Abstract
Wet atmospheric deposition (WAD) measurements during the cyclic warm and dry periods in San Francisco de Campeche City (CAM) and “La Mancha” (LM), Veracruz-Mexico are presented and compared. The behavior of the ionic species in WAD periods from 2006 and 2012 are described. Data on ion concentrations in WAD samples for the study period were analyzed to assess the effects of acid precipitation and to determine sources origin in these heritage cities. During the study the pH values averaged were 6.4 and 4.7 for CAM and LM, indicating active neutralization of the acidic species caused by the alkaline particles due to the high mineral content in the region, La Mancha site tends to be acidic, due to emissions related to fuels from oil platforms. The evaluation of SO42−/NO3− suggests emission sources with greater contribution of sulfur over nitrogen species and the formation of secondary compounds in both sites. Cluster Analysis (CA), showed for the Campeche site a strong correlation between Ca2+, K+, from soil origin; and SO42−, NO3−, suggesting vehicular emissions, and NH4+ associated with the burning of crop fields. The CA for “La Mancha” site showed that SO42−, Ca2+, Mg2+ is related to geological material, and a second group consisting of NH4+, NO3− related to fuel emissions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Atmospheric pollution is a worldwide problem, in particular atmospheric deposition which has generated considerable interest in various parts of the world due to its repercussions on the environment and on materials of cultural heritage. Emissions from combustion processes impacting air quality and historic monuments include gases such as carbon monoxide (CO), carbon dioxide (CO2), nitrogen oxides (NOx), and sulfur dioxide (SO2), the latter a precursor of black crusts and pathologies in heritage materials, which is often used in studies to explain degradation mechanisms (Kampa and Castanas, 2008). These emissions also have a negative impact on human health (Krupińska et al., 2013).
On the other hand, the formation of acid rain is caused by the reaction of sulfur and nitrogen oxides with atmospheric humidity. Causing pH values lower than 5.6, these gases react with water vapor and other atmospheric compounds forming strong acids such as sulfuric and nitric (Başak and Alagha, 2004). Acid rain adversely affects natural ecosystems where it causes a decrease in productivity, acidification of soils and water bodies as well as the deterioration of materials (Singh and Mondal, 2007). These effects are perceptible not only in the areas where their precursors are originated, but can also be observed at great distances from emission sources (Possanzini et al., 1988).
Currently, the phenomenon of acid rain represents a potential problem that has a direct impact on all ecosystems and on the deterioration of materials of historical and artistic interest. Emissions of relevant pollutants such as SO2, NOX, and NH3 are mostly of anthropogenic origin, with industrial activities, vehicle emissions and biomass burning as the main sources of these components (Gallego et al., 2012). Ions such as NH4+, NO3−, and SO42− are associated with negative environmental impacts and health problems.
In southeastern Mexico there is little information on atmospheric deposition. On the other hand, in San Francisco de Campeche (CAM), atmospheric deposition has been monitored since 2002 to evaluate acidity trends in rainfall samples. CAM is located on the west coast of the Yucatan Peninsula, along the Gulf of Mexico. It is in the middle of a small valley, limited to the north and east by small hills with heights no greater than 150 m a.s.l. To the west, the Bay of Campeche delimits its coastal area. Some of those hills are subjected to continuous erosion and construction of residential developments, or are used to extract materials for construction. These very aggressive activities disturb the land, causing dust storms, especially during the dry season, during which local levels of atmospheric aerosols are elevated. During the spring, the city is regularly affected by the traditional burning of local farmland in the region in order to prepare crops. Also, numerous natural forest fires normally occur during dry periods, something that potentially could increase the content of pollutants in the atmosphere (Jaina, 2008).
In the nearby Gulf of Mexico, various commercial activities are carried out. These include the extraction, processing and distribution of hydrocarbons, port and maritime industrial facilities, fisheries, and tourism (Botello et al., 2014). These activities are a potential source of secondary compound precursors that can give rise to the phenomenon of acid rain. Due to the economic importance of the region, wet atmospheric deposition has been studied continuously since 2002 at the station “La Mancha”, a reference site located in the state of Veracruz along the west coast of the Gulf of Mexico (Sosa et al., 2015, 2018).
Cities with tropical climates, such as CAM, show an increasing development over the years, with growing urbanization, industrialization and development of agricultural activities, leading to an increase in emissions of gases and aerosol particles. The conditions prevailing in tropical climate zones, such as high ultraviolet radiation, temperature and water vapor content in the atmosphere, lead to intense photochemical activity throughout the year, accelerating the formation of acidic species in the atmosphere (Lacaux et al., 2003).
Apart from the anthropogenic species sulfur dioxide (SO2), nitrogen oxides (NOX) and ammonia (NH3), inorganic species generally comprise between 25 to 50% of the total mass of particulate matter, the most abundant being sulfate, ammonium and nitrate (Gray et al., 1986). Sulfates (SO42−) and nitrates (NO3−) are generated mainly by the oxidation of sulfur dioxide (SO2) and nitrogen oxides (NOx), respectively, which are associated with industrial emissions and the burning of fossil fuels (Cope, 2004).
Ions such as NH4+, NO3− and SO42− are present in both rainfall and suspended particles, however. It is thus necessary to make total measurements, in order to evaluate the inputs and outputs of these components in different ecosystems, to identify the origin of these ions that contribute to atmospheric pollution, and to relate emission patterns in different areas (John et al., 1990).
This work presents and evaluates results of wet atmospheric deposition monitoring from 2006 to 2012 at the Campeche and La Mancha sampling sites. Sources of pollutants and ionic species are investigated and identified. This information is expected to enhance studies on air quality in order to evaluate natural trends of wet atmospheric deposition in this important region of the Gulf of Mexico.
2 Methodology
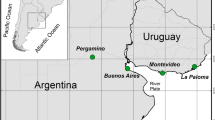
Sampling was carried out with automatic equipment (Model 301, Aerochem Metrics, Inc.) for the collection of wet deposition, using procedures based on techniques developed by the Environmental Protection Agency (USEPA) and the National Atmospheric Deposition Program of the United States of America (NADP) (NADP, 2004; US EPA, 2004). The equipment was located on the rooftop of the Instituto Nacional de Antropología e Historia (INAH) building (19.84295N, -90.53678W) in the city of San Francisco de Campeche (CAM site) Mexico, an area characterized by intense economic activity and high vehicular traffic. The LM site was located in Veracruz in the location “Centro de Investigaciones Costeras La Mancha” at the sea´s shore (Fig. 1) (Sosa-Echeverría et al., 2018).
The sampling and analysis program implemented by the University of Mexico (UNAM) laboratory includes quality assurance and quality control plans that yield reliable data complying with recommendations from the US-EPA (US-EPA, 1994), the National Atmospheric Deposition Program (NADP, 2014 and 2017), and the Global Atmosphere Watch Precipitation Chemistry Program of the World Meteorological Organization (WMO, 2004). The sampling and analysis program were adopted based on experience (Sosa-Echeverría et al., 2018; Sosa et al., 2020) and recommendations from institutions and recognized researchers (Krupa, 2002; Hautman Daniel and Munch David, 1997).
Wet deposition samples were collected using automatic wet/dry deposition collectors (Aerochem Metrics). Each sample was collected in a standard high-density polyethylene bucket, transferred to a polyethylene bottle (Nalgene©), and refrigerated at 4 °C for preservation (Hautman Daniel and Munch David, 1997). Samples were sent to the UNAM laboratory for chemical analysis. Samples greater than 1.0 mm in precipitation depth were analyzed. Rain samples were filtered through a 0.22 µm Millipore membrane (Whatman©) in order to remove particles, and all material was washed with deionized water before chemical analysis. The pH was measured within 24 h of the arrival using several pH meters (Corning 315, Methrom 827, and Orion 960). Sulfate (SO42−), nitrate (NO3−), chloride (Cl−), calcium (Ca2+), magnesium (Mg2+), potassium (K+), ammonium (NH4+), and sodium (Na+) were analyzed by Ionic Chromatography (IC). Anions were analyzed with a Perkin Elmer equipped with an isocratic LC pump 250 and conductivity detector and a Hamilton PRPX-100 analytical anion column. Cations were analyzed with a Waters liquid chromatograph equipped with an isocratic Waters 510 pump and a conductivity detector (Waters 432) using a Waters analytical cationic column. High-purity ion standards were used for calibration. This identification and quantification of ions was based on US.EPA Method 300.1 (Stumm & Morgan, 1970). Detection limits for all ions in wet atmospheric deposition analysis are presented in Table 1. Alkalinity (HCO3−) was determined using the Gran titration method with pH meters (US-EPA, 1991). Electric Conductivity (EC) was determined with YSI 32 and HORIBA D-424 conductivity instruments.
The quality of analysis of each sample was routinely validated with ion balance and specific conductance calculations on sampling days. Additionally, regular field blanks were analyzed to guarantee the cleanliness of the sampling material, and replicates were analyzed routinely to assure appropriate precision and accuracy.
2.1 Cluster Analysis (CA)
Cluster analysis was used as an exploratory tool to determine associations between elements and to assist in the identification, by means of a multivariate model, of possible sources of origin of the measured ions in wet deposition (Karson, 1982). A total of 8 variables and 311 samples were used. The analysis considered data from 2006 to 2012 for soluble ions in SFC. For the LM site, 435 samples with 8 variables were used for the same study period. To obtain the dendrograms, the Pearson correlation coefficient r and Ward’s method were applied, using R software (Maechler et al., 2012).
2.2 Importance of Atmospheric Transport at the Gulf of Mexico Region
Back trajectories to the region of the “La Mancha” (LM) sampling site in Veracruz, Mexico, show predominant transport from the east (open water) during the rainy season (Kahl et al., 2007). The region to the east of LM shows offshore petroleum operations as sources of acid rain precursors (Sosa et al., 2020). The trajectories carried out basically showed two patterns related to the season of the year in which the rain events occur: northerly winds during the dry season and easterly winds during the rainy season. The trajectories coming from the north occur in winter due to the presence of cold fronts, while in summer the east winds predominate, mainly due to the influence of the trade winds on the study area (Saranova, 2018). Back trajectories from the east during the rainy season comes from the Caribbean Sea crossing the Yucatan Peninsula and the Gulf of Mexico, and onward to the coast of Veracruz. Dominant winds at Campeche and “La Mancha” are from the land and the ocean, respectively.
3 Results
3.1 Precipitation During 2006–2012
A total of 311 rainfall samples were collected from 2006 to 2012 in the CAM site. It was observed that 2006 was the year with the highest rainfall which annual average 111.5 mm and accumulated rainfall of 1339 mm, followed by 2010 and 2012 with average value of 107.3 mm and 95.6 mm, and accumulated rainfall of 1291nmm and 1151 mm respectively, 2009 was the year with the lowest rainfall with a value of 58.1 mm and accumulated rainfall of 698 mm.
A total of 453 rainfall samples were collected for the period from 2006 to 2012 in the LM site. The site had in 2012 an annual average of 122.2 mm and accumulated rainfall of 1466 mm, the other hand the years with the highest average annual rainfall were in 2008 and 2010 with values of 106.8 mm y 117.4 mm and accumulated rainfall volume were with values of 1282.5 mm and 1409.1 mm, respectively. 2011 was the year with the lowest rainfall with a value of 68.7 mm accumulated rainfall of 824.5 mm. The comparison is valid because the devices used, the protocols for sample collection are based on the NADP and WMO (NADP., 2004; WMO, 2004). The data used for comparison correspond to the same period at both sites.
3.2 Variation of pH
The rainfall pH characteristics were analyzed to determine the acidity in wet atmospheric deposition samples. The pH values were compared between the CAM and LM sites. During the study period it can be observed that the pH behavior in CAM tends to be not acid, with average values of 6.3 (Fig. 2). Rainfall (wet deposition) is considered acidic if the pH is less than the reference value of 5.6 (Charlson and Rodhe, 1982). The observed alkalinity in wet deposition at CAM is probably due to the neutralization exerted by alkaline particles on the samples, presumably due to the presence of carbonates and silicates that are endemic minerals in the region. The pH value recorded during rainfall events ranged from 4.5 to 8.4 for the study period, and a trend towards alkaline levels was observed during all years for Campeche site (Fig. 2). The mean pH value was 6.58 which is above the widely accepted background rainfall value of 5.6 (Charlson and Rodhe, 1982).
As mentioned earlier, the CAM site is influenced by winds typically blowing from land toward the sea, supporting the hypothesis that the alkaline character in the CAM samples is due to dust contributions from soil at the sampling site. For example, Reyes et al. (2011), reported that Ca2+ from the alkaline soils of the Yucatan Peninsula is transported by wind and incorporated into rain drops, contributing to the neutralization of acidic compounds. Topcu et al. (2002) similarly reported alkaline pH values due to high calcium (CaCO3) loading from alkaline.
On the other hand, the pH values for the LM site show an acidic character during the same study period (Fig. 2). The site is influenced by winds coming from the sea containing contaminants from the oil platforms of the “Sonda de Campeche”. Back trajectories showed transport to LM sampling site from the East during rainy season (June–October) with 80% of precipitation events showing this same pattern. During the dry season (November–May), containing 20% of the precipitations events, back trajectories come from the North-East, as described by Sosa et al., 2020. Other studies conducted on the coast of Campeche and in the Caribbean region of the Yucatan Peninsula have also reported acidic values of precipitation pH. In San Antonio Cárdenas [Cerón et al., 2006] pH values of 4.6 were observed, with the acidity in the site due to the activity of a nearby gas plant. Other sites in the Caribbean region such as Puerto Morelos (Bravo et al., 2000) and Isla del Carmen [Cerón et al., 2006] reported pH values of 5.3 y 5.8, respectively; due to commercial and tourist activities that predominate in these sites.
3.3 Chemical Composition of Rainwater
Table 2 describes the ionic concentrations of rainwater at the CAM site. The most abundant ions were found in the following order: Cl− > Na+ > Ca2+ > SO42− > Mg2+ > NO3− > NH4+ > K+ > H+. The high levels of Cl− and Na+ are most likely due to the contribution of sea salt because CAM site is located on the coast of the Gulf of Mexico. The proximity of the coast to the sampling site (700 m) favors high levels of Cl− and Na+ ions due to the presence of marine aerosols. In this sense, the concentration of NaCl in the region’s atmosphere depends not only on the distance to the coast, but also on the occurrence of seasonal meteorological phenomena such as winter cold fronts and tropical storms in the fall season, which contribute to the entrainment of moisture and marine aerosols from coastal areas (Corvo et al., 2010).
The presence of Ca2+ is related to the type of soil that predominates in the southeast of Mexico, due to the high content of minerals such as carbonates and silicates that are endemically found in the Yucatan Peninsula. Mg2+ and K+ ions during the study period are observed to have similar concentrations, which suggests that they have a common source and a likely geological origin or marine aerosol.
On the other hand, the contribution of SO42− and NO3− must be related to some anthropogenic activity. The climatic conditions of the region favor the formation of secondary compounds due to high levels of solar radiation and temperature. Combined with a large vehicle fleet in Campeche city, the presence of these ionic species at the site suggests emissions related to vehicular traffic.
Table 3 shows the levels of ionic species at the LM site. The most abundant ions were: Cl− > Na+ > SO42− > NO3− > NH4+ > Ca2+ > Mg2+ > K > H+: the high levels of chlorine and sodium are due to the fact that LM is located on the shores of the Gulf of Mexico and are influenced by sea salts. The presence of SO42−, NO3− ions suggests contributions due to activities carried out in the Campeche sounding by the oil platforms, and by the wind currents that carry oil industry emissions from sea to land in the region [Sosa et al., 2020]. For the LM site, the presence of ionic species as Ca2+, Mg2+, and K+ must be associated with soil-related sources.
3.4 Ion Concentration Trends
The variation in the ionic composition of wet atmospheric deposition samples between CAM and LM sites helps to explain the significant differences in the pH values and the high values species ions as Ca2+, NH4+, SO42− and NO3− determined for these two sites.
Ca2+ ion concentrations (Fig. 3) are higher at the CAM site compared to the LM site. The average Ca2+ concentration was 40.41 μeq·L−1, with maximum and minimum values of 133.5 μeq·L−1 and 10.23 μeq·L−1, respectively. The high Ca2+ levels at CAM site indicate a contribution of dust coming from the soil, due to minerals with high carbonate content, which is endemic to the region. An analysis of wind trajectories in Campeche revealed that during the rainy season winds arrive mainly from the southeast (Kahl and Saunders, 2012), suggesting that winds cross the Yucatan peninsula carrying dust with high mineral content and impacting the CAM site.
For the LM site, an average concentration of 8.25 μeq·L−1 was determined, with maximum and minimum values of 35.53 μeq·L−1 and 2.78 μeq·L−1, respectively. The statistical test indicates significant differences between Ca2+ levels at the two sites, with higher levels in Campeche influencing the atmospheric deposition of the site.
The average NH4+ concentration at the CAM site (Fig. 4) was 7.27 μeq·L−1, with maximum and minimum values of 34.33 μeq·L−1 and 1.12 μeq·L−1, respectively. At the LM site, the average concentration was 8.68 μeq·L−1 with maximum and minimum values of 37.73 μeq·L−1 and 2.22 μeq·L−1, respectively. Major sources of ammonia are known to be natural or fertilized soils, excrements of human and animals, and wood burning. Ammonium (NH4+) thus originates mainly from agricultural sources, including animal manure and fertilized soil, intensive agriculture and livestock (Galloway & Cowling, 2002; Walker, et al., 2019). Intensive agricultural activities predominate in both sites in the region, along with a contribution from forest fires during the dry season (Jaina, 2008) and marine aerosol (Walker, et al., 2019).
The average SO42− concentration (Fig. 5) at the CAM site was 13.92 μeq·L−1. The maximum and minimum values at CAM were: 46.13 μeq·L−1 and 2.12 μeq·L−1. The highest values at the CAM site were in 2006, 2007, 2009; with average concentrations of 15.78 μeq·L−1, 16.38 μeq·L−1 and 18.8. μeq·L−1, respectively. The concentration of SO42− at LM site was 14.54 μeq·L−1 with maxima and minima of 54.59 μeq·L−1 and 5.83 μeq·L−1, respectively. The years where the highest values were presented were: 2007, 2008 and 2009, with values of 15.54 μeq·L−1, 15.81 μeq·L−1and 16.38 μeq·L−1.
The SO42− values at the CAM and LM sites are similar, however the CAM site is influenced by dust contributions and activities related to the burning of agricultural fields and activities related to vehicular traffic, the latter should directly influence SO42− levels. In recent years CAM has experienced urban growth and in the last decade the city increased its population. In addition, there has been a significant increase in the number of motor vehicles that circulate on a daily, with a vehicles number of 353 for 1000 habitats (SEMARNAT, 2018). With respect to the LM site, it is influenced by oil platforms in the Sonda de Campeche.
On the other hand, if we compare SO42− emissions in this study with data reported by the NADP (National Atmospheric Deposition Program), sulfate emissions are higher, for example stations in Florida report on average concentrations of 18 μeq·L−1 (Sosa et al., 2020; Villaseñor et al., 2003), being these lower compared to sites in Mexico, it is likely that the differences are due to the fact that in Mexico the sulfur content in fuels is 4% by weight (CFE, 2016), the excessive vehicle fleet, the activities related to the burning of biomass and forest fires at the site, may be influencing the high sulfur content in the atmospheric deposition.
The average NO3− concentration (Fig. 6) at the CAM site was 8.87 μeq·L−1, with maximum and minimum values of 28.71 μeq·L−1 and 2.11 μeq·L−1. The years with the highest NO3− levels were 2007, 2009 and 2011 with values of 9.98 μeq·L−1, 9.65 μeq·L−1 and 10.47 μeq·L−1, respectively. It can be observed that the concentrations for NO3− ion are very similar during the study period, suggesting that they come from a common source. The average NO3− concentration at the LM site was 10.59 μeq·L−1 with maximum values of 46.15 μeq·L−1 and minimum values of 5.83 μeq·L−1. The years with the highest concentrations were 2006, 2007 and 2008; with values of 11.26 μeq·L−1, 12.51 μeq·L−1 and 15.99 μeq·L−1, respectively. The origin of this environmental problem derives from the increase in emissions of reactive N (Nr), both in its oxidized form (N oxides, NOx), mainly associated with increased consumption of fossil fuels and increased agriculture and livestock (Galloway et al., 2004), also combustion processes at high temperatures and in the generation of electric power, its importance lies in the participation as a precursor of photochemical smog (SEDEMA, 2016).
3.5 SO4 2− /NO3 − Ratio
The SO42− /NO3− ratio is an indicator used to evaluate whether there is a reduction in SO2 and NOx emissions, since these primary atmospheric pollutants directly influence SO42− and NO3− ion concentrations in the atmosphere. Ratios greater than 1 indicate a major importance of the sulfur compounds as acid rain precursors and emissions come from anthropogenic sources and are related to activities involving the use of fuels. In the United States, this ratio has been applied to assess the effects of emission regulations on SO42− and NO3− in acidic wet deposition (Lehmann et al., 2015).
In Fig. 7, the results of the SO42−/NO3− ratios for each of the years in this study are shown. The results show that the average value of this ratio at CAM was 1.36, behaving similarly during the study period, suggesting that the presence of these ions is related to anthropogenic activities, and is probably due to emissions from motor vehicles. The years with the highest SO42− /NO3− levels in CAM site were 2009, 2010 and 2011 with values of 1.92, 1.62 and 1.29, respectively. The sulfate ion in Campeche has a decreasing trend during the last 3 years of this study. Finally, the results of the SO42−/NO3− ratio indicate that there is an important source of sulfur compound emissions in Campeche.
The SO42−/NO3− ratio at the LM site was 1.74 on average for the study period and is major compared to the CAM site. The years with the highest SO42−/NO3− levels in LM site were 2009, 2011 and 2012 with values of 2.03, 2.25 and 1.87, respectively. The SO42−/NO3− ratio at the LM site indicates the importance of the sources of sulfur compound emissions in Mexico in the southeastern part of the country, which is influenced by the marine winds coming from the “Sonda de Campeche” where the PEMEX oil platforms are located and which are carried towards the coast by the prevailing easterly winds. On the other hand, in Mexico, 60% of NOx emissions are anthropogenic, with transportation, electricity generation power and other industrial processes being the main contributors. (CFE, 2016).
Among the most important results are the high values of the SO42− /NO3− ratio associated with the LM site located in the State of Veracruz, which suggests a strong influence of emissions derived from sulfur compounds due to anthropogenic activities in the wet atmospheric deposition in southeastern Mexico.
3.6 NH4 +/NO3 − Ratio
The ratio of NH4+ to NO3− in wet atmospheric deposition is an essential indicator of atmospheric chemistry, reflecting the partitioning of emission sources (Du et al., 2014), and is also important regarding the environmental impacts of nitrogen deposition. There is evidence for differential effects of reduced and oxidized nitrogen deposition on vegetation independent of nitrogen load (Van den Berg et al., 2016). NH4+ deposition appears to be more effective than NO3− deposition in decreasing biodiversity and is more harmful to vegetation (Erisman et al., 2007).
In Fig. 8, the results of the NH4+/NO3− ratio for the study sites are presented. The average value at the CAM site was 0.82, with maximum and minimum values of 1.42 and 0.52, respectively. The years with the highest values were 2006 with 0.85, 2010 with 1.42 and 201 with 0.93; for the remaining years it behaved similarly. It is likely that high values of this quotient are due to emissions related to fertilizers or livestock waste due to the burning of crop fields during the dry season to prepare for planting and animal grazing (Jaina, 2008). NH4+ emissions are likely due to livestock activities and fertilizer use (CFE, 2016). The LM site recorded average values of 0.75, with maximum and minimum values of 1.06 and 0.7, respectively. The years with the highest values were 2007, 2008 and 2011, with average values of 1.01, 0.90 and 1.1, respectively.
3.7 Acidity Neutralization on Rainwater
3.7.1 Fractional Acidity
Another way to evaluate the relationship between acidic and neutralizing species in rainwater is the use of Fractional Acidity (FA):
According to Kaya and Tuncel (1997) and Balasubramanian et al. (2001), if the rainwater’s acidity is caused by strong acids like H2SO4 and HNO3 and it is not alkalinized at all, the FA should exhibit values close to one, while if the values are far from unity, it can be said that the inorganic acidity is neutralized.
Table 4 and 5 shows the variation of the fractional acidity obtained from in the precipitation samples from each of the sampling sites. The FA at the CAM site (Table 4) shows an average value of 0.13, ranging from 0.032 to 0.391, indicating that the 60% to 96% of the inorganic acidity of the precipitation is neutralized. Several studies have shown that the acidity of rainfall is directly related to H2SO4 and HNO3, while acid neutralization occurs in the presence of NH3 and CaCO3 (Topçuet al., 2002, Ramírez et al. 2010). This suggests that in southeastern Mexico species such as H2SO4 and HNO3 can be neutralized by the abundance of CaCO3 in the soils of the region and/or by the presence of atmospheric particles with Ca2+ and Mg2+ content (Balasubramanian et al., 2001). At LM site, the FA (Table 5) averages 0.72, with a range of 0.431 to 0.809, suggesting that the 19% to 57% of the inorganic rainfall acidity has been neutralized in Veracruz. This indicates that the main acid ions controlling the acidity of precipitation are sulfate and nitrate (Huang et al., 2008) and that they are related to the emissions from “Sonda de Campeche” as explained in previous sections. This behavior is mainly due to the high concentration of H+ ions at the LM site, which is 10 times higher than at the CAM site, as shown in Tables 2 and 3, suggesting that the Veracruz coastal zone favors the formation of compounds such as H2SO4 and HNO3 due to the relative abundance of H+ ions at the LM site.
3.7.2 Neutralization Factor
In order to assess the neutralizing capacity of alkaline species such as NH3 and CaCO3, the neutralization factor (NF) was determined for each cation and were calculated for all major cations (Eq. 2) to assess the neutralization capacity of precipitation (Chang et al., 2017; Zhang et al., 2007).
where [Xi] is the concentration of the alkaline component (Ca2+, NH4+, Mg2+, Na+, K+). The NF, which involves the concentrations of acids and bases that are present for wet and dry atmospheric deposition, (Balasubramanian et al., 2001; Rodhe et al., 2002; Moreda-Piñeiro et al., 2014; Sosa et al., 2015).
The NF results are shown in Tables 4 and 5 for CAM and LM sites, respectively. Values greater than unity are the indicators of higher neutralization capacity (Chang et al., 2017).
The NF results indicate that NH4+, Ca2+ and Na+ have the highest neutralizing potential, with an average NF value of 0.46, 0.30 and 0.29, respectively. The Na+ ion is the species that participates the most in the neutralization with an average value of 4.7 with a range of 2.15 to 5.64 for the years of study. On the other hand, the calcium ion must also be contributing to the neutralization of rainwater since it presented an average of 3.03 with a range of 1.35 to 4.10 during the same period. Note that this species participates significantly in the behavior of wet deposition at the CAM site, influencing the pH behavior and generating a non-acid character to the atmospheric deposition samples. It is likely that this neutralization (by Ca2+) occurs at cloud level, while below cloud level neutralization occurs due to ammonia and SO2 adsorption with suspended particles containing Ca2+ and Mg2+ (Balasubramanian et al., 2001). Also the concentration of H+ ion reflects the acidity of raindrops after the neutralization process by Ca2+ and NH4+ (Chate and Devara, 2009).
These values can be explained by the geographical characteristics of the sampling sites, since the elevated concentrations of NH4+ indicate air of marine origin transported from the Southeast Gulf of Mexico, while the significant calcium concentrations may be due to the dissolution of calcareous rocks from the neighboring mountainous regions. The contribution of Mg2+ and K+ to the neutralization process was minimal. In general, the results showed that alkaline species have a remarkable effect on acidic compounds.
Table 5 shows the NF values for the LM site, where the ionic species that mostly participate in the neutralization in the atmospheric reservoir are Na+, with an average value of 3.28 and a range of 1.61 to 3.59, and Ca2+ with an average of 3.03 and a range of 1.35 to 4.10. These ions participate strongly in the neutralization of rainwater, being marine aerosols and particles containing principally calcium ions (geological material). The difference in this behavior is mainly due to the fact that in the area of the LM, emissions from the oil platforms in the Campeche Sound are causing an acidic character in the wet atmospheric deposition samples, mainly due to sulfur-related compounds and nitrogen oxides.
3.8 The Neutralizing and Acidic Potential (NP/AP) in Rainwater
Another useful indicator to estimate the chemical nature of the rainwater is the ratio of Acidic Potential (AP) to Neutralization Potential (NP) (Roy et al., 2016). The AP is represented by the sum of the non-sea salt SO42− and NO3− (nssSO42− + NO3−), while the NP is the value of non-sea salt Ca2+ and NH 4+(nssCa2+ + NH4+). The ratio AP/NP is defined in Eq. 3:
Tables 6 and 7 show the average annual values of the AP/NP ratio for the study period at both sites in order to evaluate differences in neutralization capacity. Furthermore, the AP can be used as an indicator to identify pollution sources due to human activities, while the NP can be used as an index to demonstrate the effect of air masses of continental origin, as suggested by Fujita et al. (2000) and Wu et al., (2016).
The AP/NP at the CAM site showed an average value of 0.61, with maximum value of 1.3 in 2009 and minimum values of 0.24 in 2010. The AP/NP ratio at the CAM site indicates that the acidity was neutralized and only in 2009 was a high acidity present with a value of 1.26. The neutralization at the CAM site is influenced by the high concentrations of carbonates that predominate in the Yucatan Peninsula, as previously discussed. At the LM site the AP/NP ratio averaged 2.07, with a maximum of 2.74 in 2010 and a minimum of 0.74 in 2008. This indicates a high acidifying potential at the LM site., providing additional evidence for the influence of emissions from petroleum activities in the “Sonda de Campeche”.
3.8.1 Marine Contribution
In order to categorize chemical constituents of rainwater into natural and anthropogenic sources, we calculated sea and non-sea salt contributions of the species like SO42−, Mg2+, Ca2+, and K+ by the following equation assuming Na+ as the reference element for sea water (Keresztesi, et al., 2020):
Here ss-X and nss-X are the sea-salt and non-sea salt fractions of the desired component X, Na+ sea is the standard ratio of the component X to Na+ in sea water and X tot is the total concentration of X in rainwater. The contribution of non-sea salt (NSS) ions was calculated for both sites of the ions SO42−, Mg2+, Ca2+, and K+, according to the procedure established by Kumar et al. (2006). The results (Table 6) show that the average contribution of NSS in SO42− and K+ was 13.4% and 9.1% respectively, suggesting that the presence of these ions is due to crustal origin. The result of the NSS for Ca2+ was 54.7%, suggesting that hat the contribution of this ion is from sea salt and soil; and in the case of Mg2+ and Cl−, the NSS value suggesting that the contribution of this ion is from sea salt.
The results of non-sea salt contributions for the LM site (Table 7) show an average contribution of NSS SO42− of 60.3%, suggesting that the presence of this ion is due to anthropogenic activities related to “Sonda de Campeche” emissions. The NSS for Ca2+ was 83.91% suggesting a crustal origin, and the NSS for K+ was 55.8%, suggesting that the contribution of this ion is from sea salt and soil. The NSS values for the ions Mg2+ and Cl− suggest a contribution from sea salt.
3.9 Cluster Analysis (CA)
The CA for the CAM site (Fig. 9a) shows the presence of 3 well-defined groups. The first group is composed of ions SO42−, Ca2+, and NO3− which may be related to geological material but mainly unpaved and asphalt roads. The presence of SO42− in this component must be related to fuel burning and vehicular traffic due to the presence of S. The second group is made up of Cl−, Na+, and Mg2+ ions, suggesting a contribution due to marine aerosols (sea salt). The third group shows an association between the ions K+ and NH4+, indicating ammonium emissions related to livestock activities and the use of fertilizers (CFE, 2016). The K+ in this group must be related to the burning of crop fields in livestock activities (Espinosa et al., 2019; Galloway & Cowling, 2002; Galloway et al., 2008).
The CA for the LM site (Fig. 9b) shows the presence of 3 groups with correlations between the groups. It can be observed that one group is composed of the ions SO42−, Ca2+, and Mg2+, presumably related to resuspended dust mainly due to unpaved and asphalt roads. The second group consists of the ions Cl− and Na+ and is due to the contribution of marine aerosols (sea salt). The third group shows an association between the ions K+, NH4+ and NO3−. The latter two ions originate mainly from intensive agriculture and livestock and the burning of fossil fuels, respectively (Galloway & Cowling, 2002; Galloway et al., 2008). The K+ ion must be related to the burning of crop fields because K is used as a smoke tracer (Espinosa et al., 2019; Miranda et al., 2004).
4 Conclusions
The results of this study show that the acid rain phenomenon is not observed in Campeche during the period from 2006 to 2012. The pH values indicate alkaline levels during the six years of data studied. The presence of soil particles and high content of minerals such as carbonates in the region is influencing the non-acid character of the atmospheric deposition at the site. On the other hand, the “La Mancha” site shows pH values lower than 5.3 indicating a tendency of acid character during the period. This behavior is influenced by various industrial and commercial activities, in the region, mainly by emissions related to oil activities in the “Sonda de Campeche” that are transported to the coast of Veracruz, Mexico.
The alkaline character of the Campeche (CAM) site is influenced by wind trajectories coming from the Caribbean, which cross the Yucatan Peninsula to Gulf of Mexico, suggesting that carbonate-containing dust that is endemic to southeastern Mexico has a strong influence on the precipitation chemistry. On the other hand, the wind trajectories that crossing the Gulf of Mexico and carry the emissions from the oil platforms of Sonda Campeche, which are transported to the coasts of Veracruz by the effect of the sea breeze.
An analysis of wind trajectories in Campeche revealed that during the rainy season winds arrive mainly from the southeast (Kahl and Saunders, 2012), suggesting that winds cross the Yucatan peninsula carrying dust with high mineral content and impacting the CAM site.
At the CAM site, high levels of Cl−, Ca2+ and SO42− ions were observed, suggesting that the site is influenced by marine aerosols and geological material endemic to the Yucatan Peninsula. The Ca2+ content in Campeche is higher than the values recorded in at the “La Mancha” site, suggesting that resuspended geological material contributes significantly to the non-acidic behavior in the wet atmospheric deposition at CAM and is neutralizing the acidic species. Also, high levels of SO42− were observed at this site, related to fuel burning, commercial activities and garbage burning on the street. At La Mancha high levels of SO42− and NO3− were detected. The presence of these ions is related to commercial activities in the region, as well as emissions from the oil platforms in the Sonda de Campeche. This provides a strong motivation to continue monitoring ionic species at La Mancha and CAM site to evaluate wet atmospheric deposition trends in the region.
The SO42− /NO3− ratio for the CAM site showed an average value of 1.36, behaving similarly during the study period, further suggesting that these ions are of anthropogenic origin, probably associated with motor vehicles and commercial activities. The SO42− /NO3− ratio at the LM site was 2.52, two times higher that at the CAM site, indicating the importance of the sulfur emissions sources in southeastern of Mexico, particularly those coming from the “Sonda de Campeche” and related activities associated with the petroleum industry along the coast of the Gulf of Mexico.
The FA revealed the contribution of acidic substances in the LM site, and neutralizing compounds reducing the acidity of precipitation at the CAM site. At LM, precipitation acidity is produced by the ionic species SO42− and NO3−, with sulfur-derived compounds having a greater impact on precipitation acidity than nitric acid. Emissions originating from oil activities in the “Sonda de Campeche “ were determined to be the main factors in the formation of acid rain in the LM region. Acidifying agents at the CAM site originate mainly from agricultural activities, including vehicular emissions, which are neutralized by suspended particles containing high mineral content such as carbonates.
The cluster analysis showed an important correlation in both sites between Cl− and Na+, indicating the importance of marine aerosols. However, high levels of SO4−2 and NO3− were also found. At CAM these were due to the circulation of automotive vehicle and motorcycle taxis emissions. At LM the high levels of SO42− and NO3− are due to emissions from oil platforms. On the other hand, a significant presence of elements was observed, such as Mg2+, K+, Ca2+, that are associated with a geological origin.
4.1 Recommendations
Expanding the sampling sites along the coasts of Mexico will allow us to generate relevant information on the behavior of the wet atmospheric deposition in the Gulf of Mexico and its effects on ecosystems. Evaluate sulfate and nitrate emissions in the Gulf of Mexico and those generated by the use of fuels on the coasts of Campeche and Veracruz to determine critical loads for air and soil quality studies. Work with government authorities to use the results generated from wet atmospheric deposition studies to update the Gulf of Mexico emissions inventory and collaborate with NADP researchers to compare data to identify possible effects of acid rain.
Data Availability
Not aplicable.
References
Balasubramanian, R., Victor, T., & Chun, N. (2001). Chemical and statistical analysis of precipitation in Singapore. Water, Air and Soil Pollution, 130, 451–456. https://doi.org/10.1023/A:1013801805621
Başak, B., & Alagha, O. (2004). The chemical composition of rainwater over Büyükçekmece Lake. Istanbul. Atmospheric Research., 71(4), 275–288. https://doi.org/10.1016/j.atmosres.2004.07.001
Botello, A. V., Páez-Osuna, F., Mendez-Rodríguez, L., Betancourt-Lozano, M., Álvarez-Borrego, S., Lara-Lara, R. (2014). Pacífico Mexicano. Contaminación e impacto ambiental: diagnóstico y tendencias. UAC, UNAM-ICMYL, CIAD-MAZATLÁN, CINBOR, CICESE. 930, México.
Bravo, H. A., Saavedra, M. I. R., Sánchez, P. A., Torres, R. J., & Granada, L. M. M. (2000). Chemical composition of precipitation in a Mexican Maya region. Atmospheric Environment., 34, 1197–1204. https://doi.org/10.1016/S1352-2310(99)00305-2
Cerón, R. M., Cerón, J. G., & Muriel, M. (2006). Influence of geochemical and anthropogenic sources on rain- water chemical composition in two coastal sites impacted by the gas and oil industry in Campeche, Mexico. Enviromental Problems in Coastal Regions VI. WIT Transactions on Ecology and the Environment, 88, 419–428.
CFE. Comisión Federal de Electricidad. (2016). Guía para elaborar el inventario de emisiones de gases por la operación de centrales de generación que consumen combustibles fósiles. https://lapem.cfe.gob.mx/normas/pdfs/v/SPA00-55.pdf.
Chang, C. . Te., Wang, C. P., Chuan, J. H., Wang, L. J., Liu, C. P., & Lin, T. C. (2017). Trends of two decadal precipitation chemistry in a subtropical rainforest in East Asia. Science of the Total Environment, 605–606, 88–98. https://doi.org/10.1016/j.scitotenv.2017.06.158
Charlson, R. J., & Rodhe, H. (1982). Factors controlling the acidity of natural rain water. Nature, 295, 683–685. https://doi.org/10.1038/295683a0
Chate, D. M., & Devara, P. C. S. (2009). Acidity of raindrop by uptake of gases and aerosol pollutants. Atmospheric Environment., 43, 1571–1577. https://doi.org/10.1016/j.atmosenv.2008.06.031
Cope, W. G. (2004). Exposure classes, toxicants in air, water, soil, domestic and occupational settings. Wiley, New Jersey.
Corvo, F., Reyes, J., Valdes, C., Villaseñor, F., Cuesta, O., Aguilar, D., & Quintana, P. (2010). Influence of air pollution and humidity on limestone materials degradation in historical buildings located in cities under tropical coastal climates. Water, Air, and Soil Pollution, 205(1), 359–375. https://doi.org/10.1007/s11270-009-0081-1
Du, E., de Vries, W., Galloway, J. N., Hu, X., & Fang, J. (2014). Changes in wet nitrogen deposition in the United States between 1985 and 2012. Environmental Research Letters, 9, 095004. https://doi.org/10.1088/1748-9326/9/9/095004
Erisman, J. W., Bleeker, A., Galloway, J. N., & Sutton, M. S. (2007). Reduced nitrogen in ecology and the environment. Environmental Pollution., 150, 140–149. https://doi.org/10.1016/j.envpol.2007.06.03
Espinosa, A. A., Miranda, J., Hernández, E., Reyes, J., Alarcón, A. L., Torres, M. C., & Sosa, R. (2019). Temporal variation of suspended particles (TSP, PM 10, and PM 2.5) and chemical composition of PM 10 in a site at the coast of the Gulf of Mexico. Air Quality, Atmosphere & Health, 12(11), 1267–1277. https://doi.org/10.1007/s11869-019-00730-8
Fujita, S., Takahashi, A., Weng, J., Huang, L., Kim, H., Li, C., Huang, F. T. C., & Jeng, F. (2000). Precipitation chemistry in East Asia. Atmospheric Environment., 34, 525–537. https://doi.org/10.1016/S1352-2310(99)00261-7
Gallego, P., González, I., Sánchez, G., Fernández, P., Garcicuño, R., & Bravo, J. (2012). Contaminación Atmosférica. Madrid: UNED.
Galloway, J. N., & Cowling, E. B. (2002). Reactive nitrogen and the world: 200 years of change. Ambio, 31(2), 64–71.
Galloway, J. N., et al. (2004). Nitrogen cycles: Past, present and future. Biogeochemistry, 70, 153–226. https://doi.org/10.1007/s10533-004-0370-0
Galloway, J. N., Townsend, A. R., Erisman, J. W., Bekunda, M., Cai, Z., Freney, J. R., Martinelli, L. A., Seitzinger, S. P., & Sutton, M. A. (2008). Transformation of the nitrogen cycle: Recent trends, questions and potential solutions. Science, 320, 889–892. https://doi.org/10.1126/science.1136674
Gray, H. A., Cass, G. R., Huntzicker, J. J., Heyerdahl, E. K., & Rau, J. A. (1986). Characteristics of atmospheric organic and elemental carbon particle concentrations in Los Angeles. Environmental Science & Technology., 20(6), 580–589.
Hautman Daniel P and Munch, David J. (1997). Method 300.1 Determination of inorganic anions in drinking water by ion chromatography. EPA. Ohio.
Huang, K., Zhuang, G., Xu, C., Wang, Y., & Tang, A. (2008). The chemistry of the severe acidic precipitation in Shanghai. China. Atmospheric Research, 89(1–2), 149–160. https://doi.org/10.1016/j.atmosres.2008.01.006
Jaina. (2008). Boletín Informativo.. Universidad Autónoma de Campeche. Centro de Ecología, Pesquería y Oceanografía del Golfo de México (EPOMEX). (2008). 19, 1 JAINA. https://epomex.uacam.mx/view/paginas/13
John, W., Wall, S. M., Ondo, J. L., & Winklmayr, W. (1990). Modes in the size distributions of atmospheric inorganic aerosol. Atmospheric Environment. Part a. General Topics, 24(9), 2349–2359. https://doi.org/10.1016/0960-1686(90)90327-J
Kahl, J. D. W., Bravo-Álvarez, H., Sosa-Echeverría, R., Sánchez-Álvarez, P., Alarcón-Jiménez, A. L., & Soto-Ayala, R. (2007). Characterization of atmospheric transport to the El Tajín archaeological zone in Veracruz. México. Atmosfera, 20(4), 359–371.
Kahl, J.D.W., Rolando Olivas Saunders. (2012) Análisis Meterológico de la Depositación Ácida, Avances t Perspectivas de la Depositatión Ácida en México. Universidad Autónoma del Carmen. Ciudad del Carmen, Campeche, México. ISBN: 978–607–7826–21–7.
Kampa, M., & Castanas, E. (2008). Human health effects of air pollution. Environmental Pollution, 151(2), 362–367. https://doi.org/10.1016/j.envpol.2007.06.012
Karson, M. J. (1982). Multivariate statistical methods: An introduction. State University Press, Ames.
Kaya, G., & Tuncel, G. (1997). Trace element and major ion composition of wet and dry deposition in Ankara. Turkey. Atmospheric Environment., 31, 3985–3998. https://doi.org/10.1016/S1352-2310(97)00221-5
Keresztesi, Á., Nita, I. A., Boga, R., Birsan, M. V., Bodor, Z., & Szép, R. (2020). Spatial and long-term analysis of rainwater chemistry over the conterminous United States. Environmental Research, 188, 109872. https://doi.org/10.1016/j.envres.2020.109872
Krupa, S. V. (2002). Sampling and physico-chemical analysis of precipitation: A review. Environmental Pollution, 120(3), 565–594. https://doi.org/10.1016/S0269-7491(02)00165-3
Krupińska, B., Van Grieken, R., & De Wael, K. (2013). Air quality monitoring in a museum for preventive conservation: Results of a three-year study in the Plantin-Moretus Museum in Antwerp. Belgium. Microchemical Journal., 110, 350–360. https://doi.org/10.1016/j.microc.2013.05.006
Kumar, R., Elizabeth, A., & Gawane, A. G. (2006). Air quality profile of inorganic ionic composition of fine aerosols at two sites in Mumbai City. Aerosol Science and Technology, 40(7), 477–489. https://doi.org/10.1080/02786820600672726
J.P. Lacaux, J.P. Tathy, L. Sigha. (2003). Acid wet deposition in the tropics: two case studies using DEBITS measurements. IGACtivities Newsletter of the Intern. Global Atmosph. Chemistry Project, DEBITS, Special issue n° 27.
Lehmann, C.M., Kerschner, B., Gay, D., (2015). Impact of sulfur dioxide (SO2) and nitrogen oxide (NOx) emissions reductions on acidic deposition in the United States. Air & Waste Management Association EM. The Magazine for Environmental Managers, 6–11.
Maechler M, Rousseeuw P, Struyf A, Hubert M, Hornik K (2012). Cluster: cluster analysis basics and extensions. R package. Version 2.0. 1. 1, 56.
Miranda, J., Barrera, V. A., Espinosa, A. A., Galindo, O. S., Núñez-Orosco, A., Montesinos, R. C., & Meinguer, J. (2004). PIXE analysis of atmospheric aerosols from three sites in Mexico City. Nuclear Instruments and Methods in Physics Research Section b: Beam Interactions with Materials and Atoms, 219, 157–160. https://doi.org/10.1016/j.nimb.2004.01.045
Moreda-Piñeiro, J., Alonso-Rodríguez, E., Moscoso-Pérez, C., Blanco-Heras, G., Turnes-Carou, I., López-Mahía, P., Muniategui-Lorenzo, S., & Prada-Rodríguez, D. (2014). Influence of marine, terrestrial and anthropogenic sources on ionic and metallic composition of rainwater at a suburban site (northwest coast of Spain). Atmospheric Environment., 88, 30–38. https://doi.org/10.1016/j.atmosenv.2014.01.067
NADP. (2004). National Atmospheric Deposition Program-National Trens Network. (2004). https://www.nadp.sws.uiuc.edu
NADP, National Atmospheric Deposition Program. 2014. Site selection and installation manual. Revised 2014–11, v. 1.9 Available at: http://nadp.slh.wisc.edu/lib/manuals/NADP_Site_Selection_and_Installation_Manual_201_11.pdf.
NADP, (2017). Atmospheric integrated research monitoring network site operations manual. AIRMoN operations manual v. 2.5. National Atmospheric Deposition Program. Available at: http://nadp.slh.wisc.edu/lib/manuals/AIRMoN_Operations_Manual_v_2-5.pdf
Possanzini, M., Buttini, P., & Di Palo, V. (1988). Characterization of a rural area in terms of dry and wet deposition. Science of the Total Environment., 74, 111–120. https://doi.org/10.1016/0048-9697(88)90132-5
Ramírez Lara, E., Miranda Guardiola, R., Gracia Vásquez, Y., Balderas Rentería, I., Bravo Álvarez, H., Sosa Echeverría, R., & Kahl, J. (2010). Chemical composition of rainwater in northeastern México. Atmósfera, 23(3), 213–224.
Reyes, J., Corvo, F., Espinosa-Morales, Y., Dzul, B., Pérez, T., Valdes, C., Chmielewski, A. G. (2011). Influence of air pollution on degradation of historic buildings at the urban tropical atmosphere of San Francisco de Campeche city, Mexico. Monitoring, control and effects of air pollution (AG Chmielewski, Ed.), 201–226.
Rodhe, H., Dentener, F., & Schulz, M. (2002). The global distribution of acidifying wet deposition. Environmental Science & Technology, 36, 4382–4388. https://doi.org/10.1021/es020057g
Saranova, A. A. (2018). Identificación de regiones de procedencia de precursores de lluvia ácida en el Estado de Veracruz para el establecimiento de estrategias de prevención, minimización y control. Universidad Nacional Autónoma de México.
SEDEMA. (2016). Informe de Calidad del Aire en la Ciudad de México 2015. CDMX: Dirección General de Gestión de la Calidad del Aire, Dirección de Monitoreo Atmosférico. México.
SEMARNAT (2018). PROAIRE 2019–2028. Programa de Gestión para Mejorar la Calidad del Aire del Estado de Campeche. Dirección de Política y Economía Ambiental, Subdirección de Política Ambiental, Vinculación y Cooperación Internacional. 266. Secretaría de Medio Ambiente y Recursos Naturales de Campeche (SEMARNAT). México. https://www.semabicce.campeche.gob.mx/cambio-climatico/
Singh, A. S., & Mondal, G. C. (2007). Chemical characterization of wet precipitation events and deposition of pollutants in coal mining region. India. Journal of Atmospheric Chemistry., 59(1), 1–23. https://doi.org/10.1007/s10874-007-9092-8
Sosa E. R., Bravo A.H, Alarcón J.A.L., Torres B. M.C., Sánchez A.P., Jaimes P. M. Granados H. E. (2015). Importance and evaluation of acid rain and settled particles on the coast of the Gulf of Mexico (2003–2013). 108th Annual Conference & Exhibition of the Air & Waste Management Association. Raleigh, North Caroline, USA. June. https://doi.org/10.1016/j.scitotenv.2019.134419
Sosa E.R., Alarcòn J.A.L., Torres B.M.C., Sánchez A.P., Jaimes P.M., Granados H.E., Gay D. (2020). Sulfur and nitrogen compounds in wet atmospheric deposition on the coast of the Gulf of Mexico from 2003 to 2015. Science of the Total Environment. 700.
Sosa-Echeverría, R., Bravo-Álvarez, H., Alarcón-Jiménez, A. L., Torres-Barrera, M. D. C., Jaimes-Palomera, M., Sánchez-Álvarez, P., & Granados-Hernández, E. (2018). Acid rain in a Mexican site on the coast of the Gulf of Mexico. Atmósfera, 31(4), 317–330. https://doi.org/10.20937/atm.2018.31.04.01
Stumm W. and Morgan J.J., (1970). Aquatic chemistry: An introduction emphasizing chemical equilibria in natural waters. Wiley-Interscience, New York, 583. https://doi.org/10.1002/aheh.19730010116.
Topçu, S., Incecik, S., & Atimtay, A. T. (2002). Chemical composition of rainwater at EMEP station in Ankara. Turkey. Atmospheric Research, 65(1–2), 77–92. https://doi.org/10.1016/S0169-8095(02)00072-8
US EPA. (2004). ”Quality Assurance handbook for Air pollution Measurement System”. V.5, Precipitation Measurement System. EPA-600/R-94/038e, US Environment Protection Agency, Research triangle Park, NC
US-EPA. Guidelines: Air quality surveillance net-works. (1991). AP-98. United States Environmental Protection Agency, Research Triangle Park, NC, USA
US-EPA, (1994). Quality assurance handbook for air pollution measurement systems. Vol. V. Precipitation measurement systems. EPA-600, R-94, 038e. United States Environmental Protection Agency, Research Triangle Park, NC, USA
Van den Berg, L. J. L., Jones, L., Sheppard, L. J., Smart, S. M., Bobbink, R., Dise, N. B., & Ashmore, M. R. (2016). Evidence for differential effects of reduced and oxidized nitrogen deposition on vegetation independent of nitrogen load. Environmental Pollution., 208, 890–897. https://doi.org/10.1016/j.envpol.2015.09.017
Villaseñor, R., Magdaleno, M., Quintanar, A., Gallardo, J. C., López, M. T., Jurado, R., Miranda, A., Aguilar, M., Melgarejo, L. A., Palmerín, E., Vallejo, C. J., & Barchet, W. R. (2003). An air quality emission inventory of off-shore operations for the exploration and production of petroleum by the Mexican oil industry. Atmospheric Environment., 37, 3713–3729. https://doi.org/10.1016/S1352-2310(03)00445-X
Walker, J., Beachley, G., Amos, H., Baron, J., Bash, J., Baumgardner, R., & Zhang, L. (2019). Toward the improvement of total nitrogen deposition budgets in the United States. Science of the Total Environment., 691(15), 1328–1352. https://doi.org/10.1016/j.scitotenv.2019.07.058
WMO, (2004). Manual for the GAW precipitation chemistry programme. Guidelines, data quality objectives and standard operating procedures. GAW Report No. 160. World Meteorological Organization, 170
Wu, Y., Xu, Z., Liu, W., Zhao, T., Zhang, X., Jiang, H., Yu, C., Zhou, L., & Zhou, X. (2016). Chemical compositions of precipitation at three non-urban sites of Hebei Province, North China: Influence of terrestrial sources on ionic composition. Atmospheric Research., 181, 115–123. https://doi.org/10.1016/j.atmosres.2016.06.009
Zhang, M., Wang, S., Wu, F., Yuan, X., & Zhang, Y. (2007). Chemical compositions of wet precipitation and anthropogenic influences at a developing urban site in southeastern China. Atmospheric Research., 84, 311–322. https://doi.org/10.1016/j.atmosres.2006.09.003
Acknowledgements
The support from CONAHCyT Mexico under the program “Investigadoras e Investigadores por México” is acknowledged. This work was supported by the “Investigadoras e Investigadores por México” program (project No. 1854). The authors thank the CONAHCyT-279740-LANCIC project. The authors thanks to Universidad Nacional Autónoma de México (UNAM) Laboratory Staff, María del Carmen Torres B., Rocio Bautista B., Veronica Nequis C., Roberto Gaspariano. To the responsible of the sampling at La Mancha (INECOL) site Enrique Lopez B., and Enrique López M., as well as Jorge Lopez Portillo. To the “Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (PAPIIT) of the UNAM (IN112318) “Establecimiento de la Red Nacional de Deposito Atmosférico”.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Javier Reyes, Rodolfo Sosa, Ana Alarcón and Pablo Sánchez participated in the design of the campaign, collected and analyzed samples, read and corrected the manuscript; Alberto Espinosa designed the study, formal analysis statistics, investigation and wrote the first version of the manuscript; Jonathan Kahl read and corrected the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent for Publication
Not applicable.
Informed Consent Statement
Not applicable.
Institutional Review Board Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Reyes-Trujeque, J., Espinosa, A.A., Sosa-Echeverría, R. et al. Temporal Variation and Chemical Composition of Wet Atmospheric Deposition From Two Coastal Sites in the Gulf of Mexico. Water Air Soil Pollut 235, 443 (2024). https://doi.org/10.1007/s11270-024-07250-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-024-07250-x