Abstract
In this study, components of the food-web in Macao wetlands were quantified using stable isotope ratio techniques based on carbon and nitrogen values. The δ13C and δ15N values of particulate organic matter (δ13CPOM and δ15NPOM, respectively) ranged from −30.64 ± 1.0 to −28.1 ± 0.7 ‰, and from −1.11 ± 0.8 to 3.98 ± 0.7 ‰, respectively. The δ13C values of consumer species ranged from −33.94 to −16.92 ‰, showing a wide range from lower values in a freshwater lake and inner bay to higher values in a mangrove forest. The distinct dietary habits of consumer species and the location-specific food source composition were the main factors affecting the δ13C values. The consumer 15N-isotope enrichment values suggested that there were three trophic levels; primary, secondary, and tertiary. The primary consumer trophic level was represented by freshwater herbivorous gastropods, filter-feeding bivalves, and plankton-feeding fish, with a mean δ15N value of 5.052 ‰. The secondary consumer level included four deposit-feeding fish species distributed in Fai Chi Kei Bay and deposit-feeding gastropods in the Lotus Flower Bridge flat, with a mean δ15N value of 6.794 ‰. The tertiary consumers group consisted of four crab species, one shrimp species, and four fish species in the Lotus Flower Bridge Flat, with a mean δ15N value of 13.473 ‰. Their diet mainly comprised organic debris, bottom fauna, and rotten animal tissues. This study confirms the applicability of the isotopic approach in food web studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Traditional approaches to delineate a food web include gut and stomach contents analyses, together with field and laboratory observations. These studies can identify what animals feed on with a high degree of taxonomic precision. However, these approaches are usually labor intensive and subject to errors because the identification of materials digested and assimilated by consumers is largely speculative (Fry and Sherr 1984; Persic et al. 2004; Sun et al. 2011). The alternative stable isotope method, which is based on the selective metabolic partitioning that results in the preferential waste of lighter isotopes during respiration and excretion, can overcome some of the above-mentioned difficulties. This integrative approach distinguishes assimilated rather than ingested food, reflecting the complexity of food webs over longer periods (Persic et al. 2004). Recently, analyses of stable isotopes of carbon (δ13C) and nitrogen (δ15N) have been used to trace the flow of organic matter in lake, estuarine, and marine food webs (Rodelli et al. 1984; Kendall et al. 2001; Gu et al. 2006, Bouillon et al. 2011). Therein, δ13C can be used to evaluate the ultimate sources of carbon in an organism when the isotopic signatures of the sources are different. It has been demonstrated that δ13C is enriched approximately 0.5–1 ‰ in the animal, relative to its diet (Kwak and Zedler 1997; Middelburg 2014). The basic assumption in estimating trophic positions is the conservative enrichment of 3–4 ‰ δ15N in a consumer, relative to its diet (Persic et al. 2004). Stable isotope signatures based on δ13C and δ15N values can be used to determine the sources of food ingested by a consumer and food assimilation over a long period (Middelburg 2014).
Macao is a very densely populated modern city located on a peninsula in the south-west of the Pearl River Delta in China. The Macao wetlands are small but internationally important (Jin et al. 2008), because more than 50 individuals of black-faced spoonbills (Platalea minor), categorized as an endangered species by IUCN (IUCN, 2014), visit Macao for over-wintering (Zhang et al. 2012). These wetlands are also the only known habitat of the recently discovered bryophyte Fissidens macaoensis, which requires particular environmental conditions (Jin et al. 2008). These endangered and endemic species demonstrate the values of Macao’s urbanized wetlands for conservation and ecological function. Although the phytoplankton, zooplankton, and benthic fauna of the Macao wetlands have been studied recently (Li et al. 2009; Chen et al. 2014, 2015), there have been few studies on the food web structure of this wetland. In this study, we present the first attempt to describe the food web in the Macao wetlands using a combined stable C and N isotope method. The aims were to characterize trophic relationships among the dominant species in Macao wetlands, and to determine which factors affect the trophic status of individual organisms whose feeding histories are poorly understood.
Materials and methods
Study area
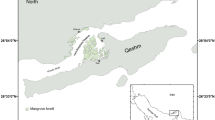
The study was conducted in four typical wetlands in Macao; Fai Chi Kei Bay (22°12′29″N; 113°32′30″E), Nam Van Lake (22°11′21″N; 113°32′19″E), Wang de Notre Dame Bay (22°9′7″N; 113°33′47″E), and Lotus Flower Bridge Flat (22°8′29″; 113°33′6″E). Fai Chi Kei, located at the northern part of the Macao peninsula, is a semi-enclosed water body with poor exchange. Nam Van Lake is an artificial lake located at the southern end of the peninsula. Wang de Notre Dame Bay is a freshwater lake with a surface area of nearly 20 hectares, and covered with Nelumbo nucifera and water hyacinth. Lotus Flower Bridge Flat is the largest wetland in Macao, and consists of about 80 ha covered by mangroves. The most abundant mangrove species in Lotus Flower Bridge Flat are Avicennia marina and Acanthus ilicifolius (Fig. 1).
Sample collection and processing
Samples for isotopic analysis were collected from December 2012 to September 2013. Particulate organic matter (POM), mixed phytoplankton/zooplankton, and suspended organic matter samples were collected with a plankton net with a mesh size of 35 µm, washed with distilled water, filtered through Whatman GF/G filters (precombusted at 500 °C for 2 h), and then dried to constant weight at 55 °C (Thimdee et al. 2004). Because the aquatic community may vary seasonally, four replicate samples in spring (March 2013), summer (June 2014), autumn (September 2013), and winter (December 2012) were conducted to study the food web. Fish samples were collected by anglers around Fai Chi Kei Bay, and by using gill nets in Lotus Flower Bridge Flat. Additional benthic invertebrates such as crabs, shrimps, and mollusks were collected by hand picking or in nets during low tides from the mangrove inter-tidal zone and Wang de Notre Dame Bay. For the mollusks, the soft tissues of 20 individuals of each species were pooled as a sample. For the decapod crustaceans, muscle tissue of around 20 individuals was extracted as a composite sample from abdominal segments or pereiopods. For fish, muscle tissue was dissected from four to 10 individuals of each species and pooled as a sample. Only the dorsal muscle of fish was taken for analysis, because its δ13C and δ15N contents are less variable than those in other tissues (Pinnegar and Polunin 1999). The muscle tissues were dried at 60 °C in an oven to constant weight. The dry tissue was ground using a mortar and pestle.
Isotopic value collection and data analysis
The δ15N and δ13C values were measured using gas chromatography–isotope ratio mass spectrometry (Thermo Finnigan Delta XL Plus GC-IRMS, Bremen, Germany). Isotopic values were expressed in the δ-unit notation as deviations from standards following the formula: δ13C or δ15N = [((R sample/R standard) − 1) ·103], where R was the corresponding ratio of 13C/12C or 15N/14N. The standard for C was Peedee Belemnite, and for N was atmospheric diatomic nitrogen. Instrument precision was 0.1 ‰ for carbon and 0.3 ‰ for nitrogen, based on replicate analyses of standard reference materials.
Trophic level (TL) was calculated using the following formula (Fry 2006; Vander Zanden and Rasmussen 2001; Iken et al. 2010)
where TL consumer and δ15Nconsumer were the TL and the δ15N value of the tested consumer, respectively, δ15N baseline was the δ15N value of the baseline biota, and TEF represented the trophic enrichment factor (=3.4 ‰). All statistical analyses were performed with EXCEL (Microsoft, Redmond, WA, USA) and SPSS 17.0 for windows (SPSS Inc., Chicago, IL, USA). One-way ANOVA (analysis of variance) was used to test for statistically significant differences in δ15N POM and δ13CPOM values among sites. Graphs were plotted using the ORIGIN 8.0 package (Origin Lab Corp., Northampton, MA, USA).
Results
Stable carbon and nitrogen isotope traits of POM in Macao wetlands
The δ13CPOM values at the four wetlands ranged from −30.64 ± 1.0 to −28.1 ± 0.7 ‰, and the mean value was −28.76 ± 1.58 ‰. The δ15NPOM values of each site ranged from −1.11 ± 0.8 to 3.98 ± 0.7 ‰, and the mean value was 2.314 ± 0.658 ‰ (Fig. 2). There was a significant difference (p < 0.05) in isotopic composition among the sampling sites.
δ13C and δ15N values of consumers in Macao wetlands
The δ13C values of 21 consumers ranged from −33.94 (Angulyagra polyzonata) to −16.92 ‰ (Uca arcuata), indicating that δ13C values varied widely among different species. Mollusk species (e.g. Neritina violacea, Ellobium chinense), which were distributed in inter-tidal areas with a certain amount of salinity, showed higher δ13C values than those of freshwater species (e.g. A. polyzonata, Pomacea canaliculata) (Fig. 3). Crustaceans showed a higher δ13C values than those of mollusks; U. arcuata showed the most enriched values (Figs. 3, 4). The δ13C values of fishes ranged from −28.15 to −21.79 ‰. The depleted carbon signatures (−28.15 to −24.72 ‰) of fishes in the Bay of Fai Chi Kei contrasted with the more enriched signatures (−24.61 to −21.79 ‰) of those in the Lotus Flower Bridge Flat. Overall, δ13C values of N. violacea, E. chinense, Exopalamon carincauda, Parasesarma plicatum, Perisesarma bidens, and Boleophthalmus pectinirostris were very similar (−22.3 to −20.37 ‰) (Figs. 2, 3, 4 and 5).
Values of δ13C and δ15N for fishes and birds in Macao wetlands (Mug = Mugil cephalus; Til = Tilapia; Peri = Periophthalmus cantonensis; Hem = Hemiculter leucisculus; Spa = Sparus latus; Bos = Bostrichthys sinensis; Lab = Labeo rohita; Tri = Tridentiger trigonocephalus; Cry = Cryprinus carpiod; Nyc = Nycticorax nycticorax)
The δ15N values of macro-invertebrates ranged from 4.62 ‰ (Achatina fulica) to 14.69 ‰ (E. carincauda) (Figs. 2, 3). The δ15N values of fishes ranged from 4.25 ‰ (Mugil cephalus) to 14.15 ‰ (Sparus latus) (Fig. 4). Among the mollusk taxa, compared with the freshwater species A. polyzonata (7.84 ‰) and P. canaliculata (7.71 ‰), the estuarine species N. violacea (11.94 ‰), E. chinense (13.48 ‰) showed more enriched δ15N values (Fig. 3). Similarly, fishes distributed in the Fai Chi Kei Bay had more depleted δ15N signatures than those in the mangroves (Fig. 5).
Food-web structure in Macao wetlands
It should be noted that POM, as the food-web baseline, is a heterogeneous food source comprising plankton, bacteria, and particulate matter, with large spatial and temporal variations in its isotopic signature. The variations arise from differences in biogeochemical processes (e.g., ammonium and nitrate availability) and in the reproductive cycle of plankton. In this study, POM at each sampling site was considered as the baseline. The trophic levels were ranked from 1.19 to 4.27 (Fig. 6). Eight species represented primary consumers, with mean TLs ranging from 1.19 to 2.94. Nine species were secondary consumers, with mean TLs ranging from 3.32 to 3.99. Four species were tertiary consumers, with mean TLs of >4.0. Ultimately, a scheme describing the feeding relationships of the analyzed dominant components of Macao wetlands trophic web (Fig. 7) was achieved by integrating the theoretical data with the results from the investigations described above.
Discussion
Signatures of δ13C and δ15N for POM in Macao wetlands
The POM is composed of allochthonous and autochthonous organic materials, and can provide a detailed, integrated record of natural and anthropogenic activities in aquatic ecosystems (Hein et al. 2003; Zhang et al. 2007). Several lines of evidence indicate that POM mainly consists of phytoplankton in eutrophic water, but consists of autochthonous organic compounds in oligotrophic water (Junes et al. 1999; Gu 2009). Allochthonous and autochthonous organic matter usually have different δ13C and δ15N signatures, which results in different δ13CPOM and δ15NPOM signatures (Savoye et al. 2003; Kendall et al. 2001). In the oceanic food web at the northwestern gulf of Mexico, δ13C values ranged from −30 to −23 ‰ for C3 terrestrial plants and from −14 to −10 ‰ for C4 plants, and the δ13CPOM values ranged from −27 to −25 ‰ (Fry and Sherr 1984). In a mangrove-fringed estuary in Thailand, the δ13C values of mangrove leaves ranged from −29.4 to −28.2 ‰ (Thimdee et al. 2004). Keely and Sandquist (1992) concluded that the δ13C values of aquatic macrophytes were often less than −27 ‰ due to respiratory CO2. In this survey, the mean δ13CPOM value was relatively low (−28.76 ‰). The inadequate water exchange in the eutrophic conditions of the inner bay of Fai Chi Kei limited the input of allochthonous organic matter; consequently, the POM in the bay contained a large proportion of phytoplankton (Li et al. 2009). In contrast with Fai Chi Kei, Nan Vam Lake was an oligotrophic water body in which allochthonous organic matter from rainfall was the main source of POM. The most depleted δ13CPOM was in Wang de Notre Dame Bay (−30.64 ± 1.0 ‰), a freshwater site extensively dominated by macrophytes. The POM at this site comprised mainly autochthonous organic matter.
Among the four wetlands, the Lotus Flower Bridge Flat showed the most enriched isotope signature with a mean δ13CPOM of −28.1 ± 0.7 ‰ and a mean δ15NPOM of 3.98 ± 0.7 ‰. A previous study demonstrated that POM in the intertidal mangrove forest consisted of both phytoplankton and mangrove detritus (Kon et al. 2007). Accordingly, the POM at the Lotus Flower Bridge Flat mainly consisted of mangrove detritus and phytoplankton. Meanwhile, the excreta of animals is known to have higher δ15N values (Kendall et al. 2001; Vizzini and Mazzola 2002). As an estuarine wetland, the Lotus Flower Bridge Flat exhibited a high density and wide biological diversity of organisms (Chen et al. 2014). The utilization of δ13C and δ15 N from animal excreta by phytoplankton may be the main reason for the high isotope signatures at this site.
δ13C values of consumers in Macao wetlands
The δ13C composition of consumers typically reflects the composition of assimilated food, plus a slight enrichment, and is often used to discriminate between consumer use of pelagic (depleted in δ13C) and littoral (near-shore) benthic resources (enriched in δ13C), with terrestrial allochthonous δ13C being intermediate (Fry and Sherr 1984; Larson et al. 2011). Tue et al. (2012) found that the δ13C value of invertebrates in an estuarine mangrove ecosystem in Vietnam ranged from −26.8 to −14.5 ‰. The wide ranges of δ13C signatures indicated that the invertebrates had heterogeneous diets, comprising benthic microalgae, marine phytoplankton, POM, sediment organic matter, mangrove detritus, meiofauna, and rotten animal tissues. In Laoshan Bay, China, the carbon isotope data confirmed that POM was the main food source of benthic filter feeders (e.g., bivalves, crustaceans). These filter feeders had a wide range of δ13C values, reflecting the diversity of their food sources. Benthic diatoms are an important part of the diet of most gastropods (Cai et al. 2011). In this study, the δ13C values of consumers ranged from −33.45 ‰ (A. polyzonata) to −16.92 ‰ (U. arcuata), and showed a wide range from lower values in the freshwater lake and inner bay to higher values in the mangrove forest. The wide variation in values was mainly because of the distinct dietary habits of the consumers and the diverse food sources in the ecosystem. Benthic microalgae in estuarine ecosystems are considered to be 13C-enriched (Doi et al. 2005). In this study, the δ13C values of gastropods distributed in mangrove mudflats (e.g., N. violacea and E. chinense) were higher than those of gastropods living in freshwater habitats (e.g., A. polyzonata and P. canaliculata). The gastropods in mangrove mudflats consumed mainly sediment organic matter and benthic microalgae, whereas those in freshwater habitats consumed mainly leaves and stems of aquatic plants. The macro-invertebrates collected from the Lotus Flower Bridge Flat (E. chinense, N. violacea, E. carincauda, P. plicatum, P. bidens, and P. cantonensis) had similar δ13C values (−22.3 to −20.37 ‰). This result suggested that the food sources were restricted in this area, and that those different types of benthic animals had similar omnivorous diets.
Food-web structure and trophic level of consumers
Naturally occurring stable isotopes of nitrogen and carbon display a stepwise enrichment between prey and consumer tissues. In particular, the heavier nitrogen isotope becomes progressively enriched from prey (3 ‰) to predator (5 ‰). The values of δ15N, therefore, provide a continuous variable that can quantify the relative trophic positioning of biota (Van der Zanden et al. 1998; Persic et al. 2004). Nitrogen isotope distributions have been shown to be robust indicators of trophic position in estuary ecosystems, where 15N enrichment increases predictably with the TL of consumers. The number of trophic levels (food chain length) and consumer trophic positions were approximated in previous studies (Minagawa and Wada 1984; Kwak and Zedler 1997). Quan et al. (2010) examined the 15N isotope distributions of consumers in the Yangtze River Delta, and estimated that there were three trophic levels in that system: primary consumers (debris/phytoplankton feeders, TL2 < 2.6), secondary consumers (omnivorous, 2.6 < TL3 < 3.4) and tertiary consumers (carnivorous animals, TL4 > 4.0). In the present study, invertebrates except for E. carincauda and S. paramamosain occupied the secondary TL and mainly fed on organic debris and phytoplankton, while E. carincauda and S. serrata may have fed on small animals and rotten animal tissues. Yu et al. (2008) reported that the trophic levels of macro-benthic fauna distributed in Chongming Island Flat ranged from 2.0 to 3.7, with P. cantonensis showing the highest value and Glaucomya chinensis the lowest. The trophic levels ranging from 2.0 to 3.0 were occupied by filter-feeding bivalves, deposit-feeding gastropods, and crustaceans. Exopalaemon modestus, E. carincauda, S. serrata, and P. cantonensis represented the secondary trophic level (TL > 3.0), suggested that they could feed on some animal baits. In this study, the TL of the consumers ranged from 1.19 (A. fulica) to 4.27 (P. cantonensis). There was a wide variation in trophic levels among different species. According to the TL division by Quan et al. (2010), the consumer 15N isotopic enrichment detected in this study indicated that there were three trophic levels. The primary consumer TL (mean δ15N value, 5.052 ‰) was represented by freshwater herbivorous gastropods (P. canaliculata and A. polyzonata), filter-feeding bivalves (Mytilopsis sallei) and plankton-feeding fish (M. cephalus). The secondary consumer level (mean δ15N value, 6.794 ‰) included four deposit-feeding fish species (Hemiculter leucisculus, Tilapia, Labeo rohita, and Cryprinus carpiod), which were distributed in Fai Chi Kei Bay, and a deposit-feeding gastropod in Lotus Flower Bridge Flat. The tertiary consumer level (mean δ15N value, 13.473 ‰) consisted of four crab species (P. plicatum, U. arcuata, P. bidens, S. paramamosain), one shrimp species (E. carincauda), and four fish species (S. latus, Tridentiger trigonocephalus, Bostrichthys sinensis, P. cantonensis) in the Lotus Flower Bridge Flat. The main diet of these species was organic debris, bottom fauna, and rotten animal tissues.
In conclusion, we quantified trophic relationships between common species that were collected from representative wetlands using a stable isotopic approach. Based on these results, we proposed food web structure and their site variations in wetlands of Macao. This study also confirms the applicability of the isotopic approach in food web studies where direct quantification is not easy. Collectively, this is the first quantitative study of food web in Macao wetlands, highly urbanized wetlands that still support endangered and endemic animal and plant species. Thus this study provides important knowledge that can contribute wetland management and conservation in Macao.
References
Bouillon S, Connolly RM, Gillikin DP (2011) Use of stable isotopes to understand food webs and ecosystem functioning in estuaries. In: Wolanski E, McLusky DS (eds) Treatise on estuarine and coastal science. Academic Press, Waltham, pp 143–173
Cai DL, Hong XG, Mao XH, Zhang SF, Han YB, Gao SL (2011) Preliminary studies on trophic structure of tidal zone in the Laoshan Bay by using carbon stable isotopes. Acta Ocean Sinica 23:41–47
Chen Q, He WT, Liu Y, Huang JR (2014) Characteristics of the zooplankton communities in four typical wetlands of Macao. J Hydroecol 35:24–30 (in Chinese with English abstract)
Chen Q, He WT, Liu Y, Xu JQ, Huang JR (2015) Characteristics of macrozoobenthic community structure in typical wetlands of Macao. South China Fish Sci 11(4):1–10 (in Chinese with English abstract)
Doi H, Matsumasa M, Toya T, Satoh N, Mizota C, Maki Y, Kikuchi E (2005) Spatial shifts in food sources for macrozoobenthos in an estuarine ecosystem: carbon and nitrogen stable isotope analyses. Estuar Coast Shelf Sci 64:316–322
Fry B (2006) Stable Isotope Ecology. Springer Press, New York, p 308
Fry B, Sherr EB (1984) δ13C measurements as indicators of carbon flow marine and freshwater ecosystems. Contrib Mar Sci 27:13–47
Gu B (2009) Variations and controls of nitrogen stable isotopes in particulate organic matter of lakes. Oecologia 160:421–431
Gu B, Chapman AD, Schelske CL (2006) Factors controlling seasonal variations in stable isotope composition of particulate organic matter in a soft water eutrophic lake. Limnol Oceanogr 51:2837–2848
Hein T, Baranyi C, Herndl GJ, Wanek W, Schiemer F (2003) Allochthonous and autochthonous particulate organic matter in floodplains of the River Danube: the importance of hydrological connectivity. Freshw Biol 48:220–232
Iken K, Bluhm B, Dunton K (2010) Benthic food-web structure under differing water mass properties in the southern Chukchi Sea. Deep-Sea Res II 57:71–85
Jin JJ, Wang ZH, Liu XM (2008) Valuing black-faced spoonbill conservation in Macao: a policy and contingent valuation study. Ecol Econ 68:328–335
Junes RI, Grey J, Sleep D, Arvola L (1999) Stable isotope analysis of zooplankton carbon nutrition in humic lakes. Oikos 86:97–104
Keely JE, Sandquist DR (1992) Carbon: freshwater plants. Plant Cell Environ 15:1021–1035
Kendall C, Silva RS, Kelly JV (2001) Carbon and nitrogen isotopic compositions of particulate organic matter in four large ricer systems across the United States. Hydrol Process 15:1301–1336
Kon K, Kurokura H, Hayashizaki K (2007) Role of microhabitats in food webs of benthic communities in a mangrove forest. Mar Ecol Prog Ser 340:55–62
Kwak TJ, Zedler JB (1997) Food web analysis of southern California coastal wetlands using multiple stable isotopes. Oecologia 110:262–277
Larson ER, Olden JD, Usio N (2011) Shoreline urbanization interrupts allochthonous subsidies to a benthic consumer over a gradient of lake size. Biol Lett 11:1–4
Li QH, He WT, Chen C (2009) Characteristics of the phytoplankton community in wetlands of Macao. Chin J Plant Ecol 33(4):689–697 In Chinese with English abstract
Middelburg JJ (2014) Stable isotopes dissect aquatic food webs from the top to the bottom. Biogeosciences 11:2357–2371
Minagawa M, Wada E (1984) Stepwise enrichment of 15N along food chains: Further evidence and the relation between δ15N and animal age. Geochim Et Cosmochim Acta 48:1135–1140
Persic A, Roche H, Ramade F (2004) Stable carbon and nitrogen isotope quantitative structural assessment of dominant species from the Vaccare`s Lagoon trophic web (Camargue Biosphere Reserve, France). Estuar Coast Shelf Sci 60:261–272
Pinnegar JK, Polunin NVC (1999) Differential fractionation of δ15N and δ13C among fish tissues: implications for the study of trophic interactions. Funct Ecol 13:225–231
Quan WM, Shi LY, Chen YQ (2010) Stable isotopes in aquatic food web of an artificial lagoon in the Hangzhou Bay, China. Chin J Oceanol Limnol 28:489–497
Rodelli MR, Gearing JN, Gearing PJ, Marshall N, Sasekumar A (1984) Stable isotope ratio as a tracer of mangrove carbon in Malaysian ecosystems. Oecologia 61:326–333
Savoye N, Aminot A, Tréguer P, Fontugne M, Naulet N, Kéroue R (2003) Dynamics of particulate organic matter δ15N and δ13C during spring phytoplankton blooms in a macrotidal ecosystem (Bay of Seine, France). Mar Ecol Prog Ser 255:27–41
Sun ZG, Mou XJ, Li XH, Wang LL, Song HL, Jiang HH (2011) Application of stable isotope techniques in studies of carbon and nitrogen biogeochemical cycles of ecosystem. Chin Geogr Sci 21:129–148
The IUCN Red List of Threatened Species. Version 2014.3. <www.iucnredlist.org>. Accessed 16 April 2015
Thimdee W, Deein G, Sangrungruang C, Matsunaga K (2004) Analysis of primary food sources and trophic relationships of aquatic animals in a mangrove-fringed estuary, Khung Krabaen Bay(Thailand) using dual stable isotope techniques. Wetl Ecol Manag 12:135–144
Tue NT, Hamaoka H, Sogabe A, Quy TD, Nhuan MT, Omori K (2012) Food sources of macro-invertebrates in an important mangrove ecosystem of Vietnam determined by dual stable isotope signatures. J Sea Res 72:14–21
Van der Zanden MJ, Hulshof M, Ridgway M, Rasmussen JB (1998) Application of stable isotope techniques to trophic studies of age-0 small mouth bass. T Am Fish Soc 127:729–739
Van der Zanden MJ, Rasmussen JB (2001) Variationin δ15 N and δ15C trophic fractionation; implications for aquatic food web studies. Limnol Oceanogr 46:2061–2066
Vizzini S, Mazzola A (2002) Seasonal variations in the stable carbon and nitrogen ratios (13C/12C and 15N/14N) of primary producers and consumers in a western Mediterranean coastal lagoon. Mar Biol 142:1009–1018
Yu J, Liu M, Hou LJ, Xu SY, Ou DN, Cheng SB (2008) Food sources of macro-faunal in east Chongming salt marsh as traced by stable isotopes. J Nat Res 23:319–325
Zhang L, Xu J, Xie P, Zang XP, Qiu GH, Zeng JF (2007) Stable isotope variations in particulate organic matter and a planktivorous fish in the Yangtze River. J Freshw Ecol 22(3):383–386
Zhang M, Cheong K, Leong KF, Zou FS (2012) Effect of traffic noise on black-faced spoonbills in the Taipa-Coloane wetland reserve, Macao. Wildl Res 39:603–610
Acknowledgments
We thank Chen Hua-sheng and Zhong Yin for their help with stable isotope analyses and valuable comments from two anonymous reviewers. This project was funded by The Science and Technology Development Fund of Macao (045/2010/A) and the special fund for Marine-scientific research in the Public Interest (201305021).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, Q., Liu, Y., Ho, WT. et al. Use of stable isotopes to understand food webs in Macao wetlands. Wetlands Ecol Manage 25, 59–66 (2017). https://doi.org/10.1007/s11273-016-9502-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11273-016-9502-2