Abstract
Among numerous sustainable advanced nanomaterials, nanocellulose is receiving increasing attention for its utilization in water treatment technologies due to its various specific properties and functionalities. The term “nanocellulose” is used for cellulosic material having a nanoscopic scale (or nanoscale) for their dimensional characteristics. It can be obtained in three different forms, which are fibrous form, crystalline form, or bacterial form. One of the benefits of this natural polymer is that it can be found abundantly on Earth, as most plants or waste contains the cellulose. For decades, this material has been widely used in various applications, mainly in water purification, with current studies focusing on surface functionalization of nanocellulose to enhance its properties. This brief review aims to provide the readers with the recent studies (year 2015 to year 2021) of nanocellulose properties, alongside the application of the material for removal of heavy metals from water and its reusability efficiency. In this regard, the main limitations for the specific applications of nanocellulose-based materials and its future prospective are identified in an effort to make possible enhancements in the future.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
There have been tremendous progress in modern, environmentally friendly, economical, and sustainable advanced materials which exhibit remarkable characteristics and functionalities primarily due to the increment in greenhouse gas emissions, crude oil depletion, and increasing global demand for advanced materials for various high-end applications (Dusastre & Martiradonna, 2017; Jiang et al., 2018; Kim et al., 2015; Moon et al., 2011). Cellulose-based materials have a well-aligned nanocellulose (NC) composition for its structural hierarchy. This material is also deemed to be the viable alternative to replace petroleum-based polymers, since it can be found abundantly on Earth, is extractable from renewable source (e.g., wood, plants, fruit peel) and biodegradable, and has ease of use in surface modification and functionalization for specific applications. This helps to decrease the dependency on non-renewable natural resources and retard the depletion percentage of non-renewable sources (Kim et al., 2015; Tang et al., 2016).
The major components in plant cell walls are cellulose, hemicellulose, and lignin; with a percentage of approximately 35–50%, 20–35%, and 10–25%, respectively, by dry weight of biomass. Lignin in the cell serves as a binder between the components and the stiffness for different plants can be described by the percentage of lignin found in the source (Moon et al., 2011). Figure 1 illustrates the standard chemical structure of cellulose. To obtain NCs, cellulose-based materials need to undergo pretreatment in order to eliminate unnecessary components, such as lignin and hemicellulose, and for material breakdown into its nanoscale structure. Reported findings mentioned that nanocellulose has a large specific area (100–200 g/m2), superior mechanical properties (7.5–7.7 GPa tensile strength and 110–220 GPa for Young’s modulus), good resistance to chemical substance, tailored crystallinity, and ease of surface modification (Chen & Hu, 2018; Thomas et al., 2018). These unique characteristics offer potentialities for various applications, such as in membrane technology, electronic devices, and energy applications. Three different types of NCs can be obtained, which are cellulose nanofiber (CNF), cellulose nanocrystal (CNC), and bacterial cellulose which depend on the method of extraction; however, bacterial cellulose is not included in this review.
Chemical structure of cellulose (Richards et al., 2012)
Over the last decade, the methods of nanocellulose synthesis and extraction have been reported by many researchers. NCs are preferred over cellulose, as they provide higher surface area-to-volume ratio and are lighter in weight, which is beneficial in the removal of pollutants from water (Lavanya et al., 2011). Rapid globalization and industrialization have led to increasing issues of having potable water in the future. Therefore, water treatment and purification for both wastewater and potable water have been some of the most important issues that need to be tackled, next to the energy issue. Water bodies are polluted with various types of impurities, such as heavy metals, dyes, and bacteria. The current method applied for water treatment is based on ion-exchange resins, which may produce secondary pollution by regeneration of resins (Dong et al., 2018; Joseph et al., 2020). This review paper aims to deliver a brief explanation on the preparation methods of nano-sized cellulose-based material and summarize the available literatures on the utilization of this green (i.e., clean) material, NC, in removing heavy metals via water treatment application.
2 Nanocellulose Properties and Extraction Method
The first step to extract NCs is by removing unwanted components, which are hemicellulose and lignin, from the lignocellulosic biomass or any cellulose source. In this step, there are two pretreatment approaches known as alkali treatment and bleaching treatment (Phanthong et al., 2018). Researchers nowadays focus on agricultural wastes as the main cellulose source due to its dual purpose in protecting the environment and reducing waste (waste valorization) (Chen & Lee, 2018). Table 1 shows the composition of major constituents in various lignocellulosic sources.
2.1 Alkali Treatment
For this step, cellulose source is processed with alkaline substance. Two main chemical substances usually used for this process as found in literature are sodium hydroxide and potassium hydroxide. These chemicals eliminate the hemicellulose and small fraction of lignin present in the fiber before dialyzed using water until neutral pH is achieved. Mohamed et al. (2015) in their paper have described the detailed methodology for this pretreatment step, in which they extracted cellulose from recycled newspaper. Other researchers have conducted experimental work to obtain CNF from rice straw (Sharma et al., 2017). In their paper, they studied different concentrations of NaOH used to soak the rice straw waste ranging from 8 to 16% before heating the material for 60 to 120 min at temperature of 90 to 160℃.
2.2 Bleaching Treatment
Bleaching treatment, or also known as delignification process, helps to remove the majority of lignin from the cellulose source through the use of two chemicals, which are sodium chlorite and acetic acid. As the name suggests, the bleaching process results in white residues (Phanthong et al., 2018). However, some of the cellulose sources will need to repeat this step several times before the white samples are obtained. If the pretreatment is done overnight, step repetition is not needed, as the white product will be obtained. Similar to alkali treatment, the sample will have to be washed until it reaches pH 7 and then dried in an oven before further treatment can be done. Removal of lignin and hemicellulose can be confirmed by Fourier transform infrared spectroscopy (FTIR) analysis (Bisla et al., 2020). Figure 2 shows the FTIR analysis presented by Bisla et al. (2020) in their published work on CNF preparation extracted from rice straw. Comparing the graph in Fig. 2(a) with Fig. 2(b), some of the peaks are reduced, while Fig. 2(c) illustrates development of new peaks due to functionalization with new functional group.
FTIR spectra for (a) rice straw, (b) pristine CNF, and (c) functionalized CNF (Bisla et al., 2020)
2.3 Nanocellulose Isolation
Acid hydrolysis and mechanical treatment process are two techniques for isolating nanocellulose from the pretreated sample. Depending on this step, the type of cellulose obtained will differ and it can be classified into two forms: crystal form or fibril form.
2.3.1 Acid Hydrolysis
Acid hydrolysis is a process that utilizes strong acid (e.g., sulfuric acid, hydrochloric acid, nitric acid) or mild acid (e.g., formic acid, phosphoric acid, acetic acid) to remove the amorphous region from cellulose fiber, leaving the material with high crystallinity structure. For example, sulfuric acid hydrolyzed the amorphous region through esterification of –OH groups by the SO42– ions. It helps to form a stable colloidal dispersion of NC. Recently, Septevani et al. (2020) published a technical report on their study on two different acids used for NC synthesis, which were sulfuric acid and phosphoric acid. Three parameters are vital for this process which can alter the end result of NC properties. The parameters are as follows: (i) temperature of reaction; (ii) time of reaction; and (iii) concentration of acid used. To stop the hydrolysis reaction, a volume of cold water is added into the mixture (Mohamed et al., 2015). After the acid hydrolysis process is completed, the suspension needs to achieve pH 7. It can be done by dialyzing the sample using water or using sodium hydroxide to increase its alkalinity until a neutral pH is obtained. Table 2 lists the experimental conditions on acid hydrolysis reported by various researchers.
2.3.2 Mechanical and Chemical Treatment
CNF can be obtained via mechanical treatment to the cellulose fibers. Different mechanical approaches were reported in literature such as high-pressure homogenization (HPH), ultrasonication, and ball milling (Klemm et al., 2011; Phanthong et al., 2018). Unfortunately, these mechanically treated fibers consume high energy input, thereby resulting in an increased capital cost for the operation. The first industrial scale-up plant for CNF production using this technique was set up in 2011 by Inventia in Sweden, where the current production units are located mainly in Europe, the United States of America (USA), Canada, and few Asian countries (India, China, Japan, and Iran). Figure 3 shows an example of the network-like structure of CNF.
SEM images of TEMPO oxidated CNF and TEMPO oxidated CNF grafted with polyethyleneimine (Zhang et al., 2016)
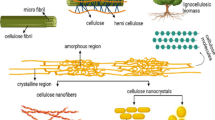
Figure 4 below demonstrates the schematic of nanocellulose extraction. The amorphous regions were removed during acid hydrolysis, leaving behind a crystalline structure, while the mechanical process breaks down the size of cellulose fibers to being nanosized in diameter. Table 3 outlines the summary of type of cellulose and its properties (Lasrado et al., 2020).
Generally, both CNF and CNC have fibrous-like structures but CNC has a sharper structure due to the removal of amorphous area and some publications noting the CNC to have whiskers-like structure, therefore explaining the other known name for CNC which is cellulose nanowhiskers. For CNC, the crystalline structure is visible when observed via TEM rather than SEM. Examples of SEM and TEM images for CNC can be referred to in Fig. 5.
Images of carboxylated CNC–PEI composite, (a) SEM and (b) TEM (Liu et al., 2017)
3 Heavy Metals in the Environment
One of the major pollutants that is still under extensive investigation by researchers are heavy metals, which can be categorized into two: anion (–) and cation ( +). Under normal conditions, the human body can usually withstand metal traces without experiencing serious health issues. However, pollution of water by heavy metals has become a serious concern worldwide. Fu and Wang stated that heavy metals are metals that have an atomic weight between 63.5 and 200.6, with a specific gravity higher than 5.0 (Fu & Wang, 2011). Threats to water quality are due to industrial activities by humans and discharge of municipal waste directly into the water streams. According to Andrews et al. (2004), there are only 1% continental freshwater available for daily use, and with the increasing trends in global population, scarcity of pollution-free water will become the next global issue in no time. The World Health Organization (WHO) and relevant government agencies have published the guideline for optimum concentration of heavy metals in drinking water but it differs according to country. Table 4 displays the limit for drinkable water standards for common heavy metals, as established by WHO.
The presence of the aforementioned toxic contaminants in water threatens the environment and all living organisms, especially marine life. Unlike organic contaminants, these heavy metals are not biodegradable and can cause serious hazards to humans and animals; Table 5 lists some of health hazards known. Commonly, heavy metals are discharged into the water bodies through industrial processing activities, such as mining, metal plating, and fertilizer industry, among others.
4 Recent Applications of NC-Based Materials in Water Treatment
Clean water resources are very important for living creatures to ensure a healthy life. Thus, contamination of water bodies is considered as a threat to the population. Rapid globalization increases the amount of wastewater containing harmful chemicals being discharged into the water streams due to escalation of agricultural and industrial activities. Several approaches have been introduced to efficiently purify the contaminated water source and wastewater. Among the extensively studied techniques, ion exchange is an approach used for the elimination of unnecessary metals from wastewater (Joseph et al., 2020). However, the disadvantage of this process is that the regeneration of resins for ion exchange needs to be done by chemical reagents, which may cause severe secondary pollution. Furthermore, this technique is costly and unfeasible for water management at a large scale. Presently, adsorption is a known technique that utilizes NC-based materials.
4.1 Adsorption
Adsorption can be defined as a process involving liquid solute or adsorbate that builds up onto the adsorbent surface (Singh et al., 2018). Figure 6 illustrates the basic adsorption mechanism (Worch, 2012). Adsorption can be divided into two categories, which are physical adsorption (physisorption) and chemical adsorption (chemisorption). To differentiate the two types of adsorption, the attraction between sorbent surface and adsorbate was evaluated. Physisorption involves natural interaction between molecules and weak van der Waals force, while chemisorption involves the chemical bonding that possesses strong attraction between molecules. Adsorption has been known as an attractive, simple, and inexpensive route for removal of heavy metals from wastewater containing low concentration of pollutants. A group of researchers has critically evaluated the suitability of adsorption method in heavy metal removal and described adsorption as an excellent operative method due to its easy operating procedure and low cost compared to other approaches (Hadi et al., 2016). Additionally, the adsorbent can be recovered by a suitable desorption method and this technique is deemed a reversible process. To efficiently eliminate excess metals, dominant charges carried by the pollutant in wastewater have to first be identified, whereby suitable modification can be done accordingly to the surface of the NC.
Simple illustration of adsorption (Worch, 2012)
4.1.1 Adsorption Parameters
Numerous factors can influence adsorption, such as the pH of solution, concentration of metal ions, and adsorbent dosage. Optimization needs to be done on the parameters to enhance the development of real-scale heavy metal removal based on this approach. Optimized condition of variables offers higher adsorption capacity, and thus developing an effective technique for elimination of metal contaminants. The initial and final concentrations of metal element studied were measured using one of the following spectrophotometers: atomic absorption spectroscopy (AAS), ultraviolet visible range (UV–Vis), inductively coupled plasma optical emission spectroscopy (ICP-OES) or its other name, inductively coupled plasma atomic emission spectroscopy (ICP-AES). Basically, ICP technology is superior compared to other method as it has higher sensitivity and is able to trace multiple elements at the same time. In terms of cost, UV–Vis offers the cheapest solution but with the drawback of being unable to differentiate elements that absorb the same wavelength. In this process, pH is considered as a critical variable, as it affects the degree of ionization of the adsorbate and solubility of metal ions, as well as the concentration of the adsorbate ions on the functional groups of the adsorbents (Awang et al., 2019; Joseph et al., 2019). Generally, most heavy metals found in wastewater are in a cationic state. At low pH, positively charged heavy metal ions compete with hydronium ions, H3O+, to be bound to the adsorbent surface. Thus, the adsorption capacity of the adsorbate to the adsorbent decreases significantly. A similar situation is observed when the adsorption reaction takes place in a higher pH condition. The performance of adsorption will decrease due to fewer active sites available for the binding of adsorbate to take place.
Another crucial parameter that influences the uptake of metal ions is the initial concentration of metal ions. The higher initial concentration of heavy metals in solution increases the adsorption capacity, as it will be driven by the concentration gradient. However, the adsorption performance declines when the initial concentration exceeds the optimum value due to insufficient binding sites available on the surface of adsorbent. Above the optimum concentration, the binding sites become saturated and excess metal ions are left unadsorbed. This leads to decreasing removal percentage of ions from the solution. Adsorbent dosage is indeed a significant element in adsorption, which determines both the removal percentage and process economics. Increasing adsorbent dosage to a certain limit offers more adsorbing sites for the metal contaminants to bind, thus increasing the effectiveness of ion removal from the solution (El-Tawil et al., 2019). On the other hand, adsorption capacity decreases with increasing adsorbent dosage due to unsaturation of metal ions on the adsorbent surface. After reaching the optimum dosage, no significant elevation of removal percentage is reported; further increase in dosage amount leads to a decrement in removal efficiency (Gorzin et al., 2018).
4.1.2 Adsorption Mechanism
According to Singh et al. (2018), adsorption isotherm conveys the linkage between concentration of heavy metal ions and amount of adsorbate on surface of adsorbent at fixed pH and temperature. To simplify, the interaction of ions and adsorbents is characterized by this isotherm. Determination of isotherm models is applicable for batch adsorptions that have varying initial concentrations of metal ions, in which different models are based on different assumptions made on the adsorption mechanism. Two commonly used isotherm models in the adsorption study are Langmuir and Freundlich models.
The Langmuir model assumes the process to be a monolayer type of adsorption, where the adsorbate binds on the surface of adsorbent only in one layer. The model equation for Langmuir isotherm (Singh et al., 2018):
where Ce (mg/L) is metal ion concentration at equilibrium, qe (mg/g) is the amount of metal ions adsorbed at equilibrium, KL (L/mg) is Langmuir constant, and qm (mg/g) is the maximum adsorption capacity. The affinity and the favorableness between the adsorbent and the adsorbate also can be predicted by using Langmuir constant KL from the dimensionless constant separation factor, RL = 1/1 + KLC0. C0 is the initial concentration of adsorbate in milligrams per liter. Ranges of RL values are as follows: (i) irreversible (RL = 0), (ii) favorable (0 < RL<1), (iii) linear (RL = 1), and (iv) unfavorable (RL > 1).
For the Freundlich isotherm, it defines the process to be a multilayer adsorption, as seen in Fig. 7. The model equation for Freundlich isotherm is (Maleki & Karimi-Jashni, 2017; Singh et al., 2018):
where Ce (mg/L) is metal ion concentration at equilibrium, qe (mg/g) is the amount of metal ions adsorbed at equilibrium, KF (L/mg) is Freundlich constant, and 1/n is heterogeneity factor. The value of heterogeneity indicates the favorability of adsorption, such as (i) irreversible (1/n = 0), (ii) favorable (0 < 1/n < 1), and lastly, (iii) unfavorable (1/n > 1). These theoretical models are employed to fit the values obtained in experimental work.
4.1.3 Recent Applications Using NC-Based Materials as Adsorbent
Sehaqui and co-workers conducted a study on effect of pH on copper (II) ion removal by TEMPO-oxidized CNF (TOCNF) adsorbent (Sehaqui et al., 2014). Increasing linear performance was observed when the pH acidity was lowered from pH 3 to pH 7. They suggested that the adsorbent gives optimum result of heavy metal adsorption when the pH is near neutral. A similar result was obtained by Zhang et al. (2016). They reported an adsorption study on Cu removal using TOCNF grafted with polyethyleneimine (PEI). From their findings, the zeta potential when the acidity of pH was lowered had decreased from + 23.40 to + 9.55 mV. This shows the deprotonation of heavy metal ions, and thus increasing performance of adsorbate on the surface adsorbent. Most studies on copper ion removal reported a similar range of optimum pH which is at weak acidic condition to nearly neutral, pH 5 to pH 7. Beyond the optimum, the metal ion started to precipitate due to the presence of hydroxyl ions. In addition, surface functionalization of NC also helps to enhance the adsorption performance, as it offers more surface area for adsorbate binding (Navarro et al., 1996). Through the isotherm study, the adsorption process was found to have fit the monolayer adsorption mechanism. Morphology structure of the adsorbent can also affect the adsorption performance. Carboxylated NC-sodium alginate beads adsorbent (Fig. 8a) for metal removal were observed to have the capacity to adsorb 338.98 mg/g of Pb ions from an initial concentration of 400 ppm (Hu et al., 2018). The injection of carboxylated CNC into the sodium alginate hydrogel helps in improving the mechanical stability as well as increasing the negative charges carried by carboxylate ions. Similarly, an experiment to employ CNF-PEI aerogel beads was done by Li et al. (2018). They conducted batch removal of Cu (II) ions and Pb (II) ions separately and both showed an excellent removal performance with more selectivity towards Pb ions.
Singh et al. (2014) attempted to remove anionic heavy metal using aminated CNC. To remove anionic metal, they incorporated the surface of NC with positive charged species, and this theory is deemed vice versa for cationic heavy metal removal. Excellent adsorption performance was observed, where it was claimed to have had a removal of 98% of chromium ions. Yu et al. (2016) prepared poly(acrylic acid)-grafted bamboo CNF to eliminate copper ions from water and the result showed a good adsorption efficiency. Other than the graft copolymerization method, NC-based composite adsorbents are currently receiving attention, as reported in Li et al. (2018). Superior Pb removal in a low amount of time was reported in the published work of Alipour et al. (2020), whereby 99.99% removal has been recorded when the adsorption was done at optimized parameters. In the adsorption mechanism of Pb ions with the thiourea-modified magnetic ZnO/nanocellulose composite, hydroxide, amino, and sulfur groups dominated the removal of the metal. The simplest explanation that can be concluded is positively charged Pb ions are attracted to the anionic groups presented in the adsorbent. Composite NC-based adsorbent showed a good potential as the replacement for current commercial adsorbent. Next, a novel technique of lignocellulosic fiber functionalization using neem oil phenolic resin emulsion was introduced by Manna et al. (2017). In the abovementioned work, it was stated that rapid adsorption had occurred due to the coupled effect of electrostatic interactions. Meanwhile, magnetic adsorbent has good performance in regeneration studies as suggested by Alipour et al. (2020). Modified CNF synthesized by Bisla et al. (2020) to remove mercury ions has also produced a significant removal performance at the optimum conditions owing to the functional groups present on the CNF backbone. The mechanism of the adsorption reaction can be seen in Fig. 9, where there would be two possible adsorbed products as both sulfides and amino appear to bond with the Hg ions. The latest achievement in nanocellulose-based adsorbent was from the work by Mo and co-workers (2021). They claimed that their adsorbent in the form of wood-inspired aerogel can eliminate Pb ions in a very short time, approximately 10 min, while other types of metals (Cu, Zn, Mn, and Cd) could also be eliminated but at a comparable rate with other reported work. The maximum adsorption capacity for Pb ions removed was 571 mg/g. The unique properties of their adsorbent with combination of TOCNF, trimethylolpropanetris-(2-methyl-1-aziridine) propionate (TMPTAP), and graphene oxide (GO) provide more active sites for the binding of metal ions, and these have resulted in excellent adsorption performance. The difference of aerogel structures between studies done by Hu et al. (2018) and Mo et al. (2021) was presented in Fig. 8. Next, Table 6 exhibits a summary on recent NC-based adsorbent for heavy metal removal done by previous researchers.
Reaction mechanism of Hg ion adsorption to l-methionine functionalized CNF (Bisla et al., 2020)
An important characteristic of adsorbent that should be highlighted is its reusability after multiple adsorption cycles, which carry a significant impact on the feasibility of the process in industrial applications. In the recent publications, only few researchers included the recovery and recyclability study, but its possibility has been mentioned by many (Mahfoudhi & Boufi, 2017). NC-based aerogel adsorbent synthesized by Mo et al. (2019) was reported to exhibit an excellent desorption-regeneration ability up to four cycles with Cu (II) ion removal efficiency of more than 80% at the 4th cycle. For ease of desorption process, NC adsorbent could be pair with magnetic compound. Adsorption performance is likely to be decreasing after each cycle but with the introduction of magnetic materials in the chemical structure, the recovery percentage of adsorbent is enhanced, and hence better efficiency of metal removal. Regeneration test of magnetic carboxylated CNC prepared by a group of researchers revealed that it was able to retain Pb (II) ion removal ratio of more than 80% after the 5th consequent adsorption–desorption cycles (Lu et al., 2016). Magnetic force was used to recover the adsorbent and the CNC was then treated using acid prior to the next adsorption cycle. Similarly, TZFNC magnetic adsorbent desorption studies were reported by Alipour et al. (2020) using nitric acid but with additional process of ultrasonification. The developed adsorbent was successfully regenerated with efficiency close to 100% attributed to the ultrasonification treatment. An excellent metal removal performance was displayed in the work by Chai et al. (2020) whereby up to eight cycles of adsorption–desorption process were conducted. Due to the acidic nature of their synthetic wastewater, the NC adsorbent crosslinked with PEI and glutaraldehyde (GA) was subjected to alkali treatment for desorption process instead of acid. The removal efficiency showed an adequate performance even after the 8th cycles. In short, NC-based adsorbent can be easily regenerated using a suitable treatment, while addition of magnetic compound increases the percentage of adsorbent recovery.
5 Conclusion and Future Perspectives
This paper has briefly discussed the extraction method of CNC and CNF, and recent studies reported on the application of NC-based materials for water treatment. NC has long proven to have excellent features and mechanical properties. However, given those outstanding qualities, few constraints and limitations still need to be addressed. One of the constraints is the NC production source, which is mostly from plant and forest resources. The depletion of natural plantation in the forestry could result in natural disaster if plants are reaped, harvested, or collected (Chen & Lee, 2018). Therefore, biomass-based materials are often seen as a better option for NC production by valorization of waste, since they help to protect the environment and reduce waste. Sulfuric acid hydrolysis approach was used for isolation in large-scale product commercialization of CNC. Benefits of sulfuric acid are its cost-effectiveness and recyclability. It should be noted that the isolation of NC is time consuming and requires high energy input, and some steps even require the usage of chemical substances that are harmful to humans and the environment (Abouzeid et al., 2019; Phanthong et al., 2018).
Copper and lead are the most common heavy metals studied for adsorption process due to large amounts of those metals found in wastewater. However, industrial wastewater contains multiple mixture of heavy metal ions along with other pollutants. Therefore, extensive research needs to be done for the optimization of process conditions and selection of suitable materials. NC surface modification and functionalization play a key role in the NC-based adsorbent development for water treatment. The limitation on NC-based adsorbent is its capability in large-scale wastewater, where the target metals are not only a single metal. Different metals have different optimum conditions and more studies need to be done for single and binary metal removal, followed by more mixture of metals. To date, most of NC-based adsorbents have shown a great potential in single metal removal but not many published on binary metal removal due to the complexity of the ionic process. For mixtures containing more than one type of metal, the morphology of the nanocellulose is the most important factor that needs to be addressed. However, the properties of the pollutant in the water must be known first, whether it is cationic or anionic, for the adsorbent to work efficiently. Improvement could be made by incorporating the NC-based adsorbent studied into membrane (adsorptive membrane). As aforementioned, one of the advantages of using NC as adsorbent is the ease of modification to the surface according to application need. NC, regardless of its type either CNC or CNF, may be transformed into membrane itself or serve as an additive, embedded in other membranes. These hybrid adsorption-membrane technology and surface modification help to alter the charge content on surface and its reactivity for an enhanced reaction with the contaminants found in water by additional incorporation of functional groups, such as amino, carboxyl, silanol, and carboxylate.
While the production of CNC and CNF has been patented and commercialized, technology development of large-scale industrial production of NC-based composites remains a challenge. To overcome this, various attempts have been made in search of new process innovations that can improve the existing technology. For a larger scale study, the additional concerns are the maintenance cost of machines and equipment, management of wastewater from the process, and total energy consumption. Future study should include brief costing analysis to evaluate the process feasibility of water treatment and purification. Simulation software, such as HYSYS, can help to evaluate the total energy used by plants and its cost. Another way to reduce the total cost is by utilizing biomass as the cellulose source and producing CNC and CNF concurrently (Bian et al., 2017). Numerous researchers and industries have performed various research activities to maximize the production in commercializing NC-based materials. NC-based materials are currently still in the developing phase, and their applications in various field areas (i.e., membrane separation, drug delivery, and packaging industry) are expected to grow rapidly. Overall, NC-based materials are sustainable materials and the utilization of these materials offers solution to many challenges in the modern world, especially in water treatment for heavy metal removal.
References
Abiaziem, C. V., Williams, A. B., Inegbenebor, A. I., Onwordi, C. T., Ehi-Eromosele, C. O., & Petrik, L. F. (2019). Adsorption of lead ion from aqueous solution unto cellulose nanocrystal from cassava peel. Journal of Physics: Conference Series, 1299, 012122.
Abouzeid, R. E., Khiari, R., El-Wakil, N., & Dufresne, A. (2019). Current State and new trends in the use of cellulose nanomaterials for wastewater treatment. Biomacromolecules, 20(2), 573–597.
Alipour, A., Zarinabadi, S., Azimi, A., & Mirzaei, M. (2020). Adsorptive removal of Pb(II) ions from aqueous solutions by thiourea-functionalized magnetic ZnO/nanocellulose composite: Optimization by response surface methodology (RSM). International Journal of Biological Macromolecules, 151, 124–135.
Andrews, J. E., Brimblecombe, P., Jickells, T. D., Liss, P. S., & Reid, B. (2004). An introduction to environmental chemistry (Second.). Blackwell Publishing.
Awang, N. A., Salleh, W. N. W., Ismail, A. F., & Hasbullah, H. (2019). Synthesis, characterization and adsorption properties of grafted cellulose for Cr (VI) removal. Materials Today: Proceedings, 19, 1777–1786.
Bian, H., Chen, L., Wang, R., & Zhu, J. (2017). Green and low-cost production of thermally stable and carboxylated cellulose nanocrystals and nanofibrils using highly recyclable dicarboxylic acids. Journal of Visualized Experiments, (119). https://doi.org/10.3791/55079
Bisla, V., Rattan, G., Singhal, S., & Kaushik, A. (2020). Green and novel adsorbent from rice straw extracted cellulose for efficient adsorption of Hg (II) ions in an aqueous medium. International Journal of Biological Macromolecules, 161, 194–203.
Chai, F., Wang, R., Yan, L., Li, G., Cai, Y., & Xi, C. (2020). Facile fabrication of pH-sensitive nanoparticles based on nanocellulose for fast and efficient As(V) removal. Carbohydrate Polymers, 245, 116511. https://doi.org/10.1016/j.carbpol.2020.116511
Chen, C., & Hu, L. (2018). Nanocellulose toward advanced energy storage devices: Structure and electrochemistry. Accounts of Chemical Research, 51(12), 3154–3165.
Chen, Y. W., & Lee, H. V. (2018). Revalorization of selected municipal solid wastes as new precursors of “green” nanocellulose via a novel one-pot isolation system: A source perspective. International Journal of Biological Macromolecules, 107, 78–92. https://doi.org/10.1016/j.ijbiomac.2017.08.143
Dobrowolski, R., Szcześ, A., Czemierska, M., & Jarosz-Wikołazka, A. (2017). Studies of cadmium(II), lead(II), nickel(II), cobalt(II) and chromium(VI) sorption on extracellular polymeric substances produced by Rhodococcus opacus and Rhodococcus rhodochrous. Bioresource Technology, 225, 113–120.
Dong, L., Hou, L., Wang, Z., Gu, P., Chen, G., & Jiang, R. (2018). A new function of spent activated carbon in BAC process: Removing heavy metals by ion exchange mechanism. Journal of Hazardous Materials, 359, 76–84.
Dusastre, V., & Martiradonna, L. (2017). Materials for sustainable energy. Nature Materials, 16(1), 15–15.
El-Tawil, R. S., El-Wakeel, S. T., Abdel-Ghany, A. E., Abuzeid, H. A. M., Selim, K. A., & Hashem, A. M. (2019). Silver/quartz nanocomposite as an adsorbent for removal of mercury (II) ions from aqueous solutions. Heliyon, 5(9), e02415.
Fu, F., & Wang, Q. (2011). Removal of heavy metal ions from wastewaters: A review. Journal of Environmental Management, 92(3), 407–418. https://doi.org/10.1016/j.jenvman.2010.11.011
Gorzin, F., Abadi, B. R., & M. . (2018). Adsorption of Cr(VI) from aqueous solution by adsorbent prepared from paper mill sludge: Kinetics and thermodynamics studies. Adsorption Science & Technology, 36(1–2), 149–169.
Hadi, M., Sanaei, D., Ali, I., & Bhatnagar, A. (2016). Removal of chromium (VI) from aqueous solution using treated waste newspaper as a low-cost adsorbent : Kinetic modeling and isotherm studies. Journal of Molecular Liquids, 215, 671–679.
Hu, Z.-H., Omer, A. M., Ouyang, X., & Yu, D. (2018). Fabrication of carboxylated cellulose nanocrystal/sodium alginate hydrogel beads for adsorption of Pb(II) from aqueous solution. International Journal of Biological Macromolecules, 108, 149–157.
JECFA. (2000). Summary Reports of the Fifty-fifth and Fifty-sixth Meetings of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). http://www.fao.org/3/Y0474E/y0474e09.htm#TopOfPage. Accessed 10 June 2020.
Jiang, F., Li, T., Li, Y., Zhang, Y., Gong, A., Dai, J., et al. (2018). Wood-based nanotechnologies toward sustainability. Advanced Materials, 30(1), 1703453.
Joseph, J., Radhakrishnan, R. C., Johnson, J. K., Joy, S. P., & Thomas, J. (2020). Ion-exchange mediated removal of cationic dye-stuffs from water using ammonium phosphomolybdate. Materials Chemistry and Physics, 242, 122488.
Joseph, L., Jun, B. M., Flora, J. R. V., Park, C. M., & Yoon, Y. (2019). Removal of heavy metals from water sources in the developing world using low-cost materials: A review. Chemosphere, 229, 142–159.
Kargarzadeh, H., Johar, N., & Ahmad, I. (2017). Starch biocomposite film reinforced by multiscale rice husk fiber. Composites Science and Technology, 151, 147–155.
Kaur, M., Kumari, S., & Sharma, P. (2020). Removal of Pb (II) from aqueous solution using nanoadsorbent of Oryza sativa husk: Isotherm, kinetic and thermodynamic studies. Biotechnology Reports, 25, e00410.
Kaur, M., Sharma, P., & Kumari, S. (2019). Equilibrium studies for copper removal from aqueous solution using nanoadsorbent synthesized from rice husk. SN Applied Sciences, 1(9), 988.
Kim, J.-H., Shim, B. S., Kim, H. S., Lee, Y.-J., Min, S.-K., Jang, D., et al. (2015). Review of nanocellulose for sustainable future materials. International Journal of Precision Engineering and Manufacturing-Green Technology, 2(2), 197–213.
Klemm, D., Kramer, F., Moritz, S., Lindström, T., Ankerfors, M., Gray, D., & Dorris, A. (2011). Nanocelluloses: A new family of nature-based materials. Angewandte Chemie (International ed. in English), 50(24), 5438–66.
Lasrado, D., Ahankari, S., & Kar, K. (2020). Nanocellulose-based polymer composites for energy applications—A review. Journal of Applied Polymer Science, 137(27), 48959.
Lavanya, D., Kulkarni, P. K., Dixit, M., Raavi, P. K., & Krishna, L. N. V. (2011). Sources of cellulose and their applications - A review. International Journal of Drug Formulation and Research, 2(6), 19–38.
Li, J., Zuo, K., Wu, W., Xu, Z., Yi, Y., Jing, Y., et al. (2018). Shape memory aerogels from nanocellulose and polyethyleneimine as a novel adsorbent for removal of Cu(II) and Pb(II). Carbohydrate Polymers, 196, 376–384.
Liu, C., Jin, R.-N., Ouyang, X., & Wang, Y.-G. (2017). Adsorption behavior of carboxylated cellulose nanocrystal—polyethyleneimine composite for removal of Cr(VI) ions. Applied Surface Science, 408, 77–87.
Liu, P., Borrel, P. F., Božic, M., Kokol, V., Oksman, K., & P, M. A. . (2015). Nanocelluloses and their phosphorylated derivatives for selective adsorption of Ag+, Cu2+ and Fe3+ from industrial effluents. Journal of Hazardous Materials, 294, 177–185.
Lu, J., Jin, R.-N., Liu, C., Wang, Y.-F., & Ouyang, X. (2016). Magnetic carboxylated cellulose nanocrystals as adsorbent for the removal of Pb(II) from aqueous solution. International Journal of Biological Macromolecules, 93, 547–556. https://doi.org/10.1016/j.ijbiomac.2016.09.004
Mahfoudhi, N., & Boufi, S. (2017). Nanocellulose as a novel nanostructured adsorbent for environmental remediation: A review. Cellulose, 24(3), 1171–1197. https://doi.org/10.1007/s10570-017-1194-0
Maleki, S., & Karimi-Jashni, A. (2017). Effect of ball milling process on the structure of local clay and its adsorption performance for Ni(II) removal. Applied Clay Science, 137, 213–224.
Malucelli, L. C., Lacerda, L. G., Dziedzic, M., & da Silva Carvalho Filho, M. A. . (2017). Preparation, properties and future perspectives of nanocrystals from agro-industrial residues: A review of recent research. Reviews in Environmental Science and Bio/technology, 16(1), 131–145.
Manna, S., Roy, D., Saha, P., Gopakumar, D., & Thomas, S. (2017). Rapid methylene blue adsorption using modified lignocellulosic materials. Process Safety and Environmental Protection, 107, 346–356.
Mo, L., Pang, H., Lu, Y., Li, Z., Kang, H., Wang, M., et al. (2021). Wood-inspired nanocellulose aerogel adsorbents with excellent selective pollutants capture, superfast adsorption, and easy regeneration. Journal of Hazardous Materials, 415, 125612.
Mo, L., Pang, H., Tan, Y., Zhang, S., & Li, J. (2019). 3D multi-wall perforated nanocellulose-based polyethylenimine aerogels for ultrahigh efficient and reversible removal of Cu(II) ions from water. Chemical Engineering Journal, 378, 122157. https://doi.org/10.1016/j.cej.2019.122157
Mohamed, M. A., Salleh, W. N. W., Jaafar, J., Asri, S. E. A. M., & Ismail, A. F. (2015). Physicochemical properties of “green” nanocrystalline cellulose isolated from recycled newspaper. RSC Advances, 5(38), 29842–29849. https://doi.org/10.1039/C4RA17020B
Moon, R. J., Martini, A., Nairn, J., Simonsen, J., & Youngblood, J. (2011). Cellulose nanomaterials review: Structure, properties and nanocomposites. Chemical Society Reviews, 40(7), 3941.
Navarro, R. R., Sumi, K., Fujii, N., & Matsumura, M. (1996). Mercury removal from wastewater using porous cellulose carrier modified with polyethyleneimine. Water Research, 30(10), 2488–2494.
Oyewo, O. A., Mutesse, B., Leswifi, T. Y., & Onyango, M. S. (2019). Highly efficient removal of nickel and cadmium from water using sawdust-derived cellulose nanocrystals. Journal of Environmental Chemical Engineering, 7(4), 103251.
Phanthong, P., Reubroycharoen, P., Hao, X., Xu, G., Abudula, A., & Guan, G. (2018). Nanocellulose: Extraction and application. Carbon Resources Conversion, 1(1), 32–43. https://doi.org/10.1016/j.crcon.2018.05.004
Richards, H. L., Baker, P. G. L., & Iwuoha, E. (2012). Metal nanoparticle modified polysulfone membranes for use in wastewater treatment: A critical review. Journal of Surface Engineered Materials and Advanced Technology, 02(03), 183–193.
Sai Prasanna, N., & Mitra, J. (2020). Isolation and characterization of cellulose nanocrystals from Cucumis sativus peels. Carbohydrate Polymers, 247, 116706.
Sehaqui, H., de Larraya, U. P., Liu, P., Pfenninger, N., Mathew, A. P., Zimmermann, T., & Tingaut, P. (2014). Enhancing adsorption of heavy metal ions onto biobased nanofibers from waste pulp residues for application in wastewater treatment. Cellulose, 21(4), 2831–2844.
Septevani, A. A., Rifathin, A., Sari, A. A., Sampora, Y., Ariani, G. N., Sudiyarmanto, & Sondari, D. (2020). Oil palm empty fruit bunch-based nanocellulose as a super-adsorbent for water remediation. Carbohydrate Polymers, 229, 115433.
Shahzad, B., Tanveer, M., Rehman, A., Cheema, S. A., Fahad, S., Rehman, S., & Sharma, A. (2018). Nickel; whether toxic or essential for plants and environment - a review. Plant Physiology and Biochemistry, 132, 641–651. https://doi.org/10.1016/j.plaphy.2018.10.014
Sharma, A., Mandal, T., & Goswami, S. (2017). Cellulose nanofibers from rice straw: Process development for improved delignification and better crystallinity index. Trends in Carbohydrate Research, 9(4), 16–27.
Sheikhi, A., Safari, S., Yang, H., & van de Ven, T. G. M. (2015). Copper removal using electrosterically stabilized nanocrystalline cellulose. ACS Applied Materials & Interfaces, 7, 11301–11308.
Singh, K., Arora, J. K., Sinha, T. J. M., & Srivastava, S. (2014). Functionalization of nanocrystalline cellulose for decontamination of Cr(III) and Cr(VI) from aqueous system: Computational modeling approach. Clean Technologies and Environmental Policy, 16(6), 1179–1191.
Singh, N. B., Nagpal, G., Agrawal, S., & Rachna. (2018). Water purification by using adsorbents: A review. Environmental Technology & Innovation, 11, 187–240. https://doi.org/10.1016/j.eti.2018.05.006
Tang, A., Liu, Y., Wang, Q., Chen, R., Liu, W., Fang, Z., & Wang, L. (2016). A new photoelectric ink based on nanocellulose/CdS quantum dots for screen-printing. Carbohydrate Polymers, 148, 29–35.
Thomas, B., Raj, M. C., B, A. K., H, R. M., Joy, J., Moores, A., , et al. (2018). Nanocellulose, a versatile green platform: From biosources to materials and their applications. Chemical Reviews, 118(24), 11575–11625.
Wang, N., Jin, R.-N., Omer, A. M., & Ouyang, X. (2017). Adsorption of Pb(II) from fish sauce using carboxylated cellulose nanocrystal: Isotherm, kinetics, and thermodynamic studies. International Journal of Biological Macromolecules, 102, 232–240.
WHO. (2003a). Chlorine in drinking-water: Background document for development of WHO guidelines for drinking-water quality. https://www.who.int/water_sanitation_health/dwq/chlorine.pdf. Accessed 12 June 2020.
WHO. (2003b). Chromium in drinking-water: Background document for development of WHO guidelines for drinking-water quality. https://www.who.int/water_sanitation_health/dwq/chemicals/chromium.pdf. Accessed 12 June 2020.
WHO. (2003c). Lead in drinking-water: Background document for development of WHO guidelines for drinking-water quality. https://www.who.int/water_sanitation_health/dwq/chemicals/lead.pdf. Accessed 12 June 2020.
WHO. (2003d). Zinc in drinking-water: Background document for development of WHO guidelines for drinking-water quality. https://www.who.int/water_sanitation_health/dwq/chemicals/zinc.pdf. Accessed 12 June 2020.
WHO. (2004). Copper in drinking-water: Background document for development of WHO guidelines for drinking-water quality. https://www.who.int/water_sanitation_health/dwq/chemicals/copper.pdf. Accessed 12 June 2020.
WHO. (2005). Nitrate and nitrite in drinking-water: Background document for development of WHO guidelines for drinking-water quality. https://www.who.int/water_sanitation_health/dwq/chemicals/nitratenitrite2ndadd.pdf. Accessed 12 June 2020.
Worch, E. (2012). Adsorption Technology in water treatment, fundamentals, processes, and modeling. De Gruyter.
Xiao, Y., Liu, Y., Wang, X., Li, M., Lei, H., & Xu, H. (2019). Cellulose nanocrystals prepared from wheat bran: Characterization and cytotoxicity assessment. International Journal of Biological Macromolecules, 140, 225–233.
Yu, H.-Y., Zhang, D.-Z., Lu, F.-F., & Yao, J. (2016). New approach for single-step extraction of carboxylated cellulose nanocrystals for their use as adsorbents and flocculants. ACS Sustainable Chemistry & Engineering, 4(5), 2632–2643.
Zhang, N., Zang, G.-L., Shi, C., Yu, H.-Q., & Sheng, G.-P. (2016). A novel adsorbent TEMPO-mediated oxidized cellulose nanofibrils modified with PEI: Preparation, characterization, and application for Cu(II) removal. Journal of Hazardous Materials, 316, 11–18. https://doi.org/10.1016/j.jhazmat.2016.05.018
Funding
The authors received the financial support from Universiti Malaysia Pahang under grant number PGRS200301 and Ministry of Higher Education Malaysia (Grant number: RDU190379).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ibrahim, H., Sazali, N., Salleh, W.N.W. et al. Nanocellulose-Based Materials and Recent Application for Heavy Metal Removal. Water Air Soil Pollut 232, 305 (2021). https://doi.org/10.1007/s11270-021-05245-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-021-05245-6