Abstract
Summer bleaching of corals has been prevalent in the coastal waters of India in recent years before the onset of monsoon. Repeated bleaching of shallow water corals has changed the benthic dynamics of reef ecosystems. In the present study two locations, Wandoor and Burmanullah in South Andaman, were identified where such changes have occurred. After 3 years of study, the shift from coral domination to macroalgae and sponge is evident. In Wandoor, fleshy macroalgae (28.80%) have become a dominated benthic substrate and in Burmanullah, sponges (19.50%) have taken over much of reef space. Observation of multispecies domination of macroalgae in Wandoor and single species domination of sponges in Burmanullah has been established through this study of shallow reefs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coral reefs around the world are in increasing threat to bleaching due to the global rise in sea temperature. Studies across all the major coral reefs in recent years have established one primary issue, which is the rapid loss of live coral cover. During the past two decades, studies have shown frequent incidences of shifting of coral domination to macroalgae dominating substrata across coral reefs worldwide (McManus et al. 2000; Hughes et al. 2007; Bruno et al. 2009; Ainsworth and Mumby 2015). Although macroalgae and sponges are vital components present in any coral reef ecosystem, healthy coral reefs have a higher percentage of live corals with a much less percentage of macroalgae and even lesser cover of sponges. According to Hoegh-Guldberg et al. (2007), community shifts from coral to macroalgae dominance are being reported globally and this trend is predicted to continue due to prevailing climate change. Change in pH levels of seawater (Hoegh-Guldberg et al. 2007; Enochs et al. 2015), eutrophication and sedimentation (Fabricius et al. 2005; De'ath and Fabricius 2010) can lead to algal dominance of coral reefs. Healthy reefs are generally characterized by the abundance of live coral cover, which keep a check on the growth of macroalgae (Hughes et al. 2007; Birrell et al. 2008). However, in degrading or degraded reefs which lack resilience for community shifts, macroalgae become the significant component thereby reducing coral recruitment (Kuffner et al. 2006) and favouring the pathogens that harm coral growth and survival (Nugues et al. 2004; Smith et al. 2006).
Another significant observation in recent years has been the shift toward sponge dominance on some coral reefs globally (Bell et al. 2013). This may occur if sponge abundance/biomass increases in comparison to decline in live coral cover due to the utilization of more space or by increased productivity (Bennett et al. 2017). Increase in sponge cover in reef areas will reduce the space available for settlement and establishment of various reef organisms, more so for coral larvae (Bell et al., 2018). Recent shreds of evidence studies suggest that there are many sponges that may be more tolerant to the impacts of climate change when compared to corals (Duckworth et al. 2012; Bennett et al. 2017, 2018). However, it is also to be noted that, compared to macroalgal phase shifts, records of sponge phase shifts have been much less (Bell et al. 2013). Andaman & Nicobar Islands are union territories under the Republic of India, which consists of 572 islands and islets. These island are known for diversity of corals reefs (geographically representing coral triangle) and vast coasts lined with mangroves. The primary objective of this study was to identify and study shallow reef habitats in South Andaman where reefs have undergone phase shifts due to repeated events of coral bleaching.
Materials and methods
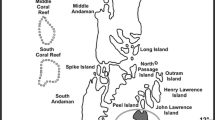
Considering the abundance and spatial distribution, line intercept transect (LIT) (3 × 20 m) was used to monitor the percentage cover and change in benthic substrates of coral reefs (English et al. 1997; Hill and Wilkinson 2004). The transects were laid parallel to the reef crest along a constant depth contour (Hill and Wilkinson 2004). This study was carried out for 3 years viz. 2014, 2015 and 2016 by snorkeling/diving. The study areas were located in South Andaman District (Fig. 1) of Andaman & Nicobar Islands (A & N Islands), India. Figure 2 depicts the study design and planning for the present study.
Survey sites
Wandoor
It is a popular tourist destination, more so because adjacent to it is one of the two marine national parks of Andaman and Nicobar Islands viz. Mahatma Gandhi Marine National Park. It is located around 17 km from Port Blair (capital of A & N Islands). The surveyed area (11°35′59.85"N, 92°36′17.32"E) is located around 100 m from the New Wandoor Beach where tourists are frequent throughout the year. Water depth around the reef ranged between 2 and 3 m depending on the tidal conditions. A small stream with mangrove vegetation opens up in the area which originates from human habitats and brings along sediment loads especially during the monsoon season. Crocodile sightings are frequent in the surrounding area, including the reef area.
Burmanullah
It is located around 7 km from Port Blair and is on the way to Chidiyatapu (a popular beach with an adjacent coral reef area). Mostly frequented by locals during the weekends and public holidays. The area is primarily rocky and rich in biodiversity of invertebrates and macroalgae. The reef (11°34′29.96″N, 92°44′27.46″E) in the area is smaller both in length and width, depth varied between 3 to 4 m depending on the tide and located at around 170 m from the shore. It is influenced by the influx of freshwater from the adjacent estuary which opens up close to the reef site. High wave action from the open sea is prevalent throughout the coastline of Burmanullah. Rocky outcrops are vital features in the study area. In recent years, a single individual of dugong (Dugong dugon) has been sighted twice near the studied reef.
Results and discussion
Boulder corals, Porites spp. dominate both the study sites. The three most common species were Porites solida, P. lobata and P. lutea in both the sites. Other corals include Pavona spp., Pachyseris spp., Galexea spp., Favia spp., Favites spp., Fungia spp. etc. Among the branching corals Pocillopora spp., Acropora spp. and Montipora spp. were common, though much less abundant than boulder corals.
In Burmanullah, sponge cover was 19.50% in the reef with live coral cover of 38.80% (Table 1). Wandoor had 31.0% of live coral (Table 1) but a significant cover of macroalgae (28.80%), which were competing with live corals and other benthic organisms for space.
Over the 3 years (Table 1), the change in live coral, macroalgae and sponge in both the stations is noteworthy as depicted in Fig. 3. In both the stations the increasing trend in cover of macroalgae (Wandoor) and sponges (Burmanullah) has been confirmed. There has been an increase in macroalgae cover in Wandoor (4.8%), and similarly, an increase in sponge cover (1.8%) was observed in Burmanullah. Live coral cover has slightly increased in Burmanullah (1.1%) but decreased in Wandoor (−2.4%).
Phase shift in Wandoor (Fig. 4) was evident as macroalgae have taken over much of the reef space. Sponges, in particular, Lamellodysidea herbacea (Fig. 5) have become a major component of the reef which is a rare phenomenon in shallow reefs. Lamellodysidea herbacea has been reported by Immanuel et al. (2015) from the Andaman Islands. 122 species of sponges are known from Andaman and Nicobar Islands out of which 49 species are known to occur in the islands of South Andaman, and most of them are recorded from coral reefs (Immanuel et al. 2015). Some other common species observed in the study sites were Cliona spp., Hyrtios erectus, Stylissa massa, Xestospongia testudinaria etc.
Macroalgae play a significant role in the coral reef ecosystem. However, overgrowth of macroalgae due to phase shifts can degrade coral reefs and cause significant changes in the ecology of reefs as was observed in Wandoor. Turbinaria spp., Padina spp., Sargassum spp., Halimeda spp. and Gracilaria spp. were in higher abundance over the reef flat in Wandoor. According to the locals, the same area where the study in Wandoor had been carried out was a popular snorkelling and coral viewing site (using glass-bottom boats) by tourists before 2010, but due to the ecological transformation, the popularity of the coral reef has diminished considerably. It is to be noted that mass bleaching of corals in Andaman and Nicobar Islands was reported in 2010 (Krishnan et al. 2011; Marimuthu et al. 2013). The fact that input of nutrients occurs from the nearby habitation through a small stream cannot be ruled out in Wandoor. Only a long term study can confirm this perception.
Two incidents of phase shift from coral dominated to sponge dominated have been reported. In the Wakatobi Marine National Park (SE Sulawesi, Indonesia), sponges have been found to dominate several reef areas which were once coral-dominated sites (Bell and Smith 2004; Powell et al. 2010). Notably, the same species of sponge (Lamellodysidea herbacea) predominated in reef areas of the Wakatobi Marine National Park as observed in Burmanullah in this study. Again, based on another study from Central Pacific, the lagoon of Palmyra Atoll has shown a shift from a coral-dominated state to sponge dominated state (Knapp et al. 2016).
In the present study, though live coral cover was found to be adequate in both the stations for reversal of phase shift, yet in the 3 years of this study no improvement in the state of the reef was observed. There are no previous reports which have highlighted such high abundance (percentage cover) of macroalgae and sponge from any other reefs in Andaman and Nicobar Islands. Bleaching of corals in shallow waters was also recorded during the three summers of the study period. Death of some coral colonies and recovery of most colonies (more than 90%) were recorded in both the stations. Bleaching of isolated coral colonies was mainly observed in the months of April and May in both the sites, just before the onset of monsoon by the first week of June. Shallow water temperature was as high as 34 °C as recorded in situ, especially in the month of May. Most of the bleached colonies would recover after the onset of monsoon when water temperature normalizes. Berkelmans (2001) has explained that shallow inshore waters generally exhibit greater extremes in temperature during summer compared to offshore reefs and more so in bleaching years. Hence, shallow reefs are more prone to phase shifts when corals die out due to bleaching.
Although shift from coral dominated to macroalgae dominated reefs seems to be more commonly reported, further research is necessary to understand shifts from coral dominated to sponge dominated reefs over a certain period of time. Long-term monitoring of the reef systems in this regard would provide evidence of a phase shift from coral to sponge dominance on the reefs. Having understood that multiple drivers always characterize the phase shift dynamics on coral reefs, our study indicates that sponge dominated reefs are formed as a result of unreversed phase shifts. It is also worth mentioning that although the current management strategies are undoubtedly useful in preventing coral–macroalgae shifts, it does not prevent phase shifts to other alternative states, like that of sponges or soft corals. Hence, we would like to assert that, management of coral reefs needs a serious understanding of the conditions under which phase shifts to different states occur, and of the mechanisms that are responsible for this phase shift. This study concludes that more reef areas with live coral cover are under threat due to competition leading to phase shift by macroalgae and sponges, with the existing state of rise in seawater temperature and climate change.
References
Ainsworth CH, Mumby PJ (2015) Coral–algal phase shifts alter fish communities and reduce fisheries production. Glob Change Biol 21:165–172
Bell JJ, Smith D (2004) Ecology of sponge assemblages (Porifera) in the Wakatobi region, south-east Sulawesi, Indonesia: richness and abundance. J Mar Biol Assoc 84(3):581–591
Bell JJ, Davy SK, Jones T, Taylor MW, Webster NS (2013) Could some coral reefs become sponge reefs as our climate changes? Glob Change Biol 19:2613–2624
Bell JJ, Rovellini A, Davy SK, Taylor MW, Fulton EA, Dunn MR, Bennett HM, Kandler NM, Luter HM, Webster NS (2018) Climate change alterations to ecosystem dominance: how might sponge-dominated reefs function? Ecology 99(9):1920–1931
Bennett HM, Altenrath C, Woods L, Davy SK, Webster NS, Bell JJ (2017) Interactive effects of temperature and pCO2 on sponges: from the cradle to the grave. Glob Change Biol 23:2031–2046
Bennett H, Bell JJ, Davy SK, Webster NS, Francis DS (2018) Elucidating the sponge stress response; lipids and fatty acids can facilitate survival under future climate scenarios. Glob Change Biol 24(7):3130–3144
Berkelmans R (2001) Bleaching, upper thermal limits and temperature adaptation in reef corals. James Cook University, Townsville, p 179
Birrell CL, McCook LJ, Willis BL, Diaz-Pulido GA (2008) Effects of benthic algae on the replenishment of corals and the implications for the resilience of coral reefs. Oceanogr Mar Biol Annu Rev 46:25–63
Bruno JF, Sweatman H, Precht WF, Selig ER, Schutte VGW (2009) Assessing evidence of phase shifts from coral to macroalgal dominance on coral reefs. Ecology 90(6):1478–1484
De'ath G, Fabricius K (2010) Water quality as a regional driver of coral biodiversity and macroalgae on the great barrier reef. Ecol Appl 20(3):840–850
Duckworth AR, West L, Vansach T, Stubler A, Hardt M (2012) Effects of water temperature and pH on growth and metabolite biosynthesis of coral reef sponges. Mar Ecol Prog Ser 462:67–77
English S, Wilkinson C, Baker V (1997) Survey manual for tropical marine resources, 2nd edn. Australian Institute of Marine Science, Townsville, p 390
Enochs IC, Manzello DP, Donham EM, Kolodziej G, Okano R, Johnston L, Young C, Iguel J, Edwards CB, Fox MD, Valentino L, Johnson S, Benavente D, Clark SJ, Carlton R, Burton T, Eynaud Y, Price NN (2015) Shift from coral to macroalgae dominance on a volcanically acidified reef. Nat Clim Change 5:1083–1089
Fabricius K, De’ath G, McCook L, Turak E, Williams DM, (2005) Changes in algal, coral and fish assemblages along water quality gradients on the inshore Great Barrier Reef. Mar Pollut Bull 51(1):384–398
Hill J, Wilkinson C (2004) Methods for ecological monitoring of coral reefs. Australian Institute of Marine Science, Townsville, p 117
Hoegh-Guldberg O, Mumby PJ, Hooten AJ, Steneck RS, Greenfield P, Gomez E, Harvell CD, Sale PF, Edwards AJ, Caldeira K, Knowlton N (2007) Coral reefs under rapid climate change and ocean acidification. Science 318(5857):1737–1742
Hughes TP, Rodrigues MJ, Bellwood DR, Ceccarelli D, Hoegh-Guldberg O, McCook L, Moltschaniwskyj N, Pratchett MS, Steneck RS, Willis B (2007) Phase shifts, herbivory, and the resilience of coral reefs to climate change. Curr Biol 17(4):360–365
Immanuel T, Krishnan P, Raghunathan C (2015) An updated report on the diversity of marine sponges of the Andaman and Nicobar Islands. In: Venkataraman K, Sivaperuman C (eds) Marine faunal diversity in India: taxonomy, ecology and conservation. Academic, Elsevier Inc, pp 3–13
Knapp ISS, Williams GJ, Bell JJ (2016) Temporal dynamics and persistence of sponge assemblages in a Central Pacific atoll lagoon. Mar Ecol 37(5):1147–1153
Krishnan P, Roy SD, George G, Srivastava RC, Anand A, Murugesan S, Kaliyamoorthy M, Vikas N, Soundararajan S (2011) Elevated sea surface temperature during May 2010 induces mass bleaching of corals in the Andaman. Curr Sci 100(1):111–117
Kuffner IB, Walters LJ, Becerro MA, Paul VJ, Ritson-Williams R, Beach KS (2006) Inhibition of coral recruitment by macroalgae and cyanobacteria. Mar Ecol Prog Ser 323:107–117
Marimuthu N, Wilson JJ, Vinithkumar NV, Kirubagaran R (2013) Coral reef recovery status in South Andaman Islands after the bleaching event 2010. J Ocean Univ China 12(1):91–96
McManus JW, Meńez LAB, Kesner-Reyes KN, Vergara SG, Ablan MC (2000) Coral reef fishing and coral-algal phase shifts: implications for global reef status. ICES J Mar Sci 57:572–578
Nugues MM, Smith GW, Hooidonk RJ, Seabra MI, Bak RP (2004) Algal contact as a trigger for coral disease. Ecol Lett 7(10):919–923
Powell AL, Hepburn LJ, Smith DJ, Bell JJ (2010) Patterns of sponge abundance across a gradient of habitat quality in the Wakatobi Marine National Park, Indonesia. Open Mar Biol J 4:31–38
Smith JE, Shaw M, Edwards RA, Obura D, Pantos O, Sala E, Sandin SA, Smriga S, Hatay M, Rohwer FL (2006) Indirect effects of algae on coral: algae-mediated, microbe-induced coral mortality. Ecol Lett 9(7):835–845
Funding
No funding source for this study as this study was a part of PhD work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Malakar, B., Venu, S., Samuel, V.D. et al. Increasing signs of degradation of shallow water coral reefs due to repeated bleaching and spatial competition among benthic substrates. Wetlands Ecol Manage 29, 669–675 (2021). https://doi.org/10.1007/s11273-020-09744-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11273-020-09744-x