Abstract
Biodegradation of pentachlorophenol (PCP) in soil by autochthonous microorganisms and in soil bioaugmented by the bacterial strain Comamonas testosteroni CCM 7530 was studied. Subsequent addition of organomineral complex (OMC) or lignite as possible sorbents for PCP immobilization has been investigated as well. The OMC was prepared from humic acids (HAs) isolated from lignite by binding them onto zeolite. Biodegradation of PCP and number of colony forming units (CFUs) were determined in the three types of soil, Chernozem, Fluvisol, and Regosol, freshly spiked with PCP and amended separately with tested sorbents. The enhancing effect of sorbent addition and bioaugmentation on PCP biodegradation depended mainly on the soil type and the initial PCP concentration. Microbial activity resulted in biotransformation of PCP into certain toxic substances, probably lower chlorinated phenols that are more soluble than PCP, and therefore more toxic to present biota. Therefore, it was necessary to monitor soil ecotoxicity during biodegradation. Addition of the OMC resulted in a more significant decrease of soil toxicity in comparison with addition of lignite. Lignite and OMC appear to be good traps for PCP with potential application in remediation technology.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organic soil pollution is an emergent environmental problem. Significant quantities of lower chlorinated phenols and pentachlorophenol (PCP) were previously used in several agricultural applications. PCP or its sodium salt has also been used as an herbicide and desiccant for feed seed crops, for non-food vegetation control, and as an insecticide for use in beehives, seed plots, and greenhouses. Moreover, PCP was formerly used in upland rice fields, particularly in Japan (Crosby et al. 1981).
The widespread use of technical PCP and its physical and chemical properties (water solubility and volatility) led to ubiquitous contamination of air, soil, water, and sediments. Depending on the soil type, PCP can be very mobile, potentially leading to contamination of groundwater and hence, of drinking water. Organic pollutants such as pesticides and phenolic compounds may be detoxified by incorporation as an integral part of the humic molecules that represent a substantial part of soil organic matter (Tan 2003). A large portion (20–70%) of a particular chemical that reaches the terrestrial system becomes sequestered or bound to soil (Calderbank 1989). The soil organic matter has significant effect on PCP adsorption as well (Otte et al. 1999). Leaching of chemicals reversibly bound on soil particles interrelates with factors such as adsorption, water solubility of the substance, soil type, moisture, percolation velocity, and pH. Many soil microorganisms produce extracellular oxidoreductases capable of catalyzing the coupling of aromatic compounds. These enzymes are classified as either peroxidases or polyphenol oxidases (Bollag 2002). The effect of the soil properties such as content of soil organic matter on PCP biodegradation was investigated by Pu and Cutright (2007). Naumova et al. (2002) assumed higher detoxification activity of the coal HAs due to higher amounts of aromatic compounds in coal and lignite HAs responsible for immobilization of the contaminants in comparison with agricultural soil.
Pentachlorophenol remains an important environmental pollutant, however, even after several decades of bioremediation research, there is still little predictability regarding how to implement soil clean-up operations. Technologies employing biological procedures aimed at detoxification of organic pollutants-contaminated soils have been widely used in an increasing scale of applications over the past few years and are viewed as being both cost-effective and environmentally friendly. The use of bioremediation depends on several factors such as the type, concentration, and bioavailability of contaminants, organic and inorganic nutrients supply, and contaminated site history. One of the bioremediation techniques is bioaugmentation, which involves the addition of external microbial strains (indigenous or exogenous) with the ability to degrade the target toxic molecules (Bae et al. 1996–1997; Bulankina et al. 2007; Odokuma and Dickson 2003).
Microorganisms can partially degrade xenobiotics converting them to non-toxic products or to more reactive derivatives that may be resistant to further degradation. Therefore, monitoring of ecotoxicity of soil during biodegradation is required. Moreover, chlorophenols may affect the fluidity of the microbial membrane (Dercová et al. 2004) and reveal their toxic effect disturbing the electrochemical proton gradient by transporting protons back across the microbial membrane and inhibiting electron flow by binding to certain components of electron transport chain (Escher et al. 1997).
This work was focused on soil bioaugmentation and subsequent sorbents addition with the aim to decrease high initial concentration of PCP by immobilization, and then to facilitate PCP degradation and consequently to decrease soil toxicity. Lignite, zeolite, and humic acids used as potential sorbents are natural substances that are cost-effective, harmless, and therefore suitable for use in the environment. Humic acids were bound onto zeolite (furtheron, organomineral complex or OMC) and then added to soil. Lignite, as a natural source of humic acids was applied separately as an alternative economical and ecological sorbent.
Materials and methods
Characteristics of soils, lignite, lignite HAs, and zeolite
The experiments were carried out in three types of soil (Calcaro-haplic Chernozem, Gleyic Fluvisol, and Arenic Regosol) collected from agricultural topsoil of key monitoring sites of the Soil Monitoring System of Slovakia, characterized according to FAO. Soils were characterized by pH, content of organic carbon Corg, and nutrients (P, K, N) using elemental analysis. They represent common soil types with a wide range of fundamental soil attributes. Soils were artificially contaminated by PCP under laboratory conditions. HAs were characterized by CHN elemental analysis, carboxylic acidity, and by 13C NMR spectroscopy used to establish the content of aliphatic (Caliph) and aromatic (Car) carbons and degree of aromaticity (α). Lignite was obtained from Hodonín (Czech Republic), and zeolite (clinoptilolite) from Nižný Hrabovec (Slovakia).
Preparation of OMC
Humic acids were dissolved in 0.01 M NaOH to achieve a concentration of 2,000 mg l−1. Fifty milliliter of HA solution was added to 10 g zeolite. Blanks were prepared by mixing zeolite with 0.01 N NaOH. The suspension was stirred for 12 h at 22°C. Solid phase (OMC) was centrifuged, washed with distilled water, and dried at 50°C.
Adsorption of PCP onto lignite
An adsorption experiment was performed with 2 g suspension of lignite and 50 ml of distilled water. For each experiment, three series of samples were used. The mixture was placed into a 100 ml Erlenmeyer flask fitted with a ground-glass stopper, and a solution of PCP in DMSO was admixed so that the resulting concentrations of PCP were 10, 100, 200, and 300 mg l−1. The samples were placed on a rotary shaker (180 rpm) at 28°C for 1 h. Solid phase was removed by centrifugation (15 min at 5,000 rpm). The supernatant was then decanted, extracted, and analyzed.
Desorption of PCP from lignite
Upon termination of the adsorption experiment and centrifugation, sediment was quantitatively transferred into a 100 ml Erlenmeyer flask fitted with a ground-glass stopper. For each experiment, three series of samples were used. The pH value of the sediment was adjusted to 2.5 (simulation of acid rains). The flask was then placed on a rotary shaker (180 rpm) at 28°C for 24 h. Subsequently, the samples were centrifuged (15 min at 5,000 rpm). Supernatant was decanted, extracted, and analyzed.
Microorganisms
Bacterial strain Comamonas testosteroni CCM 7350, an isolate from waste water treatment plant activated sludge was obtained from the Czech Collection of Microorganisms, Brno, Czech Republic.
Biodegradation of PCP
A stock solution (10 g l−1) of PCP was prepared in DMSO. The biodegradation of PCP was performed in soil in the presence of inoculum of C. testosteroni (0.5 g l−1) or without additional bioaugmentation. Microorganisms were activated by water (60% maximum capillary capacity—MCC) 24 h before PCP addition into soil. The biodegradation of PCP in soil was carried out under laboratory conditions in the real soil types (Fluvisol, Chernozem, and Regosol) with and without addition of OMC and lignite at the initial PCP concentrations of 10 and 100 mg kg−1. For each experiment, three sets of soil samples were used and analyzed after 7, 17, and 24 days. The soils were periodically moisturized with water to maintain the 60% MCC value and stirred under sterile conditions. Static cultivation was carried out in the dark at 28°C.
Abiotic elimination of PCP
Soil samples were sterilized (2 h, 120 kPa) in order to inactivate the indigenous microorganisms to allow for determination of the abiotic changes. Other conditions were the same as in the biodegradation experiment.
Extraction of PCP and purification of the extract
After bioremediation, the samples were transferred into a separation funnel and pH was adjusted to 3–5. An acidic solution was extracted twice with 15 ml of n-hexane. If organic layer foamed, 1 ml of acetone was added and the sample was gently stirred. Organic layers from both extractions were placed into a 100 ml separation funnel, which allowed for efficient phase separation. The hexane phase was poured through anhydrous Na2SO4 into a 100 ml flask fitted with a ground-glass stopper. The solvent was completely evaporated on a rotary vacuum evaporator at 40–45°C. Methanol was added to the residue left after evaporation and the sample was analyzed by HPLC (LC-10AT VP, Shimadzu). HPLC conditions: A WATREX 250 × 4 mm column packed with Nucleosil 120-5 C18; mobile phase methanol:acetic acid:water (90:10:1), flow 0.73 ml min−1, spectrophotometric detection, sensitivity 1 V/2.5 A.
CFU detection
One gram out of a soil homogenate sample was mixed with 3 ml of sterile distilled water and diluted to 104 and 105 times. For each experiment, three sets of samples were used. About 125 μl of a diluted soil extract was applied to Petri dishes with Thornton agar and incubated for 5 days at 28°C. Number of CFUs was evaluated applying the colony counter.
Thornton agar
Thornton agar is a suitable solid medium for cultivation of soil bacteria. About 5 g KNO3, 1 g K2HPO4, 0.2 g MgSO4, 0.1 g CaCl2, 0.1 g NaCl, 0.002 g FeCl3, 0.5 g aspartic acid, and 1 g mannitol were dissolved in 1 l of distilled water. The value of pH was adjusted to 7.5 by NaOH, 15 g of agar was added, sterilized (20 min, 120 kPa), and after cooling to 40°C applied to sterile Petri dishes.
Toxicity test using Lemna minor
Soil ecotoxicity was assessed applying duckweed Lemna minor growth-stimulation test (ISO 20079 2005). The biotests were carried out in 150 ml flasks filled with 100 ml Steinberg medium and soil. The value of pH was adjusted to 5.5 ± 0.2. Inoculum of L. minor used for each flask contained 10–12 fronds taken only from the plants with two or three fronds. Three control replicates and two slurry replicates were used. Tests were carried out in a climatic exposure test cabinet, calibrated at 25 ± 2°C with light intensity adjusted to 6,500 Lux. Duration of the test was 7 days. Number and area of the fronds were determined and images of the beakers were taken for analysis at the beginning of test and on the 2nd, 4th, and 7th days. Measurements and evaluations were performed using the digital image analysis system Scanalyzer (LemnaTec, GmbH, Germany). The growth-rate in the samples was compared with that observed in the control experiment, and the concentration resulting in a specific rate of growth inhibition was determined.
Results and discussion
The experiments described in this contribution are based on our recent results obtained at the determination of adsorption, desorption, and retention characteristics of OMC and lignite as potential sorbents used for decontamination of chlorophenols (Dercová et al. 2006; 2007). The previous biodegradation experiments showed different PCP degradation potentials of the indigenous (autochthonous) soil bacteria in different types of soil.
The general hypothesis of this study is that natural organic adsorbents may assist in reducing down the bioavailability of potentially toxic pollutants. The implication is that the metabolic activity of soil microorganisms is not influenced by high doses of the toxicant.
The microbial degradation of PCP that represents in our experiments pesticides or their degradation products, was studied in three different soil types (Chernozem, Fluvisol, and Regosol) by autochthonous (indigenous) microbial soil consortium and by allochthonous (inoculated) bacterial strain C. testosteroni CCM 7350 capable to use phenol as the sole source of organic carbon.
The soils were artificially contaminated (freshly spiked) by PCP (10 and 100 mg kg−1) and enriched by addition of two tested sorbents—OMC or lignite (5% w/w of the total amount of soil). The conditions used in the experiments simulated common pesticide contamination (PCP concentration 10 mg kg−1) and a markedly higher contamination (PCP concentration 100 mg kg−1) e.g. in the surroundings of the wood treatment plants. OMC was prepared by binding HAs isolated from lignite to zeolite. Lignite is the abundant source of humic substances (Veselá et al. 2005). The most important fraction of humic substances is represented by humic acids. Humic substances are considered to be energy-abundant material playing important role in the biochemical cycles in soil (Pokorný et al. 2005). In addition, lignite has growth-promoting and protective impact on microorganisms (Feifičová et al. 2005). Nam and Kim (2002) presented findings that show relative distribution of aged organic pollutants among humic substances and demonstrate potential role of humic/mineral complex and humic substances in the sequestration and reduction of bioavailability of hydrophobic organic compounds in the soil. The optimized preparation and function of OMC as a possible sorbent for the purpose of immobilization of PCP has been studied in our previous work together with adsorption, desorption, and retention of PCP onto tested OMC. The results obtained were described in our previous papers (Dercová et al. 2006; 2007). Binding of PCP onto zeolite, humic acids, and lignite is reversible. Desorption affects only a small part of adsorbed amount, thus retention capacity is very high. In the presence of microorganisms and by the action of acid rains, a part of reversibly bound amount of PCP is slowly desorbed and then becomes bioavailable for biodegradation by autochthonous or allochthonous microorganisms.
Adsorption/desorption of PCP on/from lignite
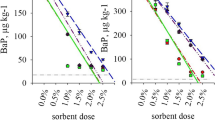
The partial aim of the work was to determine and to compare sorption properties of tested sorbents, OMC and lignite (Table 1). Adsorption/desorption characteristics of lignite were determined in the presence of PCP concentrations 10, 100, 200, and 300 mg l−1. As can be seen in Fig. 1, desorption of PCP from lignite was very low. The results confirmed excellent retention properties of lignite (79–91%). Adsorption/desorption properties of OMC were comparable with them. Other sorption materials have been already suggested for PCP sorption in the literature, e.g. inactivated biomass, peat, activated sludge, peat:bentonite mixture, chitosan, and pine bark (Bell and Tsezos 1987; Tanjore and Viraraghavan 1997; Brandt et al. 1997; Viraraghavan and Slough 1999; Zheng et al. 2004; Jianlong et al. 2000; Brás et al. 2005).
Adsorption/desorption of PCP onto soil amended with lignite
Soil particles may serve as adsorbent of a contaminant resulting in a decrease of its toxicity, as biomass growth-supporting factor, and as a source of supplementary nutrients for the microorganism colonization. This concept is of importance particularly for the production of active and resistant biomass for the biotreatment of the contaminated soils (Otte et al. 1999). The objective of this work was also to investigate the properties of lignite as a natural source of humic acids for their potential use for reinforcement of natural soil sorption capacity in soil decontamination technology.
Adsorption/desorption experiments in the presence of 10 mg kg−1 PCP were carried out with lignite addition into three soil types (Chernozem, Fluvisol, and Regosol) (Tables 1, 2). Adsorption and retention of PCP are illustrated in Fig. 2. The results emphasize the role of binding sites of lignite in adsorption/desorption processes. In soil with addition of lignite, higher retention properties (54–75%) were determined than in the soil samples with addition of zeolite (the inorganic sorbent) or OMC (HAs bound on zeolite), as has been demonstrated in our previous paper (Dercová et al. 2006).
Biodegradation of PCP in soil
The aim of this part of our work was to determine biodegradation of PCP in soil containing indigenous, autochthonous microorganisms and in bioaugmented soil inoculated by bacteria C. testosteroni CCM 7350. The biodegradation process was monitored and samples were taken after 7, 17, and 24 days. Biodegradation was performed in soil with and without OMC addition at the initial concentrations of PCP 10 and 100 mg kg−1 or in the lignite-amended soil with the initial PCP concentration 100 mg kg−1. The beneficial role of natural sorbents in improvement of PCP transformation by an indigenous soil consortium was described also by Otte et al. (1999).
The effect of the sorbents used on the improvement of PCP biodegradation in the bioaugmented soils (Fluvisol, Regosol, and Chernozem) significantly varied (Fig. 3). The differences in biodegradation of PCP were observed in soils with OMC or lignite amendments and without addition of the sorbents. The biodegradation of PCP observed in the soil samples with the addition of sorbents after 24 days decreased in the following order: lignite + soil > OMC + soil > intact soil. The soil samples with the PCP concentration 10 mg kg−1 revealed higher degradation in comparison to the soil with the higher initial PCP concentration 100 mg kg−1, which effect was already observed after 7 days. Biodegradation of PCP in the bioaugmented soils evaluated after 24 days depended on the addition of sorbent, initial PCP concentration, and the soil type. In bioaugmented Regosol and Fluvisol without sorbent addition and with the initial PCP concentration 10 mg kg−1, 72–74% biodegradation was obtained, while in Chernozem it was only 57%. Biodegradation of PCP in soils with the initial PCP concentration 100 mg kg−1 was markedly lower (in Regosol 49%, in Chernozem 39%, and in Fluvisol 34%). In the bioaugmented soils with OMC addition and PCP concentration 10 mg kg−1, a decreasing tendency of biodegradation was observed in this order: Regosol (96%) > Chernozem (77%) > Fluvisol (69%). Biodegradation of PCP in soils with the initial PCP concentration 100 mg kg−1 decreased in the order Regosol (50%) > Fluvisol (44%) > Chernozem (42%). The highest values of PCP biodegradation were obtained in soils with addition of lignite and at the initial PCP concentration 100 mg kg−1 (Chernozem 95%, Regosol 84%, and Fluvisol 61%).
Biodegradation of PCP in non-bioaugmented soils depended mainly on the soil type (Fig. 4). Biodegradation in Regosol with and without OMC and PCP concentration 10 mg kg−1 increased markedly after 7 days, and than it remained at the same level until the end of the experiment (~50%). Biodegradation in Regosol with or without addition of OMC and concentration of PCP 100 mg kg−1 increased to 81–89%. In Regosol with lignite addition, the lowest value of biodegradation was obtained (48%). The 72% biodegradation was observed in the non-bioaugmented Chernozem. In Chernozem with the addition of OMC and the initial PCP concentration 10 mg kg−1, 86% extent of biodegradation was determined. Biodegradation in Chernozem at concentration of PCP 100 mg kg−1 with or without addition of OMC reached 31% or 34%, respectively. Both values obtained were lower than the values observed in Chernozem with lignite addition (48%). Biodegradation of PCP in Fluvisol after 24 days with PCP concentration 10 mg l−1 and with OMC addition reached 64% and without OMC addition reached 78%. Significant differences were observed between the non-bioaugmented soils with OMC or lignite addition with the initial PCP concentration 100 mg kg−1. In Fluvisol with addition of lignite, 84% biodegradation was observed, whereas in Fluvisol with addition of OMC only 23% biodegradation was established. It was necessary to determine physico-chemical abiotic degradation and evaporation of PCP during biodegradation process. The results obtained demonstrate that 3–5% of PCP was irreversibly bound onto soil particles and the amount of evaporated PCP represented 1–2.5% of the initial PCP concentration (data not shown). The efficiency of PCP extraction from soils was more significantly influenced by addition of sorbents rather than by the type (soil: 65%; soil plus OMC: 70%; soil plus lignite: 53%).The biodegradation of PCP in the soil samples with addition of tested sorbents, OMC or lignite was markedly higher compared to the soil without any sorbent addition. This observation indicated that the binding of PCP was reversible, and in the presence of the microorganisms a subsequent release of low PCP concentration was observed. Bioaugmentation of soil by the external microorganisms with PCP degradation capability did not ensure higher level of pollutant degradation.
Determination of the number of colony forming units (CFUs)
The number of CFUs in the bioaugmented soils sampled after 7, 17, and 24 days (Table 3) was determined during the biodegradation of PCP. In the bioaugmented Fluvisol, 10- to 1,000-fold higher numbers of CFUs were established than in the bioaugmented Chernozem and Regosol. In Chernozem with addition of OMC and at both initial concentrations of PCP (10 and 100 mg kg−1), the number of CFUs remained constant or slightly decreased. On the contrary, after lignite addition, number of CFUs rapidly increased.
Number of CFUs was 10-fold higher in Fluvisol than in Chernozem and Regosol. In general, number of CFUs increased during biodegradation in Chernozem and Regosol. In Fluvisol with addition of OMC and in the intact Fluvisol, the number of CFUs decreased, but increased in Fluvisol with addition of lignite.
In the non-bioaugmented Regosol and Chernozem in the presence of lignite, the number of CFUs after 24 days was higher than in the bioaugmented soil. In all soil types with an exception of soil with lignite addition, higher number of CFUs was determined under the bioaugmentation conditions in comparison with non-bioaugmented soils.
Ecotoxicity of the studied soil samples before and after biodegradation of PCP using a 7-day growth inhibition test with L. minor
The biotransformation of PCP led probably to production of the more toxic metabolites in comparison with the parent compound, probably lower chlorinated phenols or quinones. These compounds are more soluble than PCP and therefore probably more bioavailable and toxic to present microbial consortium. Soil samples taken in the beginning and at the end of the biodegradation experiment were therefore evaluated for ecotoxicity applying standard plant L. minor test. Growth of L. minor was determined directly in soil samples taken at the end of biodegradation experiments (after 24 days) and mixed with Steinberg medium. The results provided the evidence that the growth of L. minor fronds was significantly affected by the presence of PCP or its degradation products. The results are demonstrated in Table 4.
In the bioaugmented Fluvisol containing 10 mg kg−1 PCP or without PCP addition and with or without addition of OMC, the growth of L. minor fronds decreased during 7-day inhibition test. In the samples with Fluvisol with 100 mg kg−1 concentration of PCP, a strong toxic effect was observed (except for the soil with lignite addition). Toxic effect was detected in soil samples taken in the beginning and at the end of biodegradation experiments. In Fluvisol with addition of lignite and the initial PCP concentration 100 mg kg−1, toxicity slightly decreased after 24 days of biodegradation, however, the samples were toxic during the whole biodegradation experiment. Similar results were obtained for bioaugmented Chernozem and Regosol. However, after 24 days in soils with lignite addition and without any PCP, low toxic effects on frond growth were also observed. It will be necessary to study a possible toxic effect of lignite added alone in the future. To eliminate low toxicity of lignite itself, it is necessary to apply it at the lower initial concentration into the soil. It might be also necessary to apply a battery of different toxicity tests, as well as the bioindicators at higher trophic levels.
In non-bioaugmented Fluvisol with OMC addition with concentration of PCP 10 mg kg−1 and without PCP addition, the frond growth stimulation decreased. Fluvisol without PCP addition and with lignite addition alone was slightly toxic toward L. minor, similarily as in bioaugmented Fluvisol. In Fluvisol with 100 mg kg−1 PCP concentration, a significant inhibition of L. minor growth was revealed. Toxic effect of PCP in the soil samples taken after 24 days of biodegradation was significantly lower and toxicity was retained only in the samples with lignite addition, not with OMC addition.
The growth of L. minor fronds decreased in the bioaugmented Chernozem with 10 mg kg−1 PCP and without PCP addition and subsequently without sorbent addition in the soils taken after 24 days of biodegradation. Chernozem with sorbent addition without PCP contamination proved to be toxic to fronds. Bioaugmented Chernozem containing PCP at 100 mg kg−1 concentration, was toxic during the whole duration of biodegradation except for the soil with OMC addition. Toxicity slightly decreased at the end of biodegradation experiments. Soils with OMC addition revealed increased toxicity toward L. minor.
Non-bioaugmented Chernozem exerted comparable toxic effect on L. minor growth as the non-bioaugmented Fluvisol. Soils with addition of OMC demonstrated stronger toxic effect on the growth of L. minor fronds than those without OMC. Chernozem without addition of PCP and with addition of lignite revealed toxic effect on L. minor growth. The addition of sorbents to bioaugmented or non-bioaugmented Chernozem exerted toxic effect on L. minor growth also without PCP addition, similarly as it was observed with Fluvisol.
Bioaugmented Regosol was assessed as well. Decrease of stimulation of L. minor frond growth in soils with lignite was observed at both tested PCP concentrations. In Regosol with 100 mg kg−1 PCP concentration, a strong permanent toxic effect during the whole degradation experiment was observed. Toxicity increased in the sample with addition of lignite.
With the non-bioaugmented Regosol similar results were obtained as with the soil with bioaugmentation, except for the samples with PCP concentration 10 mg kg−1. Samples with the initial PCP concentration 10 mg kg−1 (with and without addition of OMC) did not reveal toxic effects. Moreover, addition of OMC showed growth-promoting effect on L. minor.
PCP at a concentration of 100 mg kg−1 caused significant inhibition of L. minor frond growth and, moreover, changes in their color from green to yellow and white were observed. Results obtained applying L. minor ecotoxicity test confirmed that toxic effects of soils contaminated with PCP during biodegradation in the presence of tested sorbents OMC or lignite depended mainly on soil properties and the initial concentration of PCP. Decrease of PCP amount during biodegradation was not always associated with the considerable changes in soil ecotoxicity. We have observed that soil ecotoxicity after degradation of PCP in the majority of soil samples was higher than at the beginning of biodegradation experiment. It can be assumed that the reason of higher toxic effect was probably due to the production of toxic intermediates during biotransformation, such as lower chlorinated phenols, chlorinated catechols, or chlorinated quinones that are more water-soluble than parent PCP molecule and therefore more bioavailable and thus more toxic toward present biota.
It is necessary to stress that biodegradation of aged PCP in long term contaminated soil will be probably not comparable with freshly spiked soil. PCP is known to undergo “ageing” within soil, which is characterized by reduced bioavailability due to sequestration processes similarly to those occurring with 2,4-dichlorophenol (Shaw et al. 2000). On the other hand, Rhodes et al. (2007) established higher level of 2,4-dichlorophenol degradation in aged woodland soils in comparison with freshly spiked woodland soils.
Conclusions
The purpose of this work was to study the possibility to decrease concentration of organic chlorinated pollutants in different soil types by their immobilization onto natural sorbents added to soil and supporting consequent biodegradation of reversibly released pollutants by bioaugmentation by exogenous microorganisms with degradation ability. We assumed that combination of bioaugmentation and sorbent addition could lead to considerable decrease of PCP concentration and toxicity of PCP toward biota. The best impact on PCP biodegradation in the highly contaminated soil, simulated under the laboratory conditions by the PCP concentration 100 mg kg−1 was found in the soils with addition of lignite. Our results confirmed high efficiency of sorption ability of tested lignite toward PCP.
Addition of the tested sorbents, mainly lignite to soil had enhancing effect on the growth of the present microorganisms and their biodegradation ability. Our results also demonstrated that the effect of tested sorbents, OMC and lignite, and inoculum addition on PCP concentration depended markedly on soil type. In some cases, the positive effect of bioaugmentation or addition of sorbent on the effectiveness of biodegradation process was disputable because the standard toxicity test applying L. minor indicated the increase of soil ecotoxicity after termination of the biodegradation process. In spite of this, we suggest that the use of natural sorbents for the immobilization of PCP represents a promising technique of bioremediation technology.
Success of decontamination and detoxification depends on the soil type and mainly on the amount of the soil organic matter. During bioremediation, the monitoring of ecotoxicity is required to evaluate the success and efficacy of this process and to avoid accumulation of toxic metabolites of chlorophenol degradation in the environment. Under real environmental conditions, persistence or only transformation of the compounds are often observed. In spite of this and due to the fact that most degradation potentials are ubiquitous, activation and enhancement of natural degradation potential in the contaminated soils by bioaugmentation and the use of natural sorbents represent currently the challenge to be faced in modern environmental research and future decontamination techniques used in remediation technology.
References
Bae HS, Lee JM, Kim YB, Lee ST (1996–1997) Biodegradation of the mixtures of 4-chlorophenol and phenol by Comamonas testosteroni CPW301. Biodegradation 7(6):463–469. doi:10.1007/BF00115293
Bell JP, Tsezos M (1987) Removal of hazardous organic pollutants by biomass adsorption. J Water Pollut Control Fed 59:191–198
Bollag JM (2002) Immobilization of pesticides in soil through enzymatic reactions. In: Agathos N, Reineke W (eds) Biotechnology for the environment: soil remediation, vol 3A. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 93–101
Brandt S, Zeng AP, Deckwer WD (1997) Adsorption and desorption of pentachlorophenol on cells of Mycobacterium chlorophenolicum PCP-1. Biotechnol Bioeng 55:480–489. doi:10.1002/(SICI)1097-0290(19970805)55:3<480::AID-BIT3>3.0.CO;2-8
Brás I, Lemos L, Alves A, Pereira MFL (2005) Sorption of pentachlorophenol on pine bark. Chemosphere 60:1095–1102. doi:10.1016/j.chemosphere.2004.12.064
Bulankina MA, Lysak LV, Zvyagintsev DG (2007) Lignite microorganisms. Biol Bull 34(2):194–197. doi:10.1134/S1062359007020124
Calderbank A (1989) The occurrence and significance of bound pesticide residues in soil. Rev Environ Contam Toxicol 108:71–103
Crosby DG, Beynon KI, Greve PA (1981) Environmental chemistry of pentachlorophenol. Pure Appl Chem 53(5):1051–1080. doi:10.1351/pac198153051051
Dercová K, Čertík M, Mal’ová A, Sejáková Z (2004) Effect of chlorophenols on the membrane lipids of bacterial cells. Int Biodeter Biodegr 54: 251-254
Dercová K, Sejáková Z, Skokanová M, Barančíková G, Makovníková J (2007) Bioremediation of soil contaminated with pentachlorophenol (PCP) using humic acids bound on zeolite. Chemosphere 66:783–790. doi:10.1016/j.chemosphere.2006.06.061
Dercová K, Sejáková Z, Skokanová M, Barančíková G, Makovníková J (2006) Potential use of organomineral complex (OMC) for bioremediation of pentachlorophenol (PCP) in soil. Int Biodeter Biodegr 58:248–253. doi:10.1016/j.ibiod.2006.06.017
Escher BI, Behra R, Eggen RIL, Fent K (1997) Molecular mechanisms in ecotoxicology: an interplay between environmental chemistry and biology. Chimia (Aarau) 51:915–921
Feifičová D, Čejková A, Masák J, Siglová M, Jirků V (2005) Sorption of humic acids onto bacterial surface: factors influencing this process. In: Kalogerakis N (ed) Book of abstract of the third European bioremediation conference. Technical University of Crete, Chania, Crete, Greece, p 143
ISO 20079 (2005) Water quality. Determination of the toxic effect of water constituents and waste water to duckweed (Lemna minor)—Duckweed growth inhibition test, p 23
Jianlong W, Yi Q, Horan N, Stentiford E (2000) Bioadsorption of pentachlorophenol (PCP) from aqueous solution by activated sludge biomass. Bioresour Technol 75:157–161. doi:10.1016/S0960-8524(00)00041-9
Nam K, Kim JY (2002) Role of loosely bound humic substances and humin in the bioavalability of phenanatrene aged in soil. Environ Pollut 118(3):427–433. doi:10.1016/S0269-7491(01)00296-2
Naumova GV, Kholodov VA, Klikova NA, Zhmakova NA, Makarova NL, Ovchinikova TF, Perminova IV (2002) Detoxication of acetochlore by preparations of humic acids from different sources. In: Proceedings of humic substances—nature’s most versatile materials, Boston, pp 163–165
Odokuma LO, Dickson AA (2003) Bioremediation of crude oil polluted tropical rain forest soil. Glob J Environ Sci 2:29–40
Otte M, Comeau Y, Samson R, Greer CW (1999) Enhancement of pentachlorophenol biodegradation using organic and inorganic supports. Bioremediat J 3(1):35–45. doi:10.1080/10889869991219181
Pokorný R, Olejníková P, Balog M et al (2005) Characterization of microorganisms isolated from lignite excavated from the Záhorie coal mine (southwestern Slovakia). Res Microbiol 156(9):932–943
Pu X, Cutright TJ (2007) Degradation of pentachlorophenol by pure and mixed cultures in two different soils. Environ Sci Pollut R 14(4):244–250
Rhodes AH, Owen SM, Semple KT (2007) Biodegradation of 2,4-dichlorophenol in the presence of volatile organic compounds in soils under different vegetation types. FEMS Microbiol Lett 269:323–330
Shaw LJ, Beaton Y, Glover LA, Killham K, Osborn D, Meharg AA (2000) Bioavailability of 2,4-dichlorophenol associated with soil water-soluble humic material. Environ Sci Technol 34:4721–4726
Tan KH (2003) Humic matter in soil and the environment—principles and controversies. Marcel Dekker, New York, pp 299–300
Tanjore S, Viraraghavan T (1997) Effect of oxygen on the adsorption of pentachlorophenol by peat from water. Water Air Soil Pollut 100:151–162
Veselá L, Kubal M, Kozler J, Inemanová P (2005) Structure and properties of natural humic substances—oxihumolite. Chem Listy 99:711–717
Viraraghavan T, Slough K (1999) Sorption of pentachlorophenol on peat-bentonit mixtures. Chemosphere 39:1487–1496
Zheng S, Yang Z, Yo DH, Park YH (2004) Removal of chlorophenols from groundwater by chitosan sorption. Water Res 38:2315–2322
Acknowledgement
Financial support from the Scientific Grant Agency of Ministry of Education of Slovak Republic and Slovak Academy of Sciences VEGA (Grant No. 1/1309/04) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zuzana, S., Katarína, D. & Lívia, T. Biodegradation and ecotoxicity of soil contaminated by pentachlorophenol applying bioaugmentation and addition of sorbents. World J Microbiol Biotechnol 25, 243–252 (2009). https://doi.org/10.1007/s11274-008-9885-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-008-9885-1