciduous conifer Larix only dominates in Yakutsk (Russia), East Siberia. Deciduousness to avoid winter drought was reported (Berg and Chapin 1994); however, the annual air temperature range is relatively wide there (Fig. 7b). Therefore, the dominance of late successional species by deciduous or evergreen forests at a locality along an air temperature gradient may be explained by differences in the annual net photosynthetic patterns of deciduous and evergreen trees in relation to changes in the annual air temperature of a locality.
Monthly mean air temperature in a the Sino-Japanese geographic region and b the subarctic zone of Eurasia. In a, solid black inverted triangles Kathmandu (Nepal), solid black triangles Kunming (China), outlined triangles Changsha (China), solid black circles Yakushima Island (Japan) at 1,010-m altitude, outlined circles Karuizawa (Japan), outlined inverted triangles Hakodate (Japan). In b, solid black circles Moscow (Russia), solid black triangles Surgut (Russia), solid black inverted triangles Krasynoyarsk (Russia), solid black squares Kirensk (Russia), outlined circles Yakutsk (Russia)
References
Aiba S, Hanya G, Tsujino R, Takyu M, Seino T, Kimura K, Kitayama K (2007) Comparative study of additive basal area of conifers in forest ecosystems along elevational gradients. Ecol Res 22:439–450
Berg EE, Chapin FS III (1994) Needle loss as a mechanism of winter drought avoidance in boreal conifers. Can J For Res 24:1144–1148
Berry J, Björkman O (1980) Photosynthetic response and adaptation to temperature in higher plants. Ann Rev Plant Physiol 31:491–543
Bruhn D, Leverenz JW, Save H (2000) Effects of tree size and temperature on relative growth rate and its components of Fagus sylvatica seedlings exposed to two partial pressures of atmospheric CO2. New Phytol 146:415–425
Chabot BF, Hicks DJ (1982) The ecology of leaf life span. Annu Rev Ecol Syst 13:229–259
Eguchi T (2006) Climatic characteristics around the mountaintop. In: Ohsawa M, Tagawa H, Yamagaiwa J (eds) A world heritage: Yakushima Island—nature and ecosystem of the subtropics. Asakurashoten, Tokyo, pp 11–17 (in Japanese)
Field C, Mooney HA (1986) The photosynthesis–nitrogen relationship in wild plants. In: Givnish TJ (ed) On the economy of plant form and function. Cambridge University Press, Cambridge, pp 25–55
Gower ST, Reich PB, Son Y (1993) Canopy dynamics and aboveground production of five tree species with different leaf longevities. Tree Physiol 12:327–345
Grunderson CA, Norby RJ, Wullschleger SD (2000) Acclimation of photosynthesis and respiration to stimulated climatic warming in northern and southern populations of Acer saccharum: laboratory and field evidence. Tree Physiol 20:87–96
Hikosaka K, Hirose T (2000) Photosynthetic nitrogen-use efficiency in evergreen broad-leaved woody species coexisting in a warm-temperate forest. Tree Physiol 20:1249–1254
Hikosaka K, Nagamatsu D, Ishii HS, Hirose T (2002) Photosynthesis–nitrogen relationships in species at different altitudes on Mount Kinabalu, Malaysia. Ecol Res 17:305–313
Kikuzawa K (1983) Leaf survival of woody plants in deciduous broad-leaf forests. 1. Tall trees. Can J Bot 61:2133–2139
Kikuzawa K (1988) Leaf survival of tree species in deciduous broad-leaved forests. Plant Species Biol 3:67–76
Kikuzawa K (1991) A cost–benefit analysis of leaf habit and leaf longevity of trees and their geographical pattern. Am Nat 138:1250–1263
Kikuzawa K (1996) Geographical distribution of leaf life span and species diversity of trees simulated by a leaf-longevity model. Plant Ecol 122:61–67
Kikuzawa K, Lechowitz MJ (2006) Toward synthesis of relationships among leaf longevity, instantaneous photosynthetic rate, lifetime leaf carbon gain, and the gross primary production of forests. Am Nat 168:373–383
Kikuzawa K, Lechowitz MJ (2011) Ecology of leaf longevity. Springer, Tokyo
Kikuzawa K, Shirakawa H, Suzuki M, Umeki K (2004) Mean labor time of a leaf. Ecol Res 19:365–374
Koike T (1988) Leaf structure and photosynthetic performance as related to the forest succession of deciduous broad-leaved trees. Plant Species Biol 3:778–799
Koike T (1990) Autumn coloring, photosynthetic performance and leaf development of deciduous broad-leaved trees in relation to forest succession. Tree Physiol 7:21–32
Kudo G (1992) Effect of snow-free duration on leaf life-span of four alpine plant species. Can J Bot 70:1684–1688
Kudo G (1995) Leaf traits and shoot performance of an evergreen shrub, Ledium palustre ssp. decumbens, in accordance with latitudinal change. Can J Bot 73:1451–1456
Kudo G (1996) Intraspecific variation of leaf traits in several deciduous species in relation to length of growing season. Ecoscience 3:483–489
Kusumoto T (1978) Photosynthesis and respiration in leaves of main component species. In: Kira T, Ono Y, Hosokawa T (eds) Biological production in a warm-temperate evergreen oak forest of Japan JIBP SYNTHESIS, vol. 18. University of Tokyo, Tokyo, pp 88–98
Lewis JD, Olszyk D, Tingey DT (1999) Seasonal patterns of photosynthetic light response in Douglas-fir seedlings subjected to elevated atmospheric CO2 and temperature. Tree Physiol 19:243–252
Mediavilla S, Escudero A (2003) Photosynthetic capacity, accumulated over the lifetime of a leaf, is predicted to be independent of leaf longevity in some tree species. New Phytol 159:203–211
Menzel A, Fabian P (1999) Growing season extended in Europe. Nature 397:659
Monsi M, Saeki T (1953) Über den Lichtfackor in den Pflanzengesellschaten und seine Bedeutung für die Stoffproduktion. Jpn J Bot 14:22–52
Nomoto N (1964) Primary productivity of beech forest in Japan. Jpn J Bot 18:385–421
Ohsawa M (1990) An interpretation of latitudinal patterns of forest limits in south and east Asian mountains. J Ecol 78:326–339
Reich PB, Uhl C, Walters MB, Ellsworth DS (1991) Leaf life span as a determinant of leaf structure and function among 23 tree species in Amazonian forest communities. Oecologia 86:16–24
Reich PB, Walters MB, Elsworth DS (1992) Leaf life-span in relation to leaf, plant, and stand characteristics among diverse ecosystems. Ecol Monogr 62:365–392
Ruel JJ, Ayres MP (1996) Variation in temperature responses among populations of Betula papyrifera. Silva Fennica 30:145–158
Saxe H, Cannell MGR, Johnson Ø, Ryan MG, Vourlitis G (2001) Tree and forest functioning in response to global warming. New Phytol 149:369–399
Selaya NG, Anten NP (2010) Leaves of pioneer and later-successional trees have similar lifetime carbon gain in tropical secondary forest. Ecol 91:1102–1113
Suzuki E (1997) The dynamics of old Cryptomeria japonica forest on Yakushima Island. Tropics 6:421–428
Takada T, Kikuzawa K, Fujita N (2006) A mathematical analysis of leaf longevity of trees under seasonally varying temperatures, based on a cost–benefit model. Evol Ecol Res 8:605–615
Tang CO, Ohsawa M (1999) Altitudinal distribution of evergreen broad-leaved trees and their leaf-size pattern on a humid subtropical mountain, Mt. Emei, Sichuan. China Plant Ecol 145:221–233
Teskey RO, Will R (1999) Acclimation of loblolly pine (Pinus taeda) seedlings to high temperatures. Tree Physiol 19:519–525
Walter H (1973) Vegetation on the earth in relation to climate and the eco-physiological conditions. Springer, New York
Walther G-R (2002) Weakening of climatic constraints with global warming and its consequences for evergreen broad-leaved species. Folia Geobot 37:129–139
Walther G-R, Post E, Convey P, Menzel A, Parmesan C, Beebee TJC, Fromentin J-M, Hoegh-Guldberg O, Bairlein F (2002) Ecological responses to recent climate change. Nature 416:389–395
Wang KY, Kellomaki S, Laitinen K (1995) Effect of needle age, long-term temperature and CO2 treatments on the photosynthesis of Scots pine. Tree Physiol 15:211–218
Wang KY, Kellomaki S, Laitinen K (1996) Acclimation of photosynthetic parameters in Scots pine after three years exposure to elevated temperature and CO2. Agric For Meteorol 82:195–200
Way DA, Oren R (2010) Differential responses to changes in growth temperature between trees from different functional groups and biomes: a review and synthesis of data. Tree Physiol 30:669–688
Welp LR, Randerson JT, Liu HP (2007) The sensitivity of carbon fluxes to spring warming and summer drought depends on plant functional types in boreal forest ecosystems. Agric For Meteorol 147:172–185
Yim Y, Kira T (1975) Distribution of forest vegetation and climate in the Korean Peninsula. I. Distribution of some indices of thermal climate. Jpn J Ecol 25:77–88
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Fujita, N., Noma, N., Shirakawa, H. et al. Annual photosynthetic activities of temperate evergreen and deciduous broadleaf tree species with simultaneous and successive leaf emergence in response to altitudinal air temperature. Ecol Res 27, 1027–1039 (2012). https://doi.org/10.1007/s11284-012-0983-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-012-0983-z