Abstract
Increasing interest in the marine trophic dynamics of Pacific salmon has been motivated by the recognition of their sensitivity to changing climate and to the competitive effects of hatchery fish on wild stocks. It has become more common to use stable isotopes to supplement traditional diet studies of salmon in the ocean; however, there have been no integrated syntheses of these data to determine whether stable isotope analyses support the existing conventional wisdom of feeding strategies of the Pacific salmon. We performed a meta-analysis of stable isotope data to examine the extent of trophic partitioning among five species of Pacific salmon during their marine lives. Pink, sockeye, and chum salmon showed very high overlap in resource use and there was no consistent evidence for chum relying on alternative food webs dominated by gelatinous zooplankton. δ15N showed that Chinook and coho salmon fed at trophic levels higher than the other three species. In addition, these two species were distinctly enriched in 13C, suggesting more extensive use of coastal food webs compared to the more depleted (pelagic) signatures of pink, sockeye, and chum salmon. This paper presents the first synthesis of stable isotope work on Pacific salmon and provides δ15N and δ13C values applicable to research on the fate of the marine derived nutrients these organisms transport to freshwater and riparian ecosystems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There has been increasing interest in understanding the responses of Pacific salmon to marine climate variation (e.g., Pearcy 1992; Hare and Francis 1995; Mantua et al. 1997; Peterman et al. 1998; Hilborn et al. 2003) and to escalating hatchery stocking rates (e.g., Beamish et al. 1997; Cooney and Brodeur 1998; Ruggerone et al. 2003). Pacific salmon (Oncorhynchus spp.) spend from 1 to 5 years, depending on the species and population, feeding in the coastal and open oceans before migrating as adults to freshwater habitats to spawn (Groot and Margolis 1991). This marine stage comprises much of their lifetime mortality and most (>95%) of their growth (Groot and Margolis 1991; Quinn 2005). Traditional studies of the trophic ecology of salmon in the ocean have relied on direct diet habit studies (e.g., Pearcy et al. 1988; Brodeur and Pearcy 1992; Davis et al. 1996; Tadokoro et al. 1996; Davis 2003) and, arguably, have been relatively patchy in space and time relative to the scale of the North Pacific Ocean used by Pacific salmon for growth and maturation. In the last two decades there has been an increasing reliance on the stable isotope characteristics of salmon as a means to derive an integrated assessment of their marine foraging ecology (e.g., Welch and Parsons 1993; Kaeriyama et al. 2004). However, to date there has been no systematic synthesis of these data to determine whether the isotope derived patterns of trophic partitioning among the five North American species of Pacific salmon confirm the conventional wisdom regarding their trophic dynamics in the ocean.
The geographic range of North American Pacific salmon extends throughout the Subarctic North Pacific Ocean and Bering Sea. The migration patterns of sockeye, chum, and pink salmon have considerable overlap in the open ocean (Myers et al. 1996, reviewed in Quinn 2005). North American populations move north and west in coastal waters after entering the ocean, moving offshore into the pelagic North Pacific, Bering Sea, and Gulf of Alaska by the end of the first year at sea where they remain for approximately 1–3 years prior to their spawning migration back to freshwaters (Myers et al. 1996). Many populations of Chinook and coho, on the other hand, tend to remain in coastal waters after migration to the sea with only some populations migrating to the open ocean (Myers et al. 1996; Quinn 2005). Because there is substantial spatial overlap among salmon species, they likely compete for prey resources (Margolis et al. 1966; Godfrey et al. 1975; Burgner et al. 1992).
Marine trophic dynamics have important implications for population dynamics of Pacific salmon. For example, climatically driven changes in ocean productivity have been suggested to drive shifts in the abundance of potential prey for salmon, substantially changing long-term salmon production patterns (Mantua et al. 1997). Moreover, salmon populations may be affected through competitive interactions with other salmon species by shifting their major prey items in response to increases in inter- and intra-specific abundance of competitors (Tadokoro et al. 1996; Walker and Myers 1998; Ruggerone et al. 2003). For instance, sockeye salmon growth and survival declined in response to competition in years with high pink salmon abundance where the two species overlap spatially in the North Pacific and the Bering Sea (Kaeriyama et al. 2000; Bugaev et al. 2001; Ruggerone et al. 2003), suggesting significant overlap in prey resources.
Stomach-content analysis has been used extensively to evaluate the trophic partitioning of Pacific salmon during their marine phase. Common prey items of Pacific salmon include copepods, euphausiids, amphipods, myctophids, squid, and small fishes (Brodeur 1990; Brodeur and Pearcy 1992; Davis et al. 1996; Davis 2003). Although all salmon are considered trophic generalists, some trophic partitioning has been shown both among and within salmon species (LeBrasseur 1966; Pearcy et al. 1988; Brodeur 1990; Davis et al. 1996; Tadokoro et al. 1996). During their first year at sea, sockeye and pink salmon have similar diets that include zooplankton, small fishes, and squid (Brodeur 1990). However, during their second year at sea, pink salmon feed on larger prey than sockeye salmon (Brodeur 1990; Aydin 2000; Kaeriyama et al. 2000). Although chum salmon share many common prey items with other salmon species, they generally do not consume squid in the open ocean and they consume some unique prey items such as gelatinous zooplankton (Brodeur 1990). Coho salmon are known to be opportunistic foragers and mainly feed on prey fishes and invertebrates that are locally abundant in coastal habitats (Brodeur 1990; reviewed in Groot and Margolis 1991). Chinook salmon are mainly piscivorous and their diets reflect the regional prey abundance in coastal habitats as well (Brodeur 1990; Groot and Margolis 1991). Though the body of work is rich and informative, these stomach content analyses represent a snapshot in time, reflecting only the most recently consumed prey items (Gearing 1991) and may not necessarily reflect the diet of a single fish over time.
Stable isotopes of muscle tissues have been increasingly used to examine the trophic ecology of fishes. Muscle tissue integrates dietary composition over several months; therefore, isotope signatures may provide a different picture of dietary tendencies than diet samples (Tieszen et al. 1983; Hobson and Clark 1992). Stable isotope signatures of a consumer reflect two factors: the isotope composition of prey, and the systematic fractionation in isotope signatures that occurs during assimilation. δ15N increases by 1.3–5.3‰ (average = 3.4) per trophic transfer, while 13C trophic fractionation is subtle and δ13C increases 0–1‰ per trophic transfer (Minagawa and Wada 1984; Wada et al. 1987; Vander Zanden and Rasmussen 2001). Therefore, the δ15N signature of fish tissues is often used to infer the trophic position at which a particular fish fed during the last several months (Cabana and Rasmussen 1996; Vander Zanden and Rasmussen 1999). Because carbon fractionation is often negligible, δ13C in tissues reveal less about the trophic position of a consumer but more about the source of production in a food web (Peterson and Fry 1987; France 1995). In general, carbon in open-ocean marine phytoplankton is depleted in 13C compared to carbon fixed in coastal ecosystems (McConnaughey and McRoy 1979; Fry and Sherr 1984; Duggins et al. 1989). Differences in δ13C values can be an indicator of offshore versus coastal resources in supporting growth of consumers (Hobson et al. 1994). For example, Schell et al. (1998) showed a pattern of increasingly depleted δ13C values of zooplankton from on-shelf to pelagic regions in the Bering, Chukchi, and Beaufort seas. Thus, a combination of C and N stable isotope ratios in salmon tissue provides an integrated assessment of the degree of trophic overlap both in terms of trophic position and reliance on coastal versus open-ocean food webs.
Carbon and nitrogen stable isotopes have been used for characterizing differences in trophic ecology of Pacific salmon during their marine phase. For instance, Welch and Parsons (1993) suggested salmon form a trophic hierarchy where pink salmon feed low on the food chain followed in increasing order by sockeye, coho, and finally Chinook salmon, which feed at the highest trophic level. In addition, chum salmon used a different component of the marine food web altogether, likely one dominated by gelatinous zooplankton (Welch and Parsons 1993). Conversely, other studies have shown that chum salmon isotope signatures were similar to pink and sockeye salmon, suggesting that the trophic niches of these species overlap substantially (Satterfield and Finney 2002; Kaeriyama et al. 2004).
We performed a meta-analysis of new and previously published stable isotope data to characterize of the general trophic ecology of Chinook, coho, sockeye, chum, and pink salmon during their marine life-history phase. In particular, we evaluated the evidence for trophic partitioning among these five species of Pacific salmon and assessed the degree to which differences in isotope signature were associated with trophic position or habitat partitioning associated with pelagic versus coastal food webs.
Materials and methods
Our synthesis of stable isotopes in salmon consisted of two elements: collection and analysis of new samples from southwest Alaska salmon populations, and a meta-analysis including these data and previously published data to seek generalities in the stable isotope ecology of Pacific salmon in the ocean.
Tissue samples were collected from mature Pacific salmon as they entered spawning streams in the Wood River system of Bristol Bay, Alaska, and the Chignik River on the Alaska Peninsula. Salmon in these systems were sampled upon arrival to freshwater during their spawning season (June through September, 2002–2004). Sampling methods included angling, beach seining, and dip-netting. Locations were chosen because all five anadromous Pacific salmon species can be found in these systems.
Muscle tissue was collected from the dorsal musculature posterior to the dorsal fin and was eventually freeze dried and ground to a fine powder. Stable isotope analyses (of N and C) were performed at the University of California Davis stable isotope facility using a PDZ Europa Hydra 20–20 continuous-flow isotope ratio mass spectrometer. All δ13C and δ15N isotope values are reported versus the standard for carbon (VPDB) and nitrogen (atmospheric) as:
The measurement precision was estimated at 0.13‰ for δ15N and 0.05‰ for δ13C. Lipid content can alter δ13C values if the C:N ratio is greater than ~3.5 (Post et al. 2007). The C:N ratio for samples in this study was less than 3.5 therefore lipid correction may not be necessary for salmon muscle tissue. However, to be consistent with other studies compiled for the meta-analysis, we normalized for lipid content (δ′) according to McConnaughey (1978) and McConnaughey and McRoy (1979). Welch and Parsons (1993) data were not lipid corrected and C:N ratios were not given in the paper. Therefore, we applied a correction factor calculated for each species of our data to the same species data for Welch and Parsons (1993).
Synthesis of previous results for the meta-analysis was accomplished through a literature review of existing published Pacific salmon stable isotope (δ15N and δ13C) data. Data for Welch and Parsons (1993) were not listed in tabular format and were, therefore, digitized from Fig. 4 of their manuscript using Engauge Digitizer software. Data were included in the meta-analysis from studies where: species was given, salmon were not juveniles, sample size, and both δ13C and δ15N values were reported (Table 1).
A random-effects meta-analysis was conducted for the stable isotope data in this study (Cooper and Hedges 1994). Data were compiled using the mean and SD (standard deviation) by species in each study. The meta-analysis estimates the mean \( (\bar{\beta }) \) isotopic values for each species and the variance around that mean \( (\sigma_{\beta }^{2} ). \) We assumed individual study means were normally distributed.
The random-effects variance of \( \bar{\beta } \) for all studies \( (v_{i}^{*} ) \) is due to the variance related to measurement uncertainty (estimation variance = v i ) and the variance of each study mean (β i ) around the species mean \( \bar{\beta }(\sigma_{\beta }^{2} ): \)
The variance of \( \bar{\beta } \) among studies \( (\sigma_{\beta }^{2} ) \) is calculated from the independent measurements (k) that comprise the study mean β i and their estimation variances (v i ):
The species mean \( (\bar{\beta }) \) is calculated as a weighted average of the study means β i :
The variance of the species mean \( (\bar{\beta }) \) is:
We used reduced major axis regression analysis (RMA) to estimate the relationship between the δ15N and δ13C among the five salmon species both within each study considered in our meta-analysis, and across the species-specific means obtained from the meta-analysis. RMA is more appropriate than ordinary least squares (OLS) regression when the independent variable is measured with error, which produces a biased estimate of the slope (Sokal and Rohlf 1981). In this case, we assumed that both δ15N and δ13C were measured with error.
We compared the RMA slope between δ15N and δ13C among the five species of salmon to that which would be expected if this relationship was based on trophic fractionation of N and C isotopes alone, rather than by habitat partitioning among species. We used data from Vander Zanden and Rasmussen (1999), who compiled the trophic fractionation factors for N and C isotopes for several fishes in both field and laboratory situations. Assuming trophic fractionation factors are normally distributed and using first-order error propagation, we calculated the mean and variance of the expected slope of δ15N versus δ13C due entirely to trophic fractionation. We calculated the value of these slopes using either only field-derived data from Vander Zanden and Rasmussen (1999) or all the data they compiled. We then compared the observed value determined from the RMA regression to these distributions to assess the possibility that the observed relationship was determined by trophic fractionation of C and N isotopes alone.
Results
Stable isotope values from the samples collected from southwest Alaska salmon were within the ranges of those determined in previous studies (Table 1). Pink salmon were the least enriched and Chinook salmon were the most enriched in 15N from this region. There was much less inter-specific variation in δ13C (range ~1.4‰) than in δ15N (range ~3.4‰) and of the five species, chum salmon were the most depleted in 13C while Chinook salmon were the most enriched (Table 1).
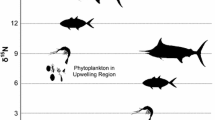
Individual studies indicated limited spatial and temporal difference in stable isotope signatures (Fig. 1) and different species exhibited relatively consistent isotope signatures across studies (Table 1). For instance, pink salmon generally had the most depleted and Chinook salmon had the most enriched δ15N signatures. Inter-study mean δ15N values ranged from 11.1 to 11.5‰ for sockeye, 10.6 to 12.0‰ for chum, 10.4 to 12.7‰ for pink, 11.6 to 13.8‰ for coho, and 13.3 to 15.2‰ for Chinook salmon. Inter-study means for δ13C ranged from −19.9 to −21.4‰ for sockeye, −20.3 to −22.5‰ for chum, −20.4 to −21.9‰ for pink, −18.6 to −21.8‰ for coho, and −17.9 to −20.5‰ for Chinook salmon. Thus, the meta-analysis revealed that Chinook exhibited the most enriched isotope signatures for both δ15N and δ13C followed by coho salmon. Sockeye, chum, and pink clustered closely together, with pink salmon tending to be the least enriched in both isotopes (Table 1, Fig. 2). Sockeye salmon showed the least variation in δ15N compared with other species (Fig. 2).
δ13C and δ15N values for studies where values for five Pacific salmon species are listed in Table 1. Each point represents the mean isotopic value for the species. Error bars are ±1 SD. Points without error bars in the Welch and Parsons (1993) are original data from paper. Points with error bars are lipid adjusted values
Average δ13C and δ15N values for Pacific salmon from a random-effects meta-analysis. Error bars are ±1 SD. Solid line is the RMA regression through the species means (slope = 1.55, p = 0.007, 95% CI = 0.75–2.11). Dashed lines represent contours of the expected relationship (slope = 17.5 per trophic level) based entirely on trophic fractionation of C and N among species
The slope of the RMA regression between δ13C and δ15N among all species means (Fig. 2) was 1.55 with a 95% confidence interval of 0.4–2.5. The compilation of trophic fractionation factors for C and N isotopes suggested that this observed slope for the five species of Pacific salmon was unlikely to be derived from trophic fractionation alone. For field studies, average trophic fractionation for δ15N was 3.4‰ (SD 0.2‰) per trophic level, and for δ13C was 0.2‰ (SD 0.5‰) per trophic level, yielding an average slope of 17.5. The uncertainty in this estimate was high (SD 10.5), yielding a distribution of likely slope values that was very broad. Based on this distribution, the slope of the observed relationship between the δ13C and δ15N among the five species of salmon (Fig. 2) fell in the lower 7% of the distribution of expected slopes based only on trophic fractionation alone (Fig. 3). Similarly, we calculated the most likely slope between δ15N and δ13C that would result only from trophic fractionation using all data from Vander Zanden and Rasmussen (2001) (i.e., field and laboratory estimates). This calculation yielded a lower slope (3.9) but with substantially less uncertainty (SD 2.3), thereby producing a much more constrained range of likely slope values (Fig. 3). The value of 1.55 obtained from our meta-analysis fell within the bottom 16% of this distribution (Fig. 3). Thus, while the relationship we observed between the average δ15N and δ13C among the five species of Pacific salmon may have been produced simply due to trophic fractionation differences in the metabolism of salmon, it is more likely that this relationship indicates differential habitat use by these species; namely that coho and Chinook salmon feed in food webs with more enriched δ13C signatures at the base of the food web.
Cumulative distributions of expected slopes describing the relationship between δ13C and δ15N based entirely on trophic fractionation. The distributions were calculated based on the data compiled by Vander Zanden and Rasmussen (1999). The solid line used data from both laboratory and field studies of isotope fractionation in fishes, while the dashed line was derived from field studies only. The arrow indicates the observed value of the slope describing the relationship between δ13C and δ15N among the five species of Pacific salmon (i.e., 1.55)
Discussion
This study is the first to synthesize the published stable isotope data for the five species of Pacific salmon. While there were small differences in isotopic characteristics of Pacific salmon among studies, there was also a distinct pattern among species across all studies.
Our analyses suggest there is a distinct pattern of trophic partitioning among species of Pacific salmon during their marine life stages. As expected, Chinook salmon occupied the highest trophic position, as indicated by enriched δ15N values, followed by coho salmon at about half a trophic level lower in the food web. Pink, sockeye, and chum salmon had remarkably high overlap in their isotopic composition, suggesting high overlap in their feeding strategies. Because comprehensive food-web data were not included in all the studies incorporated in our meta-analysis, we assumed the δ15N at the base of food webs to be roughly similar for all studies. Although this is not ideal for comparison, the pattern of trophic enrichment that we present here is consistent with that found in diet studies where coho and Chinook salmon feed at higher trophic positions than sockeye, pink, and chum salmon (Brodeur 1990; Quinn 2005).
Sockeye, pink, and chum salmon showed almost total overlap in isotopic values, which indicates that they may compete for food resources or at the very least feed on prey resources that occupy the same trophic level. Although Welch and Parsons (1993) hypothesized that chum salmon feed on a different branch of the food web dominated by jellyfish and gelatinous zooplankton, our synthesis of isotopic data from various sources reveals that chum do not consistently show distinctly different isotope values from sockeye or pink salmon. Diet studies have shown that in addition to feeding on jellyfish and gelatinous zooplankton, chum salmon share prey items common to other salmon species such as amphipods, euphausiids, pteropods, and fishes (Brodeur 1990; Tadokoro et al. 1996). However, stable isotopes may not always be able to partition gelatinous zooplankton from other food web components. Specifically, Brodeur et al. (2002) showed that hydromedusae have a δ15N signature indistinguishable from euphausiids, a common prey item of salmon and that jellyfish and gelatinous zooplankton showed a δ13C signature similar to or more enriched than salmon. Our result does not support Welch and Parsons (1993) hypothesis that the depleted δ13C carbon signature of chum salmon was due to their unique reliance on jellyfish as an energy source and highlights the need for a comprehensive study of trophic dynamics in addition to individual studies exploring spatial and temporal differences in feeding among and within species.
In general, coastal ecosystems (benthic based food webs) are enriched in δ13C compared to offshore (pelagic based food webs) (e.g., Fry 1981; Duggins et al. 1989; Jennings et al. 1997; Schell et al. 1998; Hobson 1999; Kline 1999; Kline et al. 2008). Studies of various marine organisms have shown differences in δ13C values obtained from coastal versus offshore environments (reviewed in Hobson 1999; Davenport and Bax 2000). Hobson et al. (1994) used δ13C values to identify seabirds that foraged nearshore (enriched) from those that fed offshore (depleted). Marine mammals using a nearshore environment had enriched δ13C values than those that foraged in a pelagic environment (Lusseau and Wing 2006; Sinisalo et al. 2006; Marcoux et al. 2007; Tucker et al. 2007). Studies in fishes have shown similar trends (Thomas and Cahoon 1993; Davenport and Bax 2000; Sherwood and Rose 2007).
Tissue δ13C in Pacific salmon suggested that inter-specific spatial partitioning occurs among these species. Chinook and coho salmon are widely known to use coastal regions and their more enriched δ13C values suggest they rely more heavily on organic energy sources produced in coastal habitats (Hobson et al. 1994; Schell et al. 1998) compared to the more open-ocean oriented pink, sockeye, and chum salmon that have distinctly depleted δ13C signatures. The coastal isotope signature of Chinook and coho salmon is in agreement with previous work showing that these species tend to remain in coastal waters after migration to sea, although many populations move offshore for some period of time (Groot and Margolis 1991; Quinn 2005). Additionally, maturing Chinook and coho are known to have a less direct route of migration to natal streams compared to sockeye, pink, and chum salmon; feeding more extensively in coastal waters during their migration (Quinn 2005).
In general, our results show salmon with enriched carbon signatures also had enriched nitrogen signals (Fig. 2). A positive relationship is expected based purely on trophic fractionation because both N and C become enriched in their heavier isotopes as organic matter is passed up through food webs (Peterson and Fry 1987). The expected slope of this relationship, based entirely on trophic fractionation is broadly defined based on existing data derived from field situations (Fig. 3). However, the slope of the regression through the species means was distinctly lower than the bulk of the distribution of expected values based on trophic fractionation alone (Figs. 2, 3). Although the relationship observed among the five species of salmon was more comparable to the slope calculated from data derived from both laboratory and field studies, it still falls in the lowest 16% of the expected distribution. This comparison indicates that that the species are not simply feeding at different trophic levels in the same ecosystem (Fig. 2). Rather, this indicates that species are obtaining their carbon from different sources, specifically, that Chinook and coho appear to rely more heavily on prey resources that are enriched in δ13C as is characteristic of nearshore or coastal food webs. This result suggests confirmation of conventional wisdom regarding the heavier use of coastal resources by coho and Chinook salmon (Quinn 2005).
The degree of trophic overlap among salmon species has important implications for understanding impacts of large hatchery programs on other species. There is increasing evidence that large hatchery releases of pink and chum salmon, through resource competition, may negatively impact ocean survival of wild populations of sockeye, pink, and chum salmon (e.g., Beamish et al. 1997; Ruggerone et al. 2003; Ruggerone and Nielsen 2004; Zaporozhets and Zaporozhets 2004). Specifically, the release of high densities of hatchery salmon competing for food resources could reduce the availability of prey resources for wild fish in times of diminished forage production and have negative consequences for wild stocks (Beamish et al. 1997). In fact, Cooney and Brodeur (1998) modeled the forage demand in coastal and oceanic feeding habitats by hatchery and wild pink salmon originating from Prince William Sound, Alaska, and found that annual food consumption tripled after hatchery production dominated the returns for these stocks. Our results help provide mechanistic insight into these findings—we observed a high degree of trophic overlap among species, suggesting that large releases of hatchery pink and chum salmon in the North Pacific may have negative effects on wild populations of pinks, chum and sockeye through resource competition.
A comprehensive understanding of the spatial and temporal context of trophic relationships among species is needed to model the carrying capacity of the North Pacific for salmon (Brodeur and Pearcy 1992; Pearcy 1992; Cooney and Brodeur 1998). This understanding becomes especially important when considering enhancement of stocks with hatchery fish, which may have consequences for both intra- and inter-specific competition. Generally, models assume that Chinook and coho salmon feed at higher trophic positions than pink, sockeye, and chum (Pearcy et al. 1988; Brodeur 1990; Groot and Margolis 1991) as is reflected in recent ecosystem models of the North Pacific Ocean (e.g., Aydin et al. 2005). We suggest extensive trophic overlap for chum, sockeye, and pink salmon feeding in the open ocean. Additionally, Chinook and coho salmon show trophic overlap in the coastal ocean. However, our meta-analysis, along with diet and tagging studies, show that it is unlikely that all five species spatially overlap with each other for extended periods of time during the marine phase of their life cycles. Ecosystem models should include separation between coastal and pelagic processes if these models are meant to capture the dynamics of all Pacific salmon species in the North Pacific.
In conclusion, our results suggest that there is high overlap in the trophic ecology of pink, sockeye, and chum salmon, which is relatively distinct from coho and Chinook salmon. Trophic differentiation of coho and Chinook may be a function of habitat preference (i.e., more benthic/coastal food webs compared to the pelagic food webs of pink, sockeye, and chum) corroborating previous diet studies (Brodeur 1990; Groot and Margolis 1991; Quinn 2005). Our analyses illuminate the inter-specific variation in stable isotope signatures of marine salmon and contribute to our understanding of the trophic and spatial partitioning of different salmon species feeding in the North Pacific. Although intra-specific spatial and temporal differences exist, this study highlights the overall pattern of isotope signatures in the five species of Pacific salmon and will be of use to studies examining the marine contribution of salmon to freshwater and terrestrial food webs (Gende et al. 2002; Naiman et al. 2002; Schindler et al. 2003).
References
Aydin KY (2000) Trophic feedback and carrying capacity of Pacific salmon (Oncorhynchus spp.) on the high seas in the Gulf of Alaska. PhD dissertation, University of Washington
Aydin KY, McFarlane GA, King JR, Megrey BA, Myers KW (2005) Linking oceanic foodwebs to coastal production and growth rates of Pacific salmon (Oncorhynchus spp.), using models on three scales. Deep Sea Res Pt II 52(5-6):757–780
Beamish RJ, Mahnken C, Neville CM (1997) Hatchery and wild production of Pacific salmon in relation to large-scale, natural shifts in the productivity of the marine environment. ICES J Mar Sci 54(6):1200–1215
Ben-David M (1996) Seasonal diets of mink and martens: effects of spatial and temporal changes in resource abundance. PhD dissertation, University of Alaska, Fairbanks
Bilby RE, Fransen BR, Bisson PA (1996) Incorporation of nitrogen and carbon from spawning coho salmon into the trophic system of small streams: evidence from stable isotopes. Can J Fish Aquat Sci 53(1):164–173. doi:10.1139/cjfas-53-1-164
Brodeur RD (1990) A synthesis of the food habits and feeding ecology of salmonids in marine waters of the north Pacific. FRI-UW-9016, Fisheries Research Institute, University of Washington, Seattle, USA, pp 38
Brodeur RD, Pearcy WG (1992) Effects of environmental variability on trophic interactions and food web structure in a pelagic upwelling ecosystem. Mar Ecol Prog Ser 84(2):101–119. doi:10.3354/meps084101
Brodeur RD, Sugisaki H, Hunt GL Jr (2002) Increases in jellyfish biomass in the Bering Sea: implications for the ecosystem. Mar Ecol Prog Ser 233:89–103. doi:10.3354/meps233089
Bugaev VF, Welch DW, Selifonov MM, Grachev LE, Eveson JP (2001) Influence of the marine abundance of pink salmon (Oncorhynchus gorbuscha) and sockeye salmon (O. nerka) on growth of Ozernaya River sockeye. Fish Oceanogr 10(1):26–32. doi:10.1046/j.1365-2419.2001.00150.x
Burgner RL, Light TJ, Margolis L, Okazaki T, Tautz A, Ito S (1992) Distributions and origins of steelhead trout (Oncorhynchus mykiss) in offshore waters of the North Pacific Ocean. In: Int North Pac Fish Comm Bull, vol 51
Cabana G, Rasmussen JB (1996) Comparison of aquatic food chains using stable nitrogen isotopes. Proc Natl Acad Sci USA 93(20):10844–10847. doi:10.1073/pnas.93.20.10844
Chaloner DT, Martin KM, Wipfli MS, Ostrom PH, Lamberti GA (2002) Marine carbon and nitrogen in southeastern Alaska stream food webs: evidence from artificial and natural streams. Can J Fish Aquat Sci 59(8):1257–1265. doi:10.1139/f02-084
Cooney RT, Brodeur RD (1998) Carrying capacity and North Pacific salmon production: stock-enhancement implications. Bull Mar Sci 62(2):443–464
Cooper H, Hedges LV (1994) Handbook of research synthesis. Russell Sage Foundation, New York
Davenport SR, Bax NJ (2000) A trophic study of a marine ecosystem off southeastern Australia using stable isotopes of carbon and nitrogen. Can J Fish Aquat Sci 59:514–530. doi:10.1139/f02-031
Davis ND (2003) Feeding ecology of Pacific salmon (Oncorhynchus spp.) in the central North Pacific Ocean and central Bering Sea, 1991–2000. Ph.D. dissertation, Hokkaido University, Hakodate, Japan
Davis ND, Takahashi M, Ishida Y (1996) The 1996 Japan–U.S. cooperative high-seas salmon research cruise of the Wakatake maru and a summary if 1991–1996 results. In: North Pac Anadr Fish Comm Doc. 194. FRI-UW-9617. Fish Res Inst, University of Washington, Seattle, 45 pp
Duggins DO, Simenstad CA, Estes JA (1989) Magnification of secondary production by kelp detritus in coastal marine ecosystems. Science 245(4914):170–173. doi:10.1126/science.245.4914.170
France RL (1995) Carbon-13 enrichment in benthic compared to planktonic algae: foodweb implications. Mar Ecol Prog Ser 124(1-3):307–312. doi:10.3354/meps124307
Fry B (1981) Natural carbon stable-isotope tag traces Texas shrimp migrations. Fish Bull US 79:337–345
Fry B, Sherr EB (1984) Delta-C-13 measurements as indicators of carbon flow in marine and fresh-water ecosystems. Contrib Mar Sci 27:13–47
Gearing NG (1991) The study of diet and trophic relationships through natural abundance of 13C. In: Coleman DC, Fry B (eds) Carbon isotope techniques. Harcourt (Brace Jovanovich), New York
Gende SM, Edwards RT, Willson MF, Wipfli MS (2002) Pacific salmon in aquatic and terrestrial ecosystems. Bioscience 52(10):917–928. doi:10.1641/0006-3568(2002)052[0917:PSIAAT]2.0.CO;2
Godfrey H, Henry KA, Machidori S (1975) Distribution and abundance of coho salmon in offshore waters of the North Pacific Ocean. In: Int North Pac Fish Comm Bull, vol 31. Vancouver, BC, Canada
Groot C, Margolis L (eds) (1991) Pacific salmon life histories. University of British Columbia Press, Vancouver
Hare SR, Francis RC (1995) Climate change and salmon production in the northeast Pacific Ocean. In: Beamish RJ (ed) Climate change and northern fish populations, pp 357–372. Can Spec Publ Fish Aquat Sci, p 121
Hilborn R, Quinn TP, Schindler DE, Rogers DE (2003) Biocomplexity and fisheries sustainability. Proc Natl Acad Sci USA 100(11):6564–6568. doi:10.1073/pnas.1037274100
Hobson KA (1999) Tracing origins and migration of wildlife using stable isotopes: a review. Oecologia 120:314–326. doi:10.1007/s004420050865
Hobson KA, Clark RG (1992) Assessing avian diets using stable isotopes I: turnover of 13C in tissues. Condor 94:181–188. doi:10.2307/1368807
Hobson KA, Piatt JF, Pitocchelli J (1994) Using stable isotopes to determine seabird trophic relationships. J Anim Ecol 63(4):786–798. doi:10.2307/5256
Jennings S, Renones O, Morales-Nin B, Polunin NVC, Moranta J, Coll J (1997) Spatial variation in the 15N and 13C stable-isotope composition of plants, invertebrates and fishes on Mediterranean reefs: implications for the study of trophic pathways. Mar Ecol Prog Ser 146:109–116. doi:10.3354/meps146109
Kaeriyama M, Nakamura M, Yamaguchi M, Ueda H, Anma G, Takagi S, Aydin KY, Walker RV, Myers KW (2000) Feeding ecology of sockeye and pink salmon in the Gulf of Alaska. North Pac Anadr Fish Comm Bull 2:55–63
Kaeriyama M, Nakamura M, Edpalina R, Bower JR, Yamaguchi H, Walker RV, Myers KW (2004) Change in feeding ecology and trophic dynamics of Pacific salmon (Oncorhynchus spp.) in the central Gulf of Alaska in relation to climate events. Fish Oceanogr 13(3):197–207. doi:10.1111/j.1365-2419.2004.00286.x
Kline TC (1999) Temporal and spatial variability of 13C/12C and 15N/14N in pelagic biota of Prince William Sound, Alaska. Can J Aquat Fish Sci 56 (Suppl 1):94–117. doi:10.1139/cjfas-56-S1-94
Kline TC, Boldt JL, Farley EV, Haldorson LJ, Helle JH (2008) Pink salmon (Oncorhynchus gorbuscha) marine survival rates reflect early marine carbon source dependency. Prog Oceanogr 77:194–202. doi:10.1016/j.pocean.2008.03.006
LeBrasseur RJ (1966) Stomach contents of salmon and steelhead trout in the northeastern Pacific Ocean. J Fish Res Board Can 23(1):85–100
Lusseau SM, Wing SR (2006) Importance of local production versus pelagic subsidies in the diet of an isolated population of bottlenose dolphins Tursiops sp. Mar Ecol Prog Ser 321:283–293. doi:10.3354/meps321283
Mantua NJ, Hare SR, Zhang Y, Wallace JM, Francis RC (1997) A Pacific interdecadal climate oscillation with impacts on salmon production. Bull Am Meteorol Soc 78(6):1069–1079. doi :10.1175/1520-0477(1997)078<1069:APICOW>2.0.CO;2
Marcoux M, Whitehead H, Rendell L (2007) Sperm whale feeding variation by location, year, social group and clan: evidence from stable isotopes. Mar Ecol Prog Ser 333:309–314. doi:10.3354/meps333309
Margolis L, Cleaver FC, Fukuda Y, Godfrey H (1966) Salmon of the North Pacific Ocean. VI: Sockeye salmon in offshore waters. In: Int North Pac Fish Comm Bull, vol 20. Vancouver, BC, Canada
McConnaughey T (1978) Ecosystems naturally labeled with carbon-13: applications to the study of consumer food webs. MS Thesis, University of Alaska, Fairbanks, 127 pp
McConnaughey T, McRoy CP (1979) Food-web structure and the fractionation of carbon isotopes in the Bering Sea. Mar Biol (Berl) 53(3):257–262. doi:10.1007/BF00952434
Minagawa M, Wada E (1984) Stepwise enrichment of δ15N along food chains: further evidence and the relationship between δ15N and animal age. Geochim Cosmochim Acta 48(5):1135–1140. doi:10.1016/0016-7037(84)90204-7
Myers KW, Aydin KY, Walker RV, Fowler S, Dahlberg ML (1996) Known ocean ranges of stocks of Pacific salmon and steelhead as shown by tagging experiments, 1956–1995. University of Washington, School of Fisheries, Fish Res Inst, FRI-UW-9614. Seattle, p 229
Naiman RJ, Bilby RE, Schindler DE, Helfield JM (2002) Pacific salmon, nutrients, and the dynamics of freshwater and riparian ecosystems. Ecosystems (N.Y., Print) 5(4):399–417. doi:10.1007/s10021-001-0083-3
Pearcy WG (1992) Ocean ecology of North Pacific salmonids. Washington Sea Grant Program. University of Washington Press, Seattle
Pearcy WG, Brodeur JM, Shenker JM, Smoker WW, Endo Y (1988) Food habits of Pacific salmon and steelhead trout, midwater trawl catches and oceanographic conditions in the Gulf of Alaska 1980–1985. Bull Oceanogr Res Inst 26:29–78
Peterman RM, Pyper BJ, Lapointe MF, Adkison MD, Walters CJ (1998) Patterns of covariation in survival rates of British Columbian and Alaskan sockeye salmon (Oncorhynchus nerka) stocks. Can J Fish Aquat Sci 55:2503–2517. doi:10.1139/cjfas-55-11-2503
Peterson BJ, Fry B (1987) Stable isotopes in ecosystem studies. Annu Rev Ecol Syst 18:293–320. doi:10.1146/annurev.es.18.110187.001453
Piorkowski RJ (1995) Ecological effects of spawning salmon on several southcentral Alaskan streams. PhD dissertation. University of Alaska, Fairbanks
Post DM, Layman CA, Arrington DA, Takimoto G, Quattrochi J, Montana CG (2007) Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable-isotope analyses. Oecologia 152(1):179–189. doi:10.1007/s00442-006-0630-x
Quinn TP (2005) The behavior and ecology of Pacific salmon and trout. University of Washington Press, Seattle
Ruggerone GT, Nielsen JL (2004) Evidence for competitive dominance of pink salmon (Oncorhynchus gorbuscha) over other salmonids in the North Pacific. Rev Fish Biol Fish 14(3):371–390. doi:10.1007/s11160-004-6927-0
Ruggerone GT, Zimmerman M, Myers KW, Nielsen JL, Rogers DE (2003) Competition between Asian pink salmon (Oncorhynchus gorbuscha) and Alaskan sockeye salmon (O. nerka) in the North Pacific Ocean. Fish Oceanogr 12(3):209–219. doi:10.1046/j.1365-2419.2003.00239.x
Satterfield FR, Finney BP (2002) Stable-isotope analysis of Pacific salmon: insight into trophic status and oceanographic conditions over the last 30 years. Prog Oceanogr 53(2-4):231–246. doi:10.1016/S0079-6611(02)00032-0
Schell DM, Barnett BA, Vinette KA (1998) Carbon and nitrogen isotope ratios in zooplankton of the Bering, Chukchi, and Beaufort seas. Mar Ecol Prog Ser 162:11–23. doi:10.3354/meps162011
Schindler DE, Scheuerell MD, Moore JW, Gende SM, Francis TB, Palen WJ (2003) Pacific salmon and the ecology of coastal ecosystems. Front Ecol Environ 1(1):31–37
Sherwood GD, Rose GA (2007) Stable-isotope analysis of some representative fish and invertebrates of the Newfoundland and Labrador continental shelf food web. Estuar Coast Shelf Sci 63:537–549. doi:10.1016/j.ecss.2004.12.010
Sinisalo TE, Valtonen T, Helle E, Jones RI (2006) Combining stable isotope and intestinal parasite information to evaluate dietary differences between individual ringed seals (Phoca hispida botnica). Can J Zool 84:823–831. doi:10.1139/Z06-067
Sokal RR, Rohlf FJ (1981) Biometry, 2nd edn. Freeman, New York
Tadokoro K, Ishida Y, Davis ND, Ueyanagi S, Sugimoto T (1996) Change in chum salmon (Oncorhynchus keta) stomach contents associated with fluctuations of pink salmon (O. gorbuscha) abundance in the central subarctic Pacific and Bering Sea. Fish Oceanogr 5(2):89–99. doi:10.1111/j.1365-2419.1996.tb00108.x
Thomas CJ, Cahoon LB (1993) Stable-isotope analyses differentiate between different trophic pathways supporting rocky-reef fishes. Mar Ecol Prog Ser 95:19–24. doi:10.3354/meps095019
Tieszen LL, Boutton TW, Tesdahl KG, Slade NA (1983) Fractionation and turnover of stable carbon isotopes in animal issue: implications for δ13C analysis of diet. Oecologia 57(1-2):32–37. doi:10.1007/BF00379558
Tucker S, Bowen WD, Iverson SJ (2007) Dimensions of diet segregation in grey seals Halichoerus grypus revealed through stable isotopes of carbon (δ13C) and nitrogen (δ15N). Mar Ecol Prog Ser 339:271–282. doi:10.3354/meps339271
Vander Zanden MJ, Rasmussen JB (1999) Primary consumer δ15N and δ13C and the trophic position of aquatic consumers. Ecology 80(4):1395–1404. doi:10.1890/0012-9658(1999)080[1395:PCCANA]2.0.CO;2
Vander Zanden MJ, Rasmussen JB (2001) Variation in 15N and 13C trophic fractionation: implications for aquatic food web studies. Limnol Oceanogr 46(8):2061–2066
Wada E, Terazaki M, Kabaya Y, Nemoto T (1987) 15N and 13C abundances in the Antarctic Ocean with emphasis on the biogeochemical structure of the food web. Deep Sea Res 34(5-6):829–841. doi:10.1016/0198-0149(87)90039-2
Walker RV, Myers KW (1998) Growth studies from 1956–1995 collections of pink and chum salmon scales in the central North Pacific Ocean. North Pac Anadr Fish Comm Bull 1:54–65
Welch DW, Parsons TR (1993) 13C and 15N values as indicators of trophic position and competitive overlap for Pacific salmon (Oncorhynchus spp.). Fish Oceanogr 2:11–23. doi:10.1111/j.1365-2419.1993.tb00008.x
Zaporozhets OM, Zaporozhets GV (2004) Interaction between hatchery and wild Pacific salmon in the Far East of Russia: a review. Rev Fish Biol Fish 14(3):305–319. doi:10.1007/s11160-005-3583-y
Acknowledgments
This work is a contribution of the University of Washington Alaska Salmon Program, funded by the National Science Foundation (Biological Oceanography, Biocomplexity), the Gordon and Betty Moore Foundation, the Alaska salmon processors, and the University of Washington. We thank G. Holtgrieve, J. Moore, W. Palen, L. Rogers, M. Winder, P. Westley, and J. Wittouck for assistance in obtaining salmon tissue samples, T. Essington for analytical assistance, N. Davis for comments on earlier manuscript drafts, and J. Carter and C. Ruff for assistance in sample preparation.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Johnson, S.P., Schindler, D.E. Trophic ecology of Pacific salmon (Oncorhynchus spp.) in the ocean: a synthesis of stable isotope research. Ecol Res 24, 855–863 (2009). https://doi.org/10.1007/s11284-008-0559-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-008-0559-0