Abstract
This work studies the reduction of biological and organic compounds in hospital wastewaters (HWW) by advanced oxidation process (AOP). The HWW samples were previously treated with an extended aeration process and, thereafter, a post-treatment with AOP based on UV/H2O2/O3 system with a medium pressure mercury lamp was applied. After using the AOP system, the water samples were characterized using chemical oxygen demand (COD), turbidity, color test, coliforms and E. coli test, gas chromatography coupled to mass spectrometry (GC-MS), and UV-Visible absorption spectra. The results showed that 73% of organic compounds were removed in 20 min and the HWW sample was sterilized; nevertheless, 10 persistent organic compounds and 8 by-products formed after AOP UV/H2O2/O3 were observed, some of them are toxic compounds. In this sense, current HWW treatment plants cannot eliminate all contaminants in HWW; therefore, it is necessary to improve current processes by techniques as AOP and create standards to control biological and organic compounds in HWW in Mexico
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Persistent organic compounds (POPs) in wastewater are toxic organic pollutants that cannot be eliminated by current wastewater treatments. They are a potential risk to the environment and human health because they tend to bioaccumulate in organisms and be adsorbed by soil particles (Crini et al. 2019; Om Prakash 2019; Wenjing et al. 2019). Although there are studies about the wastewater treatment in agricultural applications and industrial waste (Schuhmacher et al. 2019; Bansal and Kim 2015; Om Prakash 2019); there are still few studies investigating the impact of hospital wastewaters (HWW) into the environment and sewage system (Paulus et al. 2019; Czekalski et al. 2014; Wang et al. 2018).
HWW contain hazardous substances (Verlicchi et al. 2012; Kasprzyk-Hordern et al. 2009; Zhang et al. 2013b; Kümmerer 2008); for example, drugs are expelled from human bodies by metabolites or intact compounds such as gadolinium, a metal used as a contrast medium in radiological processes (magnetic resonance). All these compounds are discharged in concentrations greater than 95% and without chemical alterations. Similarly, analgesics such as gabapentin (100%) (Kasprzyk-Hordern et al. 2009), antibiotics as amoxicillin (60%), and β-blockers such as atenolol (90%) (Zuccato et al. 2005) were detected in HWW. In fact, all these pollutants have been detected in concentrations of ng/L to μg/L, so it is necessary to remove those pollutants from wastewater and, of course, avoid their interaction with the environment (Verlicchi et al. 2012; Kasprzyk-Hordern et al. 2009; Zhang et al. 2013a).
Although organic compounds are a risk to the environment, the biological load needs to be controlled by their negative impact in the human health. The presence of Escherichia coli (E. coli) leads to potential outbreaks of disease and it is able to survive to different environments such as soil (Xing et al. 2019); the Pseudomonas aeruginosa (P. aeruginosa) causes acute and chronic lung infections that result in significant morbidity and mortality, persist in acute and chronic infections, and include high resistance to antimicrobials (Wagner and Iglewsk 2008). Furthermore, Enterobacter aerogenes (E. aerogenes) have been reported as important opportunistic and multiresistant bacterial pathogen for humans during the last three decades in hospital wards. This Gram-negative bacterium has been largely described during several outbreaks of hospital-acquired infections (Davin-Regli and Pagès 2015).
The extended aeration process is a common method used to remove biological and organic pollutants in HWW (Diario Oficial de la Federacion 1997). However, this process cannot remove all compounds in wastewaters, this is a concern because some compounds can be chemically activated by other elements in the water body; for example, steroid estrogens (endocrine disrupters excreted in an inactive form) are activated by enzymes produced by bacteria in the sewage system and/or during the wastewater treatment (Panter et al. 1999). Most of these substances and compounds in HWW are considered mutagenic, carcinogenic, teratogenic, and embryotoxic (Kümmerer 2008). In this sense, if wastewaters are not treated properly, these substances could contaminate drinking water sources or bioaccumulate in living organisms via water bodies. Nevertheless, to improve decontamination in wastewaters treatments, some oxidants are included in the extended aeration process; this is the case of chlorine, the ozone, and the hydrogen peroxide. Chlorine is an oxidant commonly used to remove contaminants in wastewater because it has oxidation potential (OP) of 1.36 V, although oxidants with higher oxidation potentials such as ozone (O3) with OP of 2.07 V and hydrogen peroxide (H2O2) with OP of 1.78 V (Legrini et al. 1993) are more effective than chlorine in the transformation of simple and complex chemical substances. Typically, O3 and H2O2 combined with ultraviolet light reduce the pollutants in wastewaters. The oxidation process based on O3 and H2O2 is named AOP; AOP generates free radicals with an even greater oxidation potential, such as the radical hydroxyl with OP of 2.80 V (Legrini et al. 1993; Litter 2005).
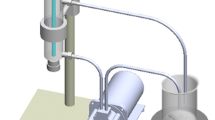
Figure 1 shows a flow diagram of wastewater decontamination using AOP. The process presented in Fig. 1 is based on the oxidants H2O2 and O3 in a closed chamber (quartz tube) with the wastewater sample; the UV-Visible lamp irradiates ultraviolet light in the quartz tube activating the oxidants in wastewater; once the oxidation process is carried out, the wastewater is decontaminated. The AOP has proven efficient in eliminating phenols (Kusic et al. 2006), pharmaceuticals acid products (Yuan et al. 2012; Hernandez et al. 2012; Mitrović et al. 2012), industrial dyes (Mitrović et al. 2012), wine wastewaters (Lucas et al. 2010), organic matter (Du et al. 2014; Lamsal et al. 2011), and furfural in aqueous solutions (Saien et al. 2017). In fact, the AOP has been a good alternative to reduce the concentration of pollutants in urban and HWW treatments (Lamsal et al. 2011; Saien et al. 2017; Giannakis et al. 2017), with advantages such as low cost and high energy efficiency (Karaca and Tasdemir 2014; Zhang et al. 2013b).
Although there are papers focused on the effectiveness of AOP for wastewater treatments, local studies are necessary to assess biological and persistent organic compounds after AOP. This work studies the reduction of biological and organic compounds by AOP in HWW treatment plant in Mexico. The HWW samples were previously treated with an extended aeration process and, thereafter, a post-treatment with AOP based on UV/H2O2/O3 system with a medium pressure mercury lamp was applied. After using the AOP system, the water samples were characterized using chemical oxygen demand (COD), turbidity, color test, coliforms and E. coli test, gas chromatography coupled to mass spectrometry (GC-MS), and UV-Visible absorption spectra.
2 Materials and Methods
This section presents a detailed description of the characterization techniques, the AOP UV/H2O2/O3 system, and the methodology applied to HWW samples.
2.1 Characterization Techniques
Quality parameters, such as COD, turbidity, and color, were monitored using a thermoreactor TR320 E. Merck and a spectrophotometer SQ118 E. Merck at wavelengths of 585, 525, and 446 nm, respectively. The COD expresses the amount of oxygen originating from potassium dichromate that reacts with oxidizable substances in the samples.
The bacterial load was determined using 20 mL of sample and inoculated in a Compact Dry Nissui EC medium (E. coli and coliforms) where colonies of coliforms (E. aerogenes and P. aeruginosa) and E. coli were grown. The medium contains two substrates of chromogenic enzymes: Magenta-GAL and X-Gluc, certified by the AOAC Performance Tested Methods. One milliliter of sample was seeded using a pipette and incubated for 24 h at 35 °C ± 2 °C; then, the colony-forming units were counted. The coliforms detected by the method were identified by a dark red-coloration, while E. coli is identified by a blue or violet-blue coloration.
GC-MS was performed using an Agilent 7890 gas chromatograph coupled to Agilent 5975C. The samples were dried over anhydrous sodium sulfate, washed with silica gel, and filtered and concentrated by rotary evaporation with a volume of 2 mL. One microliter of each extract was injected with the following chromatographic conditions: column DB-5MS of 0.5 μm × 30 μm × 0.25 μm DI, injector temperature 180 °C, Detector 220 °C, with a ramp starting at 56 °C/min, 10 °C/min, 196 °C/min, 20 °C/min, and 52 °C/min. The injector runs in Split/Spit less mode. The analysis of contaminants by chromatography was exploratory; once the chromatograms were obtained, they were compared with the NIST08 library.
2.2 Advanced Oxidation Process UV/H2O2/O3
UV-Vis absorption spectra were obtained with a spectrophotometer Lambda 20 Perkin Elmer. The UV-Vis absorption spectra allowed evaluating the removal of contaminants from the wastewater treatment with the method based on the area under the curve of UV-Vis absorption spectra versus radiation time reported in (Mejia-Morales et al. 2019), where P(t)/P0 is the normalized area under curve, P(t) is the area under curve of absorption spectra at specific times, and P0 is the area under curve of pollutant concentration before AOP. The value of the area for each absorption spectrum includes all the magnitudes of the absorption bands in the UV-Vis spectrum; this method allows comparing different samples of wastewater using AOP treatments. The pre-treated wastewater was mixed with 0.02 ml of H2O2 using a glass syringe; the mixture was placed in a Pyrex glass photoreactor containing two inlets (i.e., top and bottom) for the air or air/O3 flow. The air rate supplied was 2000 mL/min and the aeration level was 400 mL/min of O2. The lamp used was a medium pressure mercury lamp PUV-1022 Heraeus with an emission range of 200 to 460 nm. Once the lamp is turned on, the chronometer records the exposure to the radiation time. About 10 mL of wastewater sample are extracted in periods of 5 min. The H2O2 content is measured by means of indicator strips (Peroxide-test, 0.5 to 25 mg/L, Merck); if the concentration of H2O2 is zero, 0.02 mL of H2O2 is added.
2.3 Methodology
The wastewater samples are composite samples, which represent the characteristics of the total effluent discharged in water bodies; the sampling method is based on the Mexican standard NMX-AA-3-1980. Sewage samples were taken for 24 h covering variations of discharged HWW in water bodies (Mexican Standard, NMX-AA-3-1980). To characterize the wastewater samples, 1 L of sample was obtained from a hospital in the city of Puebla City, Mexico. First, the samples were decontaminated by an extended aeration process (Diario Oficial de la Federacion 1997) and filtered by using a 1.5 μm filter paper; then, a first characterization involving all the methods described in “Characterization Techniques” was carried out. Next, wastewater was treated photochemically by AOP described in “Advanced Oxidation Process UV/H2O2/O3.” Finally, once the photochemical treatment was applied, a second characterization process using the techniques described in “Characterization Techniques” was carried out.
3 Results and Discussion
Once the wastewater was previously treated with the aeration process, they were characterized following the process described in “Characterization Techniques.” Table 1 shows the results of the characterization and the values specified by the official Mexican standards NOM-001-ECOL-1996 (Diario Oficial de la Federacion 1997) and NOM-CCA-029-ECOL/1993 (Diario Oficial de la Federacion 1993). It is important to mention that NOM-001-ECOL-1996 eliminated the COD parameter of NOM-CCA-029-ECOL/1993, considering only the BOD.
The results showed that the COD level after the extended aeration process is higher than the maximum limits specified by official Mexican standards; that is, the water quality is not in accordance with the environmental specification. In fact, higher COD values represent an environmental risk, because there are increases in organic compounds in wastewater.
Table 1 shows the result of the coliforms in the wastewater after the aeration process (E. aerogenes and P. aeruginosa). The coliforms are an indicator of water quality; note that there are higher coliform levels than those proposed in Mexican standards. Additionally, the E. coli bacteria test (see Table 1) is an indicator of antibiotics in wastewater. Actually, there is some papers that report that the antibiotics such as amoxicillin and cephalothin generate biological resistance in E .coli, inhibiting its elimination by aeration process (Titilawo et al. 2015; Adefisoye and Okoh 2016; Reinthaler et al. 2013).
After the aeration process was applied, the AOP was used for the wastewater sample, following the process describe in “Advanced Oxidation Process UV/H2O2/O3.” Figure 2 shows the UV-Vis absorption spectra after the AOP UV/H2O2/O3. The UV-Visible absorption curve was evaluated every 5 min to quantify the reduction of pollutants in HWW. In Fig. 3, the area under curve (P(t)/P0) versus UV-Vis radiation time is presented; notice that after 20 min, the effectiveness of the UV-Vis radiation is neglected; this means the HWW sample cannot be decontaminated; in fact, approximately 73% of the contaminants were removed during 20 min. Thus, the results corresponding with HWW samples exposed by 20 min were named post-treated wastewater. Although AOP based on UV/H2O2/O3 cannot completely reduce contaminants in wastewater (see potable water curve in Fig. 2), decontamination with AOP is greater than the aeration process (see pre-treatment with aeration process in Fig. 2).
After using AOP UV/H2O2/O3, the sample was characterized once again following “Characterization Techniques.” Table 1 shows the CDO, the color test, the turbidity, the coliforms, and E. coli test after treatment with AOP UV/H2O2/O3. Note that after applying the AOP treatment, the test equipment cannot detect the COD in the sample; in addition, color and turbidity are less than those obtained by aeration process. On the other hand, the microbiological test does not detect the presence of coliforms and E. coli. Therefore, AOP UV/H2O2/O3 system sterilizes the HWW sample. The sterilization is caused by the lamp used in the AOP treatment; the lamp power is 1000 W. The lamp increases the temperature in the wastewater obtaining the sterilization of the bacterial load; in our setup, this took 20 min. In fact, using the aeration process, the solar radiation cannot increase the HWW temperature because the sunlight in the HWW treatment plant is affected by mechanical interferences (i.e., gratings that cover the cisterns, walls, clouds, etc.) obtaining an average temperature of 19.2 °C; in this regard, inactivation of bacteria is temporary, because total inactivation is achieved at temperatures above 45 °C (McGuigan et al. 2012).
The results of the chemical compounds obtained from GC-MS are presented in Table 2 (see “Characterization Techniques”). Notice that this compounds have been found in previous AOP for HWW treatments; however, some of them as Octadecanoic acid, N-(4-methylphenyl) acetamide, m-Tert-butylphenol, 1,2,4-Trimethylcyclohexane, N,N-Dimethyl-1-tetradecanamine, 2-Thiophenecarboxylic acid ester, 2,4-Diamin-5H-pyrrolo(3,2-d) pyrimidine, Nonane, Ethylcyclohexane, and Tetradecyloxirane have not been reported. On the other hand, after applying AOP treatments, 10 persistent organic compounds were obtained: cholesterol, octadecane, triclosan, tetrachloroethylene, cholestanol, o-xylene, hexadecane, palmitic acid, octadecanoic acid, and 4-(1,1,3,3-tetramethylbutyl) phenol. In addition, 8 by-products were formed such as hydrocarbons, aromatics, phenols, and fatty acids. In fact, these compounds are also generated as intermediates with the photolysis of organic material (Adefisoye and Okoh 2016). Note in Table 2 that the AOP UV/H2O2/O3 system allowed to reduce 50% more chemical compounds than the aeration process. In addition, the compounds were classified as toxic, persistent, estrogenic, androgenic, irritant, carcinogenic, and low risk for human health and environment, according to literature reports; some of them have not been classified, but they are reported in this work. Note that most of these are toxic compounds and represent a risk for human health and environment.
The resistance of these compounds to be degraded by the AOP depends on the molecular structure, the atoms in the system, etc. For example, the presence of strongly hydrophobic compounds such as eicosane, hexadecane, and octadecane are insoluble in water because they have long chains and are not reactive. Compounds such as polycyclic aromatic hydrocarbons with high molecular weight are more difficult to be removed (Ulubelen et al. 1994). Halogenated compounds having carbon-chloro bonds are stable to hydrolysis and have resistance to biological and photolytic degradation. Most of these compounds contribute in the formation of photochemical smog and aerosols in the atmosphere (Larson and Weber 1994).
4 Conclusions
In this work was applied AOP UV/H2O2/O3 system in wastewater samples after prolonged aeration treatment. The HWW treatment using prolonged aeration process shows 10 persistent compounds and 8 by-products; the predominant compounds were fatty acids, phenols, aromatic hydrocarbons, and saturated hydrocarbons. Furthermore, 10 compounds not reported in previous aeration processes for wastewater treatments were found, some of them are toxics. On the other hand, biological testing showed that E. coli, E. aerogenes, and P. aeruginosa present a resistance to prolonged aeration treatment; this effect is attributed to antibiotics in hospitals.
Nevertheless, after using AOP UV/H2O2/O3, it was found that the sterilization and removal of organic compounds of the HWW sample were carried out in 20 min, eliminating 73% of pollutants. In this regard, AOP UV/H2O2/O3 system results in a good post-treatment for inhibition of chemical and biological compounds after prolonged aeration treatment.
Finally, Mexican HWW treatment plants require rigorous quality inspections because the treatments for HWW cannot eliminate toxics, persistent organic compounds, endocrine disrupters, and biological microorganisms. In fact, in Mexico, there are no standards and laws to control or regulate the maximum levels of toxic substances that can be discharged into water bodies by HWW treatment plants. Based on this information, it is suggested to restructure the wastewater treatment plants by adding photochemical and biological treatment, and a reverse osmosis (RO) process to eliminate toxic compounds in the wastewater.
References
Acevedo, C. A., Sánchez, E. Y., Reyes, J. G., & Young, M. E. (2007). Volatile organic compounds produced by human skin cells. Biological Research, 347–355.
Adefisoye, M. A., & Okoh, A. I. (2016). Identification and antimicrobial resistance prevalence of pathogenic Escherichia coli strains from treated wastewater effluents in Eastern Cape, South Africa. Mycrobiologyopen, 143–151.
Andersen, F. A. (1995). Final report on the safety assessment of lauramine and stearamine. International Journal of Toxicology, 196-203.
Bansal, V., & Kim, K.-H. (2015). Review of PAH contamination in food products and their health hazards. Environment International, 84, 26–38.
K. Brigden, Iryna Labunska, David Santillo, Mengjiao Wang & Paul Johnston (2013). Organic chemical and heavy metal contaminants in wastewaters discharged from two textile manufacturing facilities in Indonesia. Greenpeace Research Laboratories, Technical report.
Crini, G., Lichtfouse, E., Wilson, L. D., & Morin-Crini, N. (2019). Conventional and non-conventional adsorbents for wastewater treatment. Environmental Chemistry Letters, 17(1), 195–213.
Czekalski, N., Gascón Díez, E., & Bürgmann, H. (2014). Wastewater as a point source of antibiotic-resistance genes in the sediment of a freshwater lake. The ISME Journal, 1381–1390.
Davin-Regli, A., & Pagès, J.-M. (2015). Enterobacter aerogenes and Enterobacter cloacae; versatile bacterial pathogens confronting antibiotic treatment. Frontiers in Microbiology., 392.
Delaquis, P. J., Stanich, K., Girard, B., & Mazza, G. (2002). Antimicrobial activity of individual and mixed fractions of dill, cilantro, coriander and eucalyptus essential oils. International Journal of Food Microbiology, 74, 101–109.
Du, E.‐. D., Feng, X.‐. X., Guo, Y.‐. Q., Peng, M.‐. G., Feng, H.‐. Q., Wang, J.‐. L., & Zhang, S. (2014). Dimethyl phthalate degradation by UV/H2O2: combination of experimental methods and quantum chemical calculation. Clean - Soil, Air, Water, 811–821.
Espina, L., Gelaw, T. K., de Lamo-Castellví, S., Pagán, R., & García-Gonzalo, D. (2013). Mechanism of bacterial inactivation by (+)-limonene and its potential use in food preservation combined processes. PLoS One, 8(2), e56769.
Fang, H., Tong, W., Branham, W. S., Moland, C. L., Dial, S. L., Hong, H., Xie, Q., Perkins, R., Owens, W., & Sheehan, D. M. (2003). Study of 202 natural, synthetic, and environmental chemicals for binding to the androgen receptor. Chemical Research in Toxicology, 16, 1338–1358.
Federacion, D. O. (1993). NOM-CCA-029-ECOL-1993, Que establece los límites máximos permisibles de contaminantes en las descargas de aguas residuales a cuerpos receptores provenientes de hospitales. NORMA Oficial Mexicana.
Federacion, D. O. (1997). NOM-001-ECOL-1996, Que establece los límites máximos permisibles de contaminantes en las descargas de aguas residuales en aguas y bienes nacionales. NORMA Oficial Mexicana.
Giannakis, S., Rtimi, S., & Pulgarin, C. (2017). Light-assisted advanced oxidation processes for the elimination of chemical and microbiological pollution of wastewaters in developed and developing countries. Molecules, 1–21.
Gil, A., Elmchaouri, A., El Mouzdahir, Y., & Korili, S. A. (2015). Removal of tetrachloroethylene from aqueous solutions by adsorption on clay minerals. Adsorption Science and Technology, 355–368.
Gusso-Choueri, P. K., Choueri, R. B. R. B., de Araújo, G. S., Cruz, A. C. F., Stremel, T., Campos, S., de Sousa Abessa, D. M., & Ribeiro, C. A. O. (2015). Assessing pollution in marine protected areas: the role of a multi-biomarker and multi-organ approach. Environmental Science and Pollution Research, 22, 18047–18065.
Helmes, C. T., Atkinson, D. L., Jaffer, J., Sigman, C. C., Thompson, K. L., Kelsey, M. I., Kraybill, H. F., & Munn, J. I. (1982). Evaluation and classification of the potential carcinogenicity of organic air pollutants. Journal of Environmental Science and Health. Part A: Environmental Science and Engineering, 17, 321–389.
Hernandez, F., Rivera, A., Ojeda, A., Zayas, T., & Cedillo, L. (2012). Photochemical degradation of the ciprofloxacin antibiotic and its microbiological validation. Journal of Environmental Science and Engineering, 448–453.
S. R. Hinkle, Rodney J. Weick, Jill M. Johnson, Jeffrey D. Cahill, Steven G. Smith, Barbara J. Rich (2005). Organic wastewater compounds, pharmaceuticals, and coliphage in ground water receiving discharge from onsite wastewater treatment systems near La Pine, Oregon: Occurrence and implications for transport. Scientific Investigations Report, 2005–5055.
Kandyala, R., Raghavendra, S. P. C., & Rajasekharan, S. T. (2010). Xylene: An overview of its health hazards and preventive measures. Journal of Oral and Maxillofacial Pathology, 14, 1–5.
Karaca, G., & Tasdemir, Y. (2014). Application of advanced oxidation processes for polycyclic aromatic hydrocarbons removal from municipal treatment sludge. Clean - Soil, Air, Water, 191–196.
Kasprzyk-Hordern, B., Dinsdale, R. M., & Guwy, A. J. (2009). The removal of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs during wastewater treatment and its impact on the quality of receiving waters. Water Research, 363–380.
Kinney, C. A., Furlong, E. T., Kolpin, D. W., Burkhardt, M. R., Zaugg, S. D., Werner, S. L., Bossio, J. P., & Benotti, M. J. (2008). Bioaccumulation of pharmaceuticals and other anthropogenic waste indicators in earthworms from agricultural soil amended with biosolid or swine manure. Environmental Science & Technology, 1863–1870.
Kümmerer, K. (2008). Pharmaceuticals in the environment: sources, fate, effects and risks. Berlin: Springer.
Kusic, H., Koprivanac, N., & Bozic, A. L. (2006). Minimization of organic pollutant content in aqueous solution by means of AOPs: UV- and ozone-based technologies. Chemical Engineering Journal, 127–137.
Lamsal, R., Walsh, M. E., & Gagnon, G. A. (2011). Comparison of advanced oxidation processes for the removal of natural organic matter. Water Research, 45, 3263–3269.
Larson, R. A., & Weber, E. J. (1994). Reaction mechanisms in environmental organic chemistry. EE. UU. CRC Press, Inc..
Legrini, O., Oliveros, E., & Braun, A. M. (1993). Photochemical processes for water treatment. Chemical Reviews, 671–698.
Litter, M. I. (2005). Introduction to photochemical advanced oxidation processes for water treatment. In D. W. P. Boule (Ed.), The handbook of environmental chemistry (pp. 325–366). Berlin: Springer.
Lucas, M. S., Peres, J. A., & Puma, G. L. (2010). Treatment of winery wastewater by ozone-based advanced oxidation processes (O3, O3/UV and O3/UV/H2O2) in a pilot-scale bubble column reactor and process economics. Separation and Purification Technology, 235–241.
McGuigan, K. G., Conroy, R. M., Mosler, H. J., du Preez, M., Ubomba-Jaswa, E., & Fernandez-Ibañez, P. (2012). Solar water disinfection (SODIS): a review from bench-top to roof-top. Journal of Hazardous Materials, 29–46.
Mejia-Morales, C., Cortés-Hernández, D. M., Hernández-Aldana, F., Peña-Moreno, R. D., & Bonilla y Fernández, N. (2019). Broadband characterization method for photochemical systems used in hospital wastewater treatment. Journal of Water Chemistry and Technology, 228–235.
Mitrović, J., Radović Vučić, M. D., Bojić, D., Anđelković, T., Purenović, M., & Bojić, A. (2012). Decolorization of the textile azo dye Reactive Orange 16 by the UV/H2O2 process. Journal of the Serbian Chemical Society, 465–481.
Norma Mexicana. (1980). NMX-AA-3-1980, “AGUAS RESIDUALES.- MUESTREO”.
Om Prakash, B. (2019). Persistent organic compounds in the environment their impact on human health: a review. GSC Biological and Pharmaceutical Sciences, 081–088.
Panter, G. H., Thompson, R. S., Beresford, N., & Sumpter, J. P. (1999). Transformation of a non-oestrogenic steroid metabolite to an oestrogenically active substance by minimal bacterial activity. Chemosphere, 3579–3596.
Paulus, G. K., Hornstra, L. M., Alygizakis, N., Slobodnik, J., Thomaidis, N., & Medema, G. (2019). The impact of on-site hospital wastewater treatment on the downstream communal wastewater system in terms of antibiotics and antibiotic resistance genes. International Journal of Hygiene and Environmental Health, 635–644.
Ra, J. S., Lee, S. H., Lee, J., Kim, H. Y., Lim, B. J., Kim, S. H., & Kim, S. D. (2011). Occurrence of estrogenic chemicals in South Korean surface waters and municipal wastewaters. Journal of Environmental Monitoring, 13, 101–109.
Reinthaler, F. F., Galler, H., Feierl, G., Haas, D., Leitner, E., Mascher, F., Melkes, A., Posch, J., Pertschy, B., Winter, I., Himmel, W., Marth, E., & Zarfel, G. (2013). Resistance patterns of Escherichia coli isolated from sewage sludge in comparison with those isolated from human patients in 2000 and 2009. Journal of Water and Health, 13–20.
Saien, J., Moradi, M., & Soleymani, A. R. (2017). Homogenous persulfate and periodate photochemical treatment of furfural in aqueous solutions. Clean - Soil, Air, Water., 1.
Schuhmacher, M., Maria, M., Nadal, M., & Domingo, J. L. (2019). Concentrations of dioxins and furans in breast milk of women living near a hazardous waste incinerator in Catalonia, Spain. Environment International, 125, 334–341.
Shaikh, H., Memon, N., Bhanger, M. I., & Nizamani, S. M. (2014). GC/MS based non-target screening of organic contaminants in river indus and its tributaries in Sindh (Pakistan). Pakistan Journal of Analytical & Environmental Chemistry, 15, 42–65.
Stackelberg, P. E., Furlong, E. T., Meyer, M. T., Zaugg, S. D., Henderson, A. K., & Reissman, D. B. (2004). Persistence of pharmaceutical compounds and other organic wastewater contaminants in a conventional drinking-water-treatment plant. Science of the Total Environment, 329, 99–113.
Titilawo, Y., Obi, L., & Okoh, A. (2015). Antimicrobial resistance determinants of Escherichia coli isolates recovered from some rivers in Osun State, South-Western Nigeria: implications for public health. Science of the Total Environment, 82–94.
Ulubelen, A., Topcu, G., Eriş, C., Sönmez, U., Kartal, M., Kurucu, S., & Bozok-Johansson, C. (1994). Terpenoids from Salvia sclarea. Phytochemistry, 971–974.
Verlicchi, P., Al Aukidy, M., Galletti, A., Petrovic, M., & Barceló, D. (2012). Hospital effluent: investigation of the concentrations and distribution of pharmaceuticals and environmental risk assessment. Science of the Total Environment, 109–118.
Wagner, V. E., & Iglewsk, B. H. (2008). P. aeruginosa biofilms in CF infection. Clinical Reviews in Allergy & Immunology, 124–134.
Wang, Q., Wang, P., & Yang, Q. (2018). Occurrence and diversity of antibiotic resistance in untreated hospital wastewater. Science of The Total Environment, 990–999.
Wenjing, G., Bohu, P., Sugunadevi, S., Gokhan, Y., Weigong, G., Wen, Z., Weida, T., & Huixiao, H. (2019). Persistent organic pollutants in food: contamination sources, health effects and detection methods. International Journal of Environmental Research and Public Health, 4361.
Xing, J., Wang, H., Brookes, P. C., Falcão Salles, J., & Xu, J. (2019). Soil pH and microbial diversity constrain the survival of E. coli in soil. Soil Biology and Biochemistry, 139–149.
Yuan, H., Zhou, X., & Zhang, Y.-L. (2012). Degradation of acid pharmaceuticals in the UV/H2O2 process: effects of humic acid and inorganic salts. Clean – Soil, Air, Water, 45–50.
Zhang, J., Chang, V. W., Giannis, A., & Wang, J. Y. (2013a). Removal of cytostatic drugs from aquatic environment: a review. Science of the Total Environment, 445–446.
Zhang, Q., Li, C., & Li, T. (2013b). UV/H2O2 process under high intensity UV irradiation: a rapid and effective method for methylene blue decolorization. Clean - Soil, Air, Water, 1201–1207.
Zuccato, E., Castiglioni, S., & Fanelli, R. (2005). Identification of the pharmaceuticals for human use contaminating the Italian aquatic environment. Journal of Hazardous Materials, 205-209.
Funding
The author received from CONACyT the support of grant number 574902, for the Master’s Degree in Environmental Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mejía-Morales, C., Hernández-Aldana, F., Cortés-Hernández, D.M. et al. Assessment of Biological and Persistent Organic Compounds in Hospital Wastewater After Advanced Oxidation Process UV/H2O2/O3. Water Air Soil Pollut 231, 89 (2020). https://doi.org/10.1007/s11270-020-4463-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-4463-8