Abstract
Rhizobial inoculation has a positive impact on plants growth; however, there is little information about its effect on soil microbial communities and their activity in the rhizosphere. It was therefore necessary to test the effect of inoculation of Acacia senegal (L.) Willd. seedlings with selected rhizobia on plant growth, structure and diversity of soil bacterial communities and soil functioning in relation to plant provenance and soil origin. In order to carry out this experiment, three A. senegal seeds provenance from Kenya, Niger, and Senegal were inoculated with selected rhizobial strains. They have been further grown during 4 months in greenhouse conditions in two non-disinfected soils, Dahra and Goudiry coming respectively from arid and semi-arid areas. The principal component analysis (ACP) showed an inoculation effect on plant growth, rhizospheric bacterial diversity and soil functioning. However, the performances of the rhizobial strains varied in relation to the seed provenance and the soil origin. The selected rhizobial strains, the A. senegal provenance and the soil origin have modified the structure and the diversity of soil bacterial communities as measured by principal component analysis/denaturing gradient gel electrophoresis analyses. It is interesting to note that bacterial communities of Dahra soil were highly structured according to A. senegal provenance, whereas they were structured in relation to rhizobial inoculation in Goudiry soil. Besides, the impact of inoculation on soil microbial activities measured by fluorescein diacetate analyses varied in relation to plant provenance and soil origin. Nevertheless, total microbial activity was about two times higher in Goudiry, arid soil than in Dahra, semi-arid soil. Our results suggest that the rhizobial inoculation is a suitable tool for improving plants growth and soil fertility. Yet, the impact is dependent on inoculants, plant provenance and soil origin. It will, therefore, be crucial to identify the appropriate rhizobial strains and plant provenance or species in relation to the soil type.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil microbes maintain the soil structure and facilitate the degradation of organic matter, nutrient cycling and carbon sequestration in all terrestrial ecosystems, (Groffman and Bohlen 1999). Zak et al. (1995) suggest that the high environmental variability and resource heterogeneity associated with arid ecosystems may increase microbial functional diversity. This means that microorganisms play a pivotal role in nutrient availability, plant growth and plant health. The soil microbial community plays a central role in the turnover of organic matter and the cycling of almost all major plant nutrients (Doran and Zeiss 2000; Xu et al. 2009). It is therefore a key factor that influences the functioning of the ecosystem and the sustainability of the soil resource (Sparling 1997). Soil microbes secrete extracellular enzymes that enhance the decomposition of organic matter and the transformation of nitrogen compounds (Koch 1916). The soil enzyme activities reflects the dynamics of microbial metabolic processes associated with nutrient cycling and are sensitive indicators of environmental stresses caused by the degradation of the quality of the soil. In this respect, microbial parameters have been used as indicators of soil health (Alkorta et al. 2003; Franchini et al. 2007) because soil microorganisms are sensitive to land use and management and can be used to indicate soil health (Sparling 1997; Chen et al. 2000; Gomez et al. 2000; Li et al. 2004; Zhang and Xu 2008).
Furthermore, various types of soil managements can increase soil microbial biomass and activity (Balota et al. 2003) and populations of beneficial microorganisms such as the nitrogen-fixing bacteria (Hungria and Vargas 2000). For example, rhizoidal inoculation of legumes can increase N2 fixation and plant yield, improve the seed quality (Khan et al. 1997; Saini et al. 2004) and the plants abiotic stress tolerance (Diouf et al. 2005). However, despite the positive impact of rhizobial inoculation on plants growth, little information is available about the effect of rhizobial inoculation on soil microbial communities and their activity in the rhizosphere. Furthermore, introduction of microbial agents into the environment may cause adverse perturbations of the native soil microbiota and the nutrient turnover processes in which they are involved (Johansen et al. 2005).
Change in soil functioning and microbial community in the rhizosphere seems to be related to the plant host. Van Dillewijn et al. (2002) has determined the effect of Ensifer meliloti on the rhizospheric microbial community of alfafa (Medicago sativa), its host plant. They made the finding that the plant appeared to have a much stronger influence on the microbial community compared with an E. meliloti inoculation. Schwieger and Tebbe (2000), on their part, studied the impact of rhizobial inoculant, E. meliloti, on the microbial communities of M. sativa rhizosphere and found out that both plant species and inoculants had an effect on the rhizosphere structure.
However, other studies have reported that shift of microbial community after inoculation would depend on the type of soil. Recently, Nimnoi et al. (2010) have concluded that the differences of bacterial community structure depend greatly on plants and soil rather than on inoculants. Therefore, the bacterial communities and diversity in rhizosphere depend highly on their ability to take advantage of a specific environment or to adapt and change conditions. On the other hand, Costa et al. (2006) have demonstrated that the plant species exert a greater influence on the rhizosphere microbial communities than the sampling sites. While, Kotani-tanoi et al. (2007) demonstrate that microbial diversity is affected not only by host plants but also by soil compositions.
Acacia senegal, a legume nitrogen-fixing species, has been chosen for this experiment as it is important for reclaiming degraded and abandoned agricultural lands because of its capability to improve soil properties (Njiti and Galiana 1996). This study therefore aims at highlighting the impact of inoculation of A. senegal seedlings with selected rhizobia on plants growth, rhizospheric soil bacteria diversity and structure and, on soil functioning in relation to the A. senegal seeds provenance and the type of soil.
Materials and methods
Soils samplings
The soils used for the experiment were collected in Senegal during the dry season in April 2008 from Dahra (Lat. 15° 21 N; Long. 15° 29 W) and Goudiry (Lat. 14° 11 N; Long. 12° 43 W). The average rainfall falling from June to October in Dahra and Goudiry zones have been represented in Table 1. The soils have been sampled from the top 25 cm of rhizospheric soil of A. senegal plantations. They have been passed through a coarse sieve (2 mm mesh) to remove stones and large pieces of organic matter. Then, physico-chemical analyses have been conducted at LAMA (Laboratoire des moyens analytiques), IRD, Dakar, Senegal (Table 2).
Plant growing and rhizobial inoculation
Acacia senegal (L.) Willd. seeds have been collected from Dahra in Senegal, Tera in Niger and Makueni in Kenya. Seeds scarification and germination have been achieved as described by Fall et al. (2008). Seedlings have been transplanted individually into 12 cm x 8 cm plastic bags filled with non-sterilized soil from Dahra and Goudiry. The planted bags have been kept in greenhouse conditions (25 °C day, 20 °C night, 10 h photoperiod). The seedlings have then be inoculated with 5 ml bacterial suspension, containing about 109 cells ml−1 of each strain, or 5 ml of the culture medium without incorporated bacteria for the control treatments. The nine rhizobial strains used in this study for the inoculation tests have been isolated from A. senegal rhizospheric soils in Dahra and Goudiry (Table 2) and representing the IGS profiles defined by RFLP (data not given). These rhizobial strains were selected on the basis of their symbiotic infectivity and effectiveness. The 16S sequences of rhizobial strains were then submitted to Genbank and were allocate with the following accession numbers: ORS 3573 (JQ039728), ORS 3574 (JQ039729), ORS 3588 (JQ039735), ORS 3593 (JQ039736), ORS3600 (JQ039741), ORS 3604 (JQ039739), ORS 3607 (JQ039737), ORS 3610 (JQ039732), ORS 3628 (JQ039740). Two rhizobial strains have been added in the study as reference, CiradF 300 (Sarr et al. 2005) and ORS 3416 (Fall et al. 2008).
Experimental design
The experimental design was a randomized complete block. Each block was divided into seven plots; two plots represented soil origins (Dahra and Goudiry); three plots represented seed provenances (Senegal, Niger and Kenya); two plots represented the inoculation treatment (inoculated separately with eleven rhizobial strains and non-inoculated control). Each plot had twelve repetitions. After 4 months of growth, shoots, roots and nodules have been separated and weighed after drying (at 80 °C for 3 days) and the dry weight recorded. Shoot nutrient concentrations (total N, total P and total C) have been evaluated on crushed shoot dry matter. Thus, for each A. senegal provenance associated to a soil origin, four inoculation treatments were selected for their efficiency on the basis of plants total dry matter and non-inoculated control. The analysis was carried out at LAMA (Laboratoire des Moyens Analytiques) IRD, Dakar, Senegal. The data were analysed using SPSS software version 13 and the means were compared with the Student–Newman–Keuls range test (P < 0.05). A principal component analysis (PCA) has also been carried out on the basis of the following parameters: inoculation treatments, plants total dry matter, shoot nutrient concentrations (N, P and C), rhizospheric bacterial diversity and soil functioning using XLSTAT version 2010.
PCR/DGGE of rhizospheric soil samples of plants
The aim of the DGGE analysis was to highlight the impact of inoculation with selected rhizobia on rhizospheric bacterial community diversity and structure in relation to plant provenance and soil origin.
After 4 months of growth, structure and diversity of bacterial community in rhizospheric soil of seedlings were evaluated for the most efficient four inoculation treatments and the control as nutrient concentrations analysis. A composite soil sample was obtained after pooling the rhizospheric soil of the twelve plants inoculated with the same rhizobial strain. The soil samples have been sieved through a 2 mm grid, crushed and then stored at 4 °C in clean plastic bags to stabilize the microbial activity. Bacterial community diversity in soil samples collected from bags has then been investigated using denaturing gradient gel electrophoresis (DGGE) of amplified 16S rRNA gene fragments.
Extraction of soil total DNA
Three replicates have been made for each soil sample. The total DNA was extracted from 0.5 g aliquots of soil by adding 0.2 g glass beads (Sigma, 0.1 mm) and 1 ml lysing buffer (0.25 M NaCl, 0.1 M EDTA; pH 8) and by bead-beating the suspension (Bead-beater, Biospec products, Qiagen) twice for 2 min with 2 min heat treatment (65 °C) in between. The subsequent steps have been carried out as described by Porteous et al. (1997) and as modified by Fall et al. (2004). The crude DNA was purified with the fast DNA purification kit, Wizard DNA clean-up System (Promega) in accordance with the manufacturer’s instructions. The DNA quantification was performed by fluometry with Geldoc software version 1000 (Bio-Rad Laboratories) using the DNA quantification kit fluorescence assay (Sigma, DNA-QF).
PCR/DGGE analysis
The PCR amplification targeting total 16S rDNA bacterial community has been performed using the eubacterial primer pair 338f-GC (Ovreas et al. 1997) and 518r (Muyzer et al. 1993). The DNA extracted from the three replicates was pooled for each soil sample. PCR-DGGE analysis of total soil bacterial community was then performed as described by Assigbetse et al. (2005). DGGE analysis has been carried out by using 8 % acrylamide gels [acrylamide- bisacrylamide 40 % (37.5:1)] with a 45–70 % denaturant gradient, where 100 % denaturant was defined as 7 M urea plus 40 % formamide. Total Lab 120 version 2006 software (Nonlinear Dynamics Ltd) has then been used to calculate the percentage of similarity among lanes by taking into account the migration distance and the relative intensity of all the bands. DGGE profiles were then compared and unweighted pair group method cluster analysis has been used to produce the dendrograms.
The species richness on DGGE gels (R) has been calculated as the number of bands presents (Vivas et al. 2009). The structural diversity of the bacterial community has then been examined with the Shannon index of general diversity H′ (Shannon and Weaver 1963). In order to carry out these analyses, each band has been presumed to represent the ability of the given bacterial species to be amplified (Vivas et al. 2009). The intensity of the bands was reflected as peak heights in the densitometry curve. The Shannon H′ has been calculated from the following equations:
Pi = ni N−1; ni = height of peak and N = sum of all peak heights in the curve.
Soil microbial activity
The soil functioning has been studied on the same samples that were used for shoot nutrient concentrations analysis and bacterial diversity studies. The total microbial activity in soil samples has been measured with the fluorescein diacetate (3′, 6′,-diacetylfluorescein [FDA]) hydrolysis assay according to the method of Alef (1998) in which fluorescein released has been assessed calorimetrically at 490 nm, after 1 h of soil incubation. The total microbial activity is expressed as μg of product adjusted for background fluorescence per hour and per gram of soil.
Results
Total dry weight of A. senegal provenances
The degree of A. senegal seedlings response to inoculation with selected rhizobial strains varied according to the rhizobial strain, the provenance and the soil origin (Fig. 1). For each soil, the efficiency of rhizobial strains varied depending on the provenances used. Rhizobial inoculation increased Senegalese seedlings provenance growth regardless of the soil origin. However, the highest inoculation effect was observed for soil originating from the arid area of Dahra (Fig. 1a). Save from the inoculation with the strain ORS 3628, the total dry matter was significantly greater in both inoculated treatments in comparison with the non-inoculated control in Goudiry soil. The highest growth (92 % compared to the non-inoculated control) was observed for seedlings inoculated with the strain ORS 3604.
Total dry weight of seedlings of 3 A. senegal provenances collected from Senegal (a), Niger (b), and Kenya (c) as influenced by inoculation with selected rhizobia strains at the end of four months of culture under greenhouse conditions on non sterile Senegalese soils sampled from arid (Dahra) and semi-arid areas (Goudiry). For each soil origin, means of values affected to the same letter are not significantly different at 5 % Student–Newman–Keuls test
Rhizobial inoculation increased the growth of the Niger’s seedlings provenance, regardless of the soil origin (Fig. 1b). However, and similarly to the Senegalese provenance, the inoculation effect was more notable for Dahra soil. Indeed, a significant difference was observed for the growth of seedlings inoculated with the rhizobial stains ORS 3573 and ORS 3604. The seedlings growth was increased by 62 and 68 %, respectively by inoculation with the strains ORS 3573 and ORS 3604 when compared to the non-inoculated control seedlings. For the Niger’s provenance the inoculation effect was less marked in Goudiry soil. The total dry weight was significantly improved only when seedlings were inoculated with the strain ORS 3604 (91 %) as compared to the non-inoculated control seedlings. The negative effects of inoculation were shown on growth of seedlings grown in Goudiry soil and inoculated with the strains ORS 3573 and ORS 3628, where the total dry weight was reduced by 23 and 17 %, respectively, as compared to the non-inoculated plants.
For the Kenyan provenance, it was interesting to note that the rhizobial inoculations treatments improved significantly the total dry matter of plants in Goudiry soil (Fig. 1c). The best performance was observed for seedlings inoculated with the strain ORS 3593. The plant total dry weight was increased by 124 % in comparison to the non-inoculated control. On Dahra soil, inoculation enhanced the plants total dry weight. However, there was a significant difference between the treatments.
Inoculation impact on shoot nutrient concentrations
Results presented in Table 4 show that the overall shoot nutrient contents (total N, P and C) of inoculated seedlings was higher as compared to non-inoculated control. However, the degree of improvement varied according to the plant provenance and soil origin. The nutrient contents were obviously higher in the Kenyan A. senegal provenance, irrespective of the soil origin.
In Dahra soil, total P and total C contents of the Senegalese seedlings provenance were significantly increased by the rhizobial inoculation. Inoculations with the strains CiradF 300, ORS 3574, and ORS 3604 improved total N by 128, 160 and 183 %, respectively. As concerns the Niger’s provenance, both inoculation treatments significantly improved concentrations of total N, total P and total C. For the Kenyan provenance, a significant effect was noted only on treatments inoculated with the strain ORS 3607 on total N (118 %) and total P (135 %) concentrations. The total C content was increased by inoculation with the strains ORS 3416 (102 %), CiradF 300 (116 %) and ORS 3607 (118 %) but inoculation with the strain ORS 3610 reduced total C by 102 %.
Inoculation treatments of the Senegalese seedlings provenance grown in Goudiry soil significantly increased total P, total C and total N concentrations in the seedlings shoot. However, rhizobial inoculation with the strain ORS 3604 reduced the total N content by 114 %. In contrast, for the Niger’s provenance, concentrations of total N and total C were significantly enhanced by inoculation with the strain ORS 3604. Nevertheless, inoculation has improved the total N, P and C contents when compared to the non-inoculated control. For the Kenyan provenance rhizobial inoculation improved significantly the shoot nutrient contents (N, P and C), except for the strain ORS 3604.
Structure of bacterial community
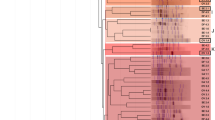
Figures 2 and 3 showed the comparative effects of inoculation of A. senegal seedlings with selected rhizobial strains on bacterial structure of rhizospheric soils after 4 months in greenhouse conditions in Dahra and Goudiry soils, respectively. The rhizobial strain, the A. senegal provenance and the soil modified the structure of soil bacterial communities.
a Denaturing gradient gel electrophoresis (DGGE) of 16S rDNA of total soil bacterial communities in rhizosphere soil of seedlings of 3 A. senegal provenances collected from Senegal, Niger, and Kenya as influenced by inoculation with selected rhizobia strains at the end of four months of culture under greenhouse conditions on non sterile Senegalese soil sampled from arid area (Dahra). b Similarities between PCR-DGGE profiles obtained from bacterial communities
a Denaturing gradient gel electrophoresis (DGGE) of 16S rDNA of total soil bacterial communities in rhizosphere soil of seedlings of 3 A. senegal provenances collected from Senegal, Niger, and Kenya as influenced by inoculation with selected rhizobia strains at the end of four months of culture under greenhouse conditions on non sterile Senegalese soil sampled from semi-arid area (Goudiry). b Similarities between PCR-DGGE profiles obtained from bacterial communities
The dendrogram in Fig. 2 shows the relatedness of PCR-DGGE fingerprints from Dahra rhizospheric soil displaying two clusters with about 91 % similarity. It is interesting to note that bacterial communities of Dahra soil were highly structured according to the A. senegal provenance. In Dahra soil, the rhizobial inoculation has had less effect on the bacterial structure. However, differences were observed among bacterial communities of the inoculation treatments for each provenance. Bacterial communities of rhizospheric soil samples from the Senegalese provenance formed one cluster. While, bacterial communities of Kenyan and Nigerien provenances formed another cluster. For the sub-cluster formed by Kenyan provenance, inoculation with the strain CiradF 300 influenced the structure of the bacterial community as compared with the control. However, in the sub-cluster of the Nigerien provenance, all the strains inoculated affected the bacterial structure. In the second cluster formed by Senegalese provenance, inoculation with the strains ORS 3593 and ORS 3574 influenced the structure of bacterial community.
The dendrogram in Fig. 3 shows three clusters with about 92 % similarity. In contrast to Dahra soil, the provenance effect on the structure of bacterial community in Goudiry soil was less marked. The structure of the bacterial community corresponded to the strain used as inoculum. The first cluster was composed of treatments of the Kenyan provenance inoculated with the strains ORS 3416 and ORS 3607 and the Senegal provenance inoculated with the strains ORS 3600 and ORS 3604. The first sub-cluster of the second cluster was formed by the non-inoculated control Kenyan seedlings provenance and two others inoculation treatments. The second sub-cluster of cluster two was made up of inoculated treatments of Niger provenance and its control and also the two inoculated treatments of Senegalese provenance. The control of Senegalese provenance was the sole component of cluster three.
Diversity of bacterial community
The results showed that the species richness had the same tendency as the diversity of bacterial communities in the rhizospheric soil (Tables 3, 4). Quite independently from the inoculation treatment, the species richness (R) diversity indexes values for DGGE profiles were higher in Goudiry than in Dahra rhizosphere soils: 60 and 50, respectively. Some inoculation treatments increased the genetic diversity of bacterial community regardless of their efficiency. Inoculation of the Senegalese provenance with the strain ORS 3574 increased the bacterial diversity of rhizospheric Dahra soil while an antagonistic effect was observed for the genetic diversity of the bacterial community of rhizospheric soil of Niger’s provenance when inoculated with the same strain (Table 3).
Soil functioning
The impact of inoculation on soil microbial activities varied depending on the plant provenances. This means that the rhizobial inoculation may improve the soil microbial activity. However, this activity varied according to the origin of the soil. Similarly to the diversity of bacterial community, the total microbial activity was about two times higher in semi-arid Goudiry soils than in arid Dahra soils. As evidenced by the values of the species richness (R) diversity indexes, the values of total microbial activities in Dahra soil were higher with Senegalese and Kenyan seedlings provenances (Fig. 4). For Niger’s seedlings provenance, any difference could be drawn between inoculation treatments and non- inoculated control. Total microbial activity of Senegalese provenance rhizospheric soil was increased by 8 and 10 % when inoculated, respectively with the rhizobial strains ORS 3593 and CiradF 300. In contrast, total microbial activity was reduced by 2 % when seedlings were inoculated with the strain ORS 3604, even though this strain improved the plant growth and nutrient content. Inoculation of the Kenyan seedlings provenance with the strain ORS 3607 increased the total microbial activity by 5 %. However, inoculation with the other strains obviously reduced the total microbial activity when compared with the control (Table 5).
Total microbial activity as measured by fluorescein diacetate method (FDA) in rhizosphere soil of seedlings of 3 A. senegal provenances collected from Senegal, Niger, and Kenya as influenced by inoculation with selected rhizobia strains at the end of 4 months of culture under greenhouse conditions on non sterile Senegalese soil sampled from arid area (Dahra)
For Goudiry soil, inoculation increased the total microbial activity in plant rhizospheric soil of the Senegalese provenance (Fig. 5). The effect was notable for all strains used as inoculums but was higher for the strains ORS 3600 and ORS 3416 whose activity was respectively increased by 38 and 33 %. Niger’s A. senegal provenance showed the same tendency. The total microbial activities of treatments inoculated with the strain ORS 3593 and ORS 3604 were increased by 24 and 27 %, respectively for Kenya provenance.
Total microbial activity as measured by fluorescein diacetate method (FDA) in rhizosphere soil of seedlings of 3 A. senegal provenances collected from Senegal, Niger, and Kenya as influenced by inoculation with selected rhizobia strains at the end of four months of culture under greenhouse conditions on non sterile Senegalese soil sampled from semi-arid area (Goudiry)
Correlation between inoculation, plants and soil parameters
Figure 6 shows the component analysis carried out on soil originating from Dahra and Goudiry with correlation matrixes using the following parameters: inoculation, plants total dry matter, shoot nutrient contents (C, N, P), rhizospheric bacterial diversity and soil functioning. These variables were condensed into two principal components which were both extracted and accounted for 54.89 and 56.08 % of the variance for Dahra and Goudiry soils, respectively. The principal component analysis showed an average effect of inoculation on plant growth, rhizospheric bacterial diversity and soil functioning. The results showed that the performances of rhizobial strains varied according to the origin of the soil.
Principal component analysis (PCA) distribution of inoculation treatments, plants total dry matter and nutrient contents, and culture soil properties. Plants subjected to inoculation with selected rhizobia strains and soils were harvested at the end of four months of culture under greenhouse conditions on non sterile Senegalese soils sampled from Dahra (a) and Goudiry (b). TDM: plants total dry matter; N: N concentration (%); P: P concentrations (%); C: C concentration (gkg−1); H′: Shannon index; R: species richness; FDA: total microbial activity. DS: Dahra Senegal; DN: Dahra Niger; DK: Dahra Kenya; GD: Goudiry; Dahra: GN: Goudiry Niger; GK: Goudiry Kenya; their letters were followed by rhizobial strains or Control (C)
For Dahra soil, the results presented in Fig. 6a show that the positive values of the axis 1 (PC1), diversity index (H′) and species richness (R) were significantly related to each other. While, shoot N content was correlated negatively with the soil bacterial diversity. The axis 2 (PC2) was positively and significantly correlated with C and negatively correlated with plants total dry matter. The first component may be interpreted as soil rhizospheric bacterial diversity while the second may be interpreted as plants C concentrations. The diagram resolves in four major clusters. Cluster A (treatments of Senegalese provenance inoculated with the strain ORS 3593, Kenyan provenance inoculated with the strains ORS 3416 and CiradF 300) was related to high soil bacterial diversity. Cluster B (treatments of Niger’s provenance inoculated with the strains ORS 3573, ORS 3604, ORS 3588 and the non-inoculated control) was separated from cluster A and linked to N concentrations. Cluster C with the non-inoculated control seedlings of the Kenyan provenance was linked to C concentrations. Treatments of Senegalese provenance inoculated with the strain ORS 3604 was linked to plants total dry matter in Cluster D.
On the soil originating from Goudiry (Fig. 6b), plants total dry matter were positively and significantly linked to PC1, more than with C concentrations however, the diversity index (H′) and (R) were negatively and significantly correlated to PC1. The FDA was positively and significantly correlated to PC2. PC1 would explain plants total dry matter while PC2 may be interpreted as soil total microbial activity (FDA). Three different clusters were defined in PCA plane. Cluster A (treatments of Kenyan provenance inoculated with the strains ORS 3604, ORS 3607 and ORS 3416) associated to plants total dry matter and C concentrations. Cluster B including treatments of Senegalese provenance inoculated with the strain ORS 3588, Kenyan and Senegalese control were linked to soil bacterial diversity and was inversely proportional to cluster A. Nigerien and Kenyan provenances inoculated respectively with the strains ORS 3607 and ORS 3593 formed cluster C, which was characterized by high soil total microbial activities (FDA).
Discussion
The stated aim of this study was to assess the effect of inoculation of A. senegal seedlings by selected rhizobial strains on plant growth, structure and diversity of soil bacterial communities and soil functioning in relation to plant provenance and soil origin.
The results show that inoculation with selected rhizobial strains has increased the total dry matter of A. senegal seedlings grown in greenhouse conditions. In addition, the importance of the increase in total dry matter depends on seed provenance which indicates that the effectiveness of a rhizobial strain varies not only according to plant species but also according to plant provenances. Therefore, in order to enhance the success of inoculation, the plant genotype has to be taken into account as suggested by Sarr et al. (2005) and Bala and Giller (2006). In addition to the compatibility between the rhizobial strain and the plant genotype, the success of rhizobial inoculation may often be limited by several factors, such as environmental conditions and the presence of competing indigenous rhizobia. This could explain the negative effect of inoculation of some rhizobial strains in plant growth for the Niger’s provenance in Goudiry soil.
Our results have also shown that rhizobial inoculation have enhanced shoot total N, total P and total C concentrations. They also show that as what happens with plant growth, the values of nutrient contents depends on the seed provenances. The values of nutrient concentrations of Kenyan provenance were higher regardless of the soil origin which suggests that A. senegal provenances used in this study have different mineral status.
In Dahra soil, originating from an arid area, the bacterial community was highly structured accordingly to the A. senegal provenance, showing that in this soil, A. senegal influences the bacterial community structure in the rhizosphere (Fig. 2). Similar results were reported by van Dillewijn et al. (2002) and Junier et al. (2009) with alfafa and bean, respectively. The rhizosphere community may be altered by changes in root exudates composition caused by the plant nutritional status (Sandnes et al. 2005). In effect, Smalla et al. (2001) have reported that different plant species select bacterial community in the vicinity of their roots and that these plants specific enrichments may be increased by repeated cultivation of the plant species in the same field.
The results of this experiment show that, the structure of bacterial community also varies according to the origins of the soil. Similar results were arrived at by Nimnoi et al. (2010), who suggested that the difference of the microbial community structures depended heavily on plants and soils rather than on inoculants. The difference on the bacterial community structure of the same provenance observed between Dahra and Goudiry soils might be due to the difference in soils particle size (Sessitsch et al. (2001) or soil composition (Kotani-tanoi et al. 2007). In effect, Goudiry soil contains a rate of clay significantly higher than in Dahra soil (Table 2).
In this study, the dendrogram generated with Goudiry semi-arid soil has shown a dispersion of the plant provenance samples inoculated with rhizobia strains (Fig. 3). Hence, the structure of bacterial communities in the rhizosphere of A. senegal was not correlated to the plant provenance. This indicates that the shift of the structure of bacterial communities is influenced by inoculation as well as by soil physico-chemical characteristics.
In contrast to data presented by Zhang et al. (2010) which reporting a reduction of the microbial biomass C in faba bean rhizosphere, our results show that, rhizobial inoculation can improve soil total microbial activity. Rhizobial inoculation could improve microbial biomass or diversity through favorable microenvironment caused by plant root exudates and then, enhances the total microbial activity in the soil. The results show that the values of total microbial activity are two times higher on clay soils originating from Goudiry. These results match those reported by Dick (1994) and Acosta-Martinez et al. (2008), which demonstrated that many soil enzyme activities increase with clay content. Clay content provides physical protection to organic matter and enzymes, and can both directly and indirectly affect the soil’s biological status as suggested by Aşkın and Kızılkaya (2006).
The results of component analysis show that the correlation between inoculation, plant growth, nutrient contents, rhizospheric bacterial diversity and soil functioning varies according to the origin of the soil. A shift has been noted on the behavior of rhizobial strains regarding their performances to improve plants growth and soil parameters. These results suggest that the impact of microbial inoculation on plant or rhizospheric soil may change with environmental conditions. Some of rhizobial strains used as inoculums were very effective for increasing one or two parameters. For example, the strain ORS 3604 was correlated to N concentrations in Dahra soil however it was associated to total dry matter and C concentrations in Goudiry soil. It is therefore suggested that microbial inoculation with a rhizobial mixture would be most beneficial for expecting to enhance plants and soil parameters when respective rhizobial performances are taken into account. PCA shows that species richness is positively correlated to diversity of bacterial communities for each provenance and soil origin as shown by Felske and Osborn (2005), who define diversity as the function of the species richness and the relative abundance of individual species.
To conclude, this study carried out on A. senegal provenances shows that differences on bacterial diversity, structure and soil functioning depend on inoculants, plant provenances and soil origin. Therefore, inoculation with selected rhizobial strains is a suitable tool for increasing plant growth, bacterial structure and diversity and soil functioning in plant rhizosphere. However, for each soil origin, the plant species or provenances and rhizobial strain must be objectively selected. It will be interesting to use an inoculums mixture for taking account plant growth, soil bacterial diversity and functioning.
Abbreviations
- DGGE:
-
Denaturing gradient gel electrophoresis
- EDTA:
-
Ethylenediamine tetraacetic
- FDA:
-
Fluorescein diacetate
- PCA:
-
Principal component analysis
- PCR:
-
Polymerase chain reaction
- RFLP:
-
Restriction fragment length polymorphism
References
Acosta-Martinez V, Acosta-Mercado D, Sotomayor-Ramirez D, Cruz-Rodriguez L (2008) Microbial communities and enzymatic activities under different management in semi-arid soils. Appl Soil Ecol 38:249–260
Alef K (1998) Estimation of hydrolysis of fluorescein diacatate. In: Alef K, Nannipieri P (eds) Methods in applied soil microbiology and biochemistry. Acacdemic Press, London, pp 232–233
Alkorta I, Amezaga I, Albizu I, Aizpurua A, Onaindia M, Buchner V, Garbisu C (2003) Molecular microbial biodiversity assessment: a biological indicator of soil health. Rev Environ Health 18:131–151
Aşkın T, Kızılkaya R (2006) Assessing spatial variability of soil enzyme activities in pasture topsoils using geostatistics. Eur J Soil Biol. doi:10.1016/j.ejsobi.2006.02.002
Assigbetse K, Gueye M, Thioulouse J, Duponnois R (2005) Soil bacterial diversity responses to root colonization by an ectomycorrhizal fungus are not root-growth-dependent. Microbiol Ecol 50:350–359
Bala A, Giller KE (2006) Relationships between rhizobial diversity and host legume nodulation and nitrogen fixation in tropical ecosystems. Nutr Cycl Agroecosys 76:319–330
Balota EL, Colozzi-Filho A, Andrade DS, Dick RP (2003) Microbial biomass in soils under different tillage and crop rotation systems. Biol Fert Soils 38:15–20
Chen C, Condron LM, Davis M, Sherlock RR (2000) Effects of afforestation on phosphorus dynamics and biological properties in a New Zealand grassland soil. Plant Soil 220:151–163
Costa R, Götz M, Mrotzek N, Lottmann J, Berg G, Smalla K (2006) Effects of site and plant species on rhizosphere community structure as revealed by molecular analysis of microbial guilds. FEMS Microbiol Ecol 56:236–249
Dick RP (1994) Soil enzyme activities as indicators of soil quality. In: Doran JW, Jones AJ (eds) Defining soil quality for a sustainable environment. Soil Sci Soc Am J Madison WI, p. 107
Diouf D, Duponnois R, Ba AT, Neyra M, Lesueur D (2005) Symbiosis of Acacia auriculiformis and A. mangium with mycorrhizal fungi and Bradyrhizobium sp. improves salt tolerance in greenhouse conditions. Funct Plant Biol 32:1143–1152
Doran JW, Zeiss MR (2000) Soil health and sustainability: managing the biotic component of soil quality. Appl Soil Ecol 15:3–11
Fall S, Nazaret S, Chotte JL, Brauman A (2004) Bacterial density and community structure associated with aggregate size fractions of soil-feeding termite mounds. Microb Ecol 48:191–199
Fall D, Diouf D, Ourarhi M, Faye A, Abdelmoumen H, Neyra M, Sylla SN, Missbah El Idrissi M (2008) Phenotypic and genotypic characteristics of root-nodulating bacteria isolated from A. senegal (L.) Willd. In the dryland part of Senegal. Lett Appl Microbiol 47:85–97
Felske A, Osborn AM (2005) DNA fingerprint of microbial communities. In: Osborn AM, Smith CJ (eds) Molecular microbial ecology, 1st edn. Taylor and francis group, New York, pp 65–90
Franchini JC, Crispino CC, Souza RA, Torres E, Hungaria M (2007) Microbiological parameters as indicators of soil quality under various soil management and crop rotation systems in southern Brazil. Soil Till Res 92:18–29
Gomez E, Bisaro V, Conti M (2000) Potential C-source utilization patterns of bacterial communities as influenced by clearing and land use in a vertic soil of Argentina. Appl Soil Ecol 15(2):73–281
Groffman PM, Bohlen PJ (1999) Soil and sediment biodiversity: cross-system comparisons and large scale effects. Bioscience 49:139–148
Hungria M, Vargas MAT (2000) Environmental factors affecting N2 fixation in grain leguminous in the tropics, with an emphasis on Brazil. Field Crop Res 65:151–164
Johansen A, Jensen L, Knudsen IMB, Binnerup SJ, Winding A, Johansen JE, Jensen LE, Andersen KS, Svenning MM, Bonde TA (2005) Non-target effects of the microbial control agents Pseudomonas fluorescens DR54 and Clonostachys rosea IK726 in soils cropped with barley followed by sugar beet: a greenhouse assessment. Soil Biol Biochem 37:2225–2239
Junier P, Junier T, Witzel KP, Caru M (2009) Composition of diazotrophic bacterial assemblages in bean-planted soil compared to unplanted soil. Eur J Soil Sci 45:153–162
Khan MS, Zaidi A, Amil M (1997) Associative effect of Bradyrhizobium sp. (Vigna) and phosphate solubilizing bacteria on mungbean [Vigna radiata (L.) Wilczek]. Biojournal 9:101–106
Koch GP (1916) Diastase activity and invertase activity of bacteria. Soil Sci 1:179–196
Kotani-tanoi T, Nishitama M, Otsuka S, Senoo K (2007) Single particle analysis reveals that bacterial community structures are semi-specific to the type of soil particle. Soil Sci Plant Nutr 53:740–743
Li Q, Lee AH, Wollum AG (2004) Microbial biomass and bacterial functional diversity in forest soils: effects of organic matter removal, compaction, and vegetation control. Soil Biol Biochem 36:571–579
Muyzer G, de Wall E, Uitterlinden A (1993) Profiling of complex microbial populations by DGGE of PCR-amplified genes coding for 16S rRNA. Appl Environ Microbiol 59:695–700
Nimnoi P, Lumyong S, Pongsilp N (2010) Impact of rhizobial inoculants on rhizosphere bacterial communities of three medicinal legumes assessed by denaturing gradient gel electrophoresis (DGGE). Ann Microbiol. doi:10.1007/s13213-010-0128-y
Njiti CF, Galiana A (1996) Symbiotic properties and rhizobium requirements for effective nodulation of five tropical dry zone Acacias. Agrofor Syst 43:265–275
Ovreas L, Forney L, Daae FL, Torsvik V (1997) Distribution of bacterioplankton in meromictic Lake Saelenvannet, as determined by denaturing gradient gel electrophoresis of PCRamplified gene fragments coding for 16S rRNA. Appl Environ Microbiol 63:3367–3373
Porteous LA, Seidler RJ, Watrud LS (1997) An improved method for purifying DNA from soil for polymerase chain reaction amplification and molecular ecology applications. Mol Ecol 6:787–791
Saini VK, Bhandari SC, Tarafdar JC (2004) Comparison of crop yield, soil microbial C, N and P, N-fixation, nodulation and mycorrhizal infection in inoculated and non-inoculated sorghum and chickpea crops. Field Crop Res 89:39–47
Sandnes A, Eldhuset TD, Wollebæk G (2005) Organic acids in root exudates and soil solution of Norway spruce and silver birch. Soil Biol Biochem 37:259–269
Sarr A, Diop B, Peltier R, Neyra M, Lesueur D (2005) Effect of rhizobial inoculation methods and host plant provenances on nodulation and growth of Acacia senegal and Acacia nilotica. New For 29:75–87
Schwieger F, Tebbe CC (2000) Effect of field inoculation with the strain E. meliloti L33 on the composition of bacterial communities in rhizosphere of a target plant (Medicago sativa) and non-target plant (Chenopodium album)-linking of 16S rRNA gene-based single stand conformation polymorphism community profiles to the diversity of cultivated bacteria. Appl Environ Microbiol 66:3556–3565
Sessitsch A, Weilharter A, Gerzabek MH, Kirchmann H, Kandeler E (2001) Microbial population structures in soil particle size fractions of a long-term fertilizer field experiment. Appl Environ Microbiol 67:4215–4224
Shannon CE, Weaver W (1963) The mathematical theory of communication. The University of Illinois Press, Urbana
Smalla K, Wieland G, Buchner A, Zock B, Parzy J, Kaiser S, Roskot N, Heuer H, Berg G (2001) Bulk and rhizosphere soil bacterial communities studied by denaturing gradient gel electrophoresis: plant-dependent enrichment and seasonal shifts revealed. Appl Environ Microbiol 67:4742–4751
Sparling GP (1997) Soil microbial biomass, activity and nutrient cycling as indicators of soil health. In: Pankhurst CE, Doube BM, Gupta VVSR (eds) Biological indicators of soil health. CAB International, pp 97–119
Van Dillewijn P, Villadas PJ, Toro N (2002) Effect of a Sinorhizobium meliloti strain with a modified putA gene on the rhizosphere microbial community of alfafa. Appl Environ Microbiol 68:4201–4208
Vivas A, Moreno B, García-Rodriguez S, Benítez E (2009) As assessing the impact of composting and vermicomposting on structural diversity of bacterial communities and enzyme activities of an olive-mill waste. Bioresource Technol 100:1319–1326
Xu ZH, Chen CR, He J, Liu J (2009) Trends and challenges in soil research 2009: linking global climate change to local long-term forest productivity. J Soil Sediment 9:83–88
Zak JC, Sinsabaugh R, MacKay W (1995) Windows of opportunity in desert ecosystems: their implications to fungal community development. Can J Bot 73:S1407–S1414
Zhang L, Xu ZH (2008) Assessing bacterial diversity in soil: a brief review. J Soil Sediment 8:379–388
Zhang NN, Sun YM, Li L, Wang ET, Chen WX, Yuan HL (2010) Effects of intercropping and Rhizobium inoculation on yield and rhizosphere bacterial community of faba bean (Vicia faba L.). Biol Fert Soils 46:625–639
Acknowledgments
This work was funded by ACACIAGUM INCO STREP project Contract Number 032233. The authors are grateful to French Embassy in Senegal (SCAC) for a fellowship. We thank Mr. Lamine Dieng for his technical assistance in PCR/DGGE analysis and; Mr. Ibou Sene (Dahra) and Mr. Dembele (Goudiry) for soil sampling.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bakhoum, N., Ndoye, F., Kane, A. et al. Impact of rhizobial inoculation on Acacia senegal (L.) Willd. growth in greenhouse and soil functioning in relation to seed provenance and soil origin. World J Microbiol Biotechnol 28, 2567–2579 (2012). https://doi.org/10.1007/s11274-012-1066-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-012-1066-6