Abstract
This study investigates the effects of lead (Pb) on earthworm Eisenia fetida and its potential to recover from Pb exposure. Adult earthworms E. fetida were exposed for 4 weeks to lead (40–2500 mg Pb kg−1) in soil, and after the period of exposure, earthworms were transferred to clean unpolluted soil for 4 weeks to recover. Pb had no effect on the earthworm’s survival but inhibited earthworm growth; growth rate decreased with Pb concentration in the soil. During the recovery period, Pb pre-exposed earthworms did not manage to recover completely their growth. Lead had a highly significant effect on the malondialdehyde (MDA) concentration during both exposure and recovery periods. Pb showed concentration dependent toxicity relationships (weight, lipid peroxidation) for total earthworm Pb concentration. However, earthworm Pb bioconcentrations after recovery period could not explain the higher MDA concentration and lower earthworm fresh weight. Earthworms pre-exposed to low Pb levels have the potential to recover their growth and decrease Pb bioconcentrations, though more prolonged recovery period is needed to full recovery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Soil contamination is listed among the major threats to soil quality, and it is estimated that there is a total of 250,000 contaminated sites in EU (European Commission, 2012). Anthropogenic lead (Pb) fraction in the environment is estimated to be in the range from 10% to 90% (Reimann et al. 2012). Pb is released into the environment from point and diffuse sources. The main anthropogenic sources of lead are mining, smelting, industrial and agricultural activities, transport (fuels containing antiknock compounds), old lead pigment paints, batteries, wastewater, and waste disposal (Jung 2008; Laidlaw et al. 2012). In addition, the concern of increasing the amount of heavy metals due to the application of sewage sludge in agriculture and forestry is rising.
Metal pollution may disturb soil ecosystems by affecting the structure of soil invertebrate communities. Being a soil keystone species, earthworms constitute the dominant biomass of the soil fauna; they are important in the terrestrial food chain and play a key role in nutrient and energy cycling. Earthworms are permanently exposed to soil contaminants via skin and digestive tract. Therefore, earthworms are among the most widely used species to evaluate various contaminants impact to soil quality and fauna; and they are relevant bioindicators of soil pollution (Römbke et al. 2005).
Numerous studies have investigated heavy metals impact to earthworms. Heavy metals have been shown to reduce earthworm growth (Spurgeon et al. 1994), to delay their sexual maturation (Žaltauskaitė and Sodienė 2014), to reduce reproduction (Reinecke et al. 2001), and to cause mortality (Spurgeon et al. 1994; Davies et al. 2003). Metals, such as lead, cadmium, copper, and zinc, were shown to disturb gene expression and enzyme activities (Laszczyca et al. 2004; Li et al. 2009; Zhang et al. 2009), to induce oxidative stress (Spurgeon et al. 2004a; Sanchez-Hernandez 2006; Berthelot et al. 2008), and to be genotoxic (Fourie et al. 2007; Wu et al. 2012). Field studies have revealed that soil metal contamination disturbs earthworm communities, abundance, and species diversity and affects demography (Lévêque et al. 2015).
Much work has been done to investigate metals acute and chronic toxicity to earthworms (Spurgeon et al. 2005; Garg et al. 2009; Hooper et al. 2011) and to analyze metals bioaccumulation in the earthworms (Conder and Lano 2003; Andre et al. 2010, Sinha et al. 2010; Wang et al. 2018). However, there is little information on the earthworm recovery after their exposure to chemicals. Recovery is defined as the return of an impacted population or community to its pre-disturbance state or range of control systems (Gergs et al. 2016). Recovery is species and endpoint dependent. The main determinants of recovery are contaminant’s toxic mode of action, duration and magnitude of exposure, contaminant’s persistence in the environment, and organism’s ability to metabolize and detoxify the chemical. The review made by Kattwinkel et al. (2012) revealed that earthworm populations were able to recover after pesticides application; however, detailed studies analyzing this phenomenon are lacking. To our knowledge, recovery of earthworms after their exposure to heavy metals was not studied at all. Toxicokinetic studies mainly focusing on mechanistic evaluation of metals uptake and elimination rates show high variability (Spurgeon et al. 2011; González-Alcaraz et al. 2018) and do not provide any insight whether and how metals’ elimination rates translate into other biochemical and physiological parameters. In this study, we aimed to study chronic lead toxicity and bioaccumulation in earthworms Eisenia fetida and to determine the earthworms’ recovery after lead exposure.
2 Materials and Methods
The dry OECD artificial soil (OECD 1984) was moistened with distilled water to obtain approximately half of the final required water content. Solutions of lead nitrate (Pb(NO3)2) were mixed with soil to obtain the final required water content (50% of the maximum water holding capacity) and metal concentrations (40, 250, 500, 1000, and 2500 mg Pb kg−1) in the soil. The same volume of distilled water was added to the control sample. Three replicates were used for each of the soil metal concentration treatment and control.
Ten washed and weighed adult earthworms Eisenia fetida (cultured in the laboratory) were added to each container, and the earthworms were exposed to lead for 4 weeks (exposure period). The soil moisture in test containers was monitored and controlled during the whole experiment. After the exposure period, five earthworms from each container were transferred to new containers with fresh clean OECD soil and kept for 4 weeks (recovery period). Both the exposure and the recovery periods were conducted in the same environmental conditions at 20 °C ± 1 °C under continuous illumination (600 lx). To ensure earthworm growth, the earthworms were fed weekly with approximately 0.5 g of oatmeal per each earthworm. The unconsumed food was removed prior to resupplying a new portion.
Earthworm’s mortality and growth were measured every week by counting and measuring the individual fresh weight of the survived earthworms in each container. The earthworms were considered alive if they were able to respond to mechanical stimulus. Lipid peroxidation was measured as concentration of malondialdehyde (MDA) according to Buege and Aust (1978).
For Pb bioconcentration determination, the earthworms were removed from the soil, cleaned, and placed on moistened filter paper in Petri dishes for 48 h to void their gut content. After the depuration, the earthworms were frozen at − 80 °C until further analysis. Weighed earthworms were digested in the mixture of HNO3 and H2O2 using Milestone Ethos One closed vessel microwave system. Pb concentrations were measured with Shimadzu AA-6800 atomic absorption spectrometer. Standard certified reference material ISE sample 918 was also included in the analysis. Bioconcentration factor (BCF) was calculated as the ratio of Pb concentration in earthworm to the total soil Pb concentration.
A one-way analysis of variance (ANOVA) was used to assess the effect of Pb concentration on the estimated endpoints. Significant differences between the control and contaminated samples were determined by the Dunnett’s test. Significant differences between treatments were determined by LSD test, and the level of significance was established at p < 0.05. Earthworms growth (measured as fresh weight) rate during the study period was determined by linear regression and the slope of the curve (b) was used as a prediction of growth rate (g week−1). Significance of difference between the linear regression slopes for different Pb concentrations was assessed using Z-test (Clogg et al. 1995). All the statistical analysis was carried out using Statistica software.
3 Results
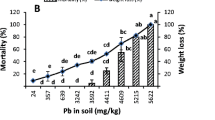
Pb had no significant effect on the survival of earthworms (ANOVA, p > 0.05). Low earthworm mortality (reaching up to 10%) was recorded only in the treatment with 40 mg Pb kg−1 (data not shown). During the recovery period all the earthworms have survived.
Lead has evoked significant effect on the fresh weight of earthworms during the 4-week exposure period (ANOVA, F = 14.74, p < 0.001), though weight loss was not recorded. Earthworms exposed to Pb in the soil grew slower during the exposure period, and the final earthworm fresh weight exposed to Pb was by 15.8–40.0% lower than that of control earthworms (Dunnett, p < 0.01) (Fig. 1a). During the recovery period, Pb pre-exposed earthworms did not manage to recover completely their growth, and final fresh earthworm weight after 4-week recovery period was by 11.1–17.6% lower than that of control earthworms (Dunnett, p < 0.01) (Fig. 1b). Earthworm fresh weight decreased along with Pb concentration in the soil both during the exposure and recovery periods (R2exposure = 0.18, R2recovery = 0.13, p < 0.05).
As some differences in the temporal pattern of earthworms’ growth during the exposure and recovery phases were observed, we calculated earthworm growth for these two periods. Earthworm weight growth rate (g week−1) was determined by linear regression b coefficient value. Earthworm growth rate decreased with Pb concentration in the soil (Table 1). Comparison of earthworm growth rates (Z-test) throughout the experiment indicated significant differences between the Pb treatments and control both for exposure and recovery period. Earthworms exposed to 40 mg kg−1 of Pb the soil grew significantly faster than those in the treatments with higher Pb concentration, and no significant differences (Z-test) between the treatments of 250–2500 mg kg−1 were observed. During the recovery period, the earthworms grew slower compared to exposure period, indicating no complete recovery in their growth. Only earthworms pre-exposed to the highest Pb concentration (2500 mg kg−1) during the recovery period started to grow faster, and the growth rate was almost the same as in the control.
Pb had a highly significant effect on the membrane lipid peroxidation, measured as MDA concentration, both during the exposure and recovery periods (ANOVA, Fexposure = 1070.17, Frecovery = 463.50, p < 0.001) (Fig. 2). During the exposure period, MDA concentration increased linearly with Pb concentration in the soil (R2 = 0.70, p < 0.01) resulting in up to 2.5-fold higher concentration than in control earthworms. During the recovery period, MDA concentrations were significantly lower (p < 0.05) compared to that after the exposure period, though did not reach the level of the control and remained 1.2–1.9-fold higher than in control earthworms. Accumulation of MDA during the recovery period indicates that E. fetida did not manage to fully recover from oxidative stress, and their cell membranes were damaged.
The amount of Pb accumulated by the earthworms linearly increased with Pb concentration in the soil during the exposure period (R2 = 0.94, p < 0.001) (Fig. 3). Bioconcentration factor was in the range from 0.14 ± 0.002 to 0.30 ± 0.001 indicating low Pb bioavailability in the soil and/or low earthworm ability to accumulate Pb from the environment. An inverse relationship between BCF and Pb exposure concentration was detected (r = − 0.92, p < 0.01). During the 4-week recovery period, Pb concentration in the earthworm was by 2.07–5.34 times lower than that after exposure period. Nevertheless, the significant linear relationship between Pb internal concentration with Pb soil concentration during the exposure period remained (R2 = 0.91, p < 0.001). Declined internal Pb indicate possible Pb elimination. However, earthworms pre-exposed to the highest Pb concentration (2500 mg kg−1) did not manage to eliminate Pb from the body (t-test, p > 0.05), and Pb concentration did not change significantly during 4-week period.
Since accumulated Pb content may be related with induced lipid peroxidation and reduced earthworm growth, therefore we examined the relationship between Pb internal concentration and MDA concentration and earthworm fresh weight. It was revealed that higher accumulated Pb concentration during the exposure period has led to a higher lipid peroxidation (r = 0.86, p = 0.03) and lower fresh weight of earthworms (r = − 0.85, p = 0.03). However, remained Pb level in the earthworms after recovery period could not explain the higher MDA concentration (r = 0.78, p = 0.07) and lower fresh weight (r = − 0.49, p = 0.33).
4 Discussion
In our study, Pb had no significant effect on the survival of adult earthworms. Our results are in line with the data of other studies showing low earthworm mortality due to Pb exposure (Chang et al. 1997; Davies et al. 2003; Neuhauser et al. 1985; Spurgeon et al. 1994; Bradham et al. 2006; Žaltauskaitė and Sodienė 2010). After 28-day E. andrei exposure to five different soils taken from the shooting ranges, high mortality was seen only in the soils with higher than 2153 mg kg−1 of total Pb (Luo et al. 2014), whereas L. terrestris exposed for 8 weeks to 12 contaminated field collected urban soils containing 30–7100 mg Pb kg−1 showed no mortality (Kennette et al. 2002). During the recovery period, all Pb pre-exposed earthworms survived as well. This might indicate that Pb exposure induced sublethal effects did not translate into lethal consequences after some time.
Unlike the survival, Pb significantly affected earthworm growth. Significant decrease in E. fetida weight compared to control was observed from the first week of exposure, indicating that even short-term exposure to Pb may inhibit earthworm growth. Longer exposure to Pb has led to more detrimental effect on the growth, and the difference between control and Pb exposed earthworms fresh weight increased (Fig. 1a). Earthworm weight loss was also observed after 7–42 days exposure to field metal-polluted soils, and significant weight loss was recorded in the two highest Pb-contaminated soils (Nahmani et al. 2007), and weight loss partially was explained by very low content of organic carbon in soil. Earthworm weight loss after Pb exposure was also recorded in our previous 28-day study; though in the latter study, the earthworms were not fed during the experiment (Žaltauskaitė and Sodienė 2010). Luo et al. (2014) have found no significant earthworm weight loss under the concentrations up to 656 mg Pb kg−1. Previously reported EC50 values for Pb (> 6670 mg kg−1) (Spurgeon and Hopkin 1995, Nahmani et al. 2007) are higher than the concentrations tested in our study. However, this was not a case in our study with E. fetida juveniles exposed to Pb for 14 weeks (Žaltauskaitė and Sodienė 2014). We have found that Pb did not induce weight loss, though dramatically reduced juvenile weight growth with 14-week EC50 value of 179 ± 35 μg Pb g−1. However, juveniles (young individuals) generally are more sensitive to metals or other contaminants exposure than adults.
In our study, the earthworms exposed to Pb grew slower, and it resulted in lower final weight (Table 1, Fig. 1a). During the recovery period, the growth rate was even lower compared to that during the exposure period. It might indicate that the reduced growth rate has a legacy effect and might remain reduced even after the cease of exposure to toxicants. Reduced earthworm growth rate was also reported by other researchers (Spurgeon and Hopkin 1996; Spurgeon et al. 2004b, Anderson et al. 2013; Žaltauskaitė and Sodienė 2014).
Pb induced oxidative stress in the earthworms, and significant increase of MDA concentration along with increasing Pb soil concentration was observed. The significant increase in MDA level indicates the presence of harmful active oxygen species and oxidative stress, which may lead to reduced earthworm growth, as was the case in our study (MDA was negatively related to the earthworm fresh weight). During the recovery period, MDA concentrations in Pb pre-exposed earthworms decreased by 6.05–18.71% with the highest decrease in the treatment with the lowest Pb concentration (40 mg kg−1). Notwithstanding this decrease, the MDA concentration after the recovery period was by 19.67–91.80% (p < 0.05) higher than in the control earthworms. These results indicate that during the 4-week recovery period earthworms did not manage to recover and oxidative damage to cell membranes remained. Insufficient decrease in MDA concentration during the recovery period may be due to some delay in MDA accumulation or that longer recovery period might be needed to reach the redox homeostasis (Chen et al. 2017).
Body Pb concentrations increased with Pb concentration in the soil, though low BCF indicate low Pb accumulation from the soil. Our calculated BCF’s values are in line with those reported in other studies (Nahmani et al. 2009; Kavehei et al. 2018). McGeer et al. (2003) reported that Pb exhibited the lowest BCF among other metals (Zn, Cu, Cd, Ni, and Ag). Higher bioaccumulation factors at lower heavy metal exposure levels, as in our case, were also determined in the study investigating Zn and Cd toxicokinetics in E. andrei (González-Alcaraz et al. 2018). Low Pb accumulation might be explained by low Pb bioavailability in the soil as Pb binds with soil organic matter (Nannoni et al. 2011). Therefore, the authors suggest that intestine Pb uptake is likely the most important uptake route, whereas dermal uptake is negligible. In addition, close relationship with Pb concentrations in earthworm food (soil, litter, etc.) was determined (Ernst et al. 2008). The body Pb concentrations after the recovery period were lower than that after the exposure period; however, earthworms did not eliminate the whole accumulated Pb content. Slow or even no elimination of Pb was also found by Davies et al. (2003) and Giska et al. (2014), and they suggested that it could be due to Pb storage in a nontoxic form. Predicted half-lives for lead in the contaminated artificial OECD soils polluted with Pb were reported to be 14.9 and 28.8 days, though no excretion was found over 100 days in earthworms exposed to field collected contaminated soil (Spurgeon and Hopkin 1999). In our study, earthworms pre-exposed to the highest Pb concentration have not eliminated Pb from their body, and it is consistent with the observations that at high pollution level, regulatory mechanism breaks down resulting in other adverse effects such as mortality (Luo et al. 2014) or reduced growth.
5 Conclusion
E. fetida chronic exposure to Pb has lowered the earthworms’ growth rate, evoked oxidative stress, and has led to significant bioaccumulation of lead. Earthworm tissue Pb concentrations were linearly related with MDA concentrations and inversely related with earthworm fresh weight. The study has showed that E. fetida pre-exposed to low levels of lead have the potential to recover their growth and decrease Pb bioconcentrations. However, the growth rate during the recovery period was lower than during the exposure period, suggesting incomplete recovery in growth. Moreover, even earthworm Pb body content reduced during the recovery period, though it was insufficient to full earthworm recovery as the impact on the growth and oxidative stress had a legacy effect, though remained Pb level after recovery period could not explain the higher MDA concentration and lower fresh weight. Our results clearly show that more prolonged recovery period is needed to full recovery.
References
Anderson, C. J., Kille, P., Lawlor, A. J., & Spurgeon, D. J. (2013). Life-history effects of arsenic toxicity in clades of the earthworm Lumbricus rubellus. Environmental Pollution, 172, 200–207.
Andre, J., Stürzenbaum, S. R., Killea, P., Morgan, A. J., & Hodson, M. E. (2010). Metal bioaccumulation and cellular fractionation in an epigenic earthworm (Lumbricus rubellus): The interactive influences of population exposure histories, site-specific geochemistry and mitochondrial genotype. Soil Biology and Biochemistry, 42(9), 1566–1573.
Berthelot, Y., Valton, É., Auroy, A., Trottier, B., & Robidoux, P. Y. (2008). Integration of toxicological and chemical tools to assess the bioavailability of metals and energetic compounds in contaminated soils. Chemosphere, 74, 166–177.
Bradham, K. D., Dayton, E. A., Basta, N. T., Schroder, J., Payton, M., & Lanno, R. P. (2006). Effect of soil properties on lead bioavailability and toxicity to earthworms. Environmental Toxicology and Chemistry, 25, 769–775.
Buege, J. A., & Aust, S. D. (1978). Microsomal lipid peroxidation. Methods in Enzymology, 52, 302–310.
Chang, L. W., Meier, J. R., & Smith, M. K. (1997). Application of plant and earthworm bioassays to evaluate remediation of lead-contaminated soils. Archives of Environmental Contamination and Toxicology, 32, 166–171.
Chen, X., Wang, X., Gu, X., Jiang, Y., & Ji, R. (2017). Oxidative stress responses and insights into the sensitivity of the earthworms Metaphire guillelmi and Eisenia fetida to soil cadmium. Science of the Total Environment, 574, 300–306.
Clogg, C. C., Petkova, E., & Haritou, A. (1995). Statistical methods for comparing regression coefficients between models. American Journal of Sociology, 100, 1261–1293.
Conder, J. M., & Lanno, R. P. (2003). Lethal critical body residues as measures of cd, Pb, and Zn bioavailability and toxicity in the earthworm Eisenia fetida. Journal of Soils and Sediments, 3(1), 13–20.
Davies, N. A., Hodson, M. E., & Black, S. (2003). The influence of time on lead toxicity and bioaccumulation determined by the OECD earthworm toxicity test. Environmental Pollution, 121, 55–61.
Ernst, G., Zimmermann, S., Christie, P., & Frey, B. (2008). Mercury, cadmium and lead concentrations in different ecophysiological groups of earthworms in forest soils. Environmental Pollution, 156, 1304–1313.
European Commission. 2012. The implementation of the soil thematic strategy and ongoing activities. Report COM (2012) 46, p. 15.
Fourie, F., Reinecke, S. A., & Reinecke, A. J. (2007). The determination of earthworm species sensitivity differences to cadmium genotoxicity using the comet assay. Ecotoxicology and Environmental Safety, 67, 361–368.
Garg, P., Satya, S., & Sharma, S. (2009). Effect of heavy metal supplementation on local (Allolobophora parva) and exotic (Eisenia fetida) earthworm species: A comparative study. Journal of Environmental Science and Health, Part A, 44(10), 1025–1032.
Gergs, A., Classen, S., Strauss, T., Ottermanns, R., Brock, T. C. M., Ratte, H. T., Hommen, U., & Preuss, T. G. (2016). Ecological recovery potential of freshwater organisms: Consequences for environmental risk assessment of chemicals. Reviews of Environmental Contamination and Toxicology, 236, 259–294.
Giska, I., van Gestel, C. A. M., Skip, B., & Laskowski, R. (2014). Toxicokinetics of metals in the earthworm Lumbricus rubellus exposed to natural polluted soils – Relevance of laboratory test to the field situation. Environmental Pollution, 190, 123–132.
González-Alcaraz, M. N., Loureiro, S., & van Gestel, C. A. M. (2018). Toxicokinetics of Zn and cd in the earthworm Eisenia andrei exposed to metal-contaminated soils under different combinations of air temperature and soil moisture content. Chemosphere, 197, 26–32.
Hooper, H. L., Jurkschat, K., Morgan, A. J., Bailey, J., Lawlor, A. J., Spurgeon, D. J., & Svendsen, C. (2011). Comparative chronic toxicity of nanoparticulate and ionic zinc to the earthworm Eisenia veneta in a soil matrix. Environment International, 37, 1111–1117.
Jung, M. C. (2008). Heavy metal concentrations in soils and factors affecting metal uptake by plants in the vicinity of a Korean cu-W mine. Sensors, 8, 2413–2423.
Kattwinkel, M., Römbke, J., & Liess, M. (2012). Ecological recovery of populations of vulnerable species driving the risk assessment of pesticides. Supporting Publications 2012, EN-338.
Kavehei, A., Hose, G. C., & Gore, D. B. (2018). Effects of red earthworms (Eisenia fetida) on leachability of lead minerals in soil. Environmental Pollution, 237, 851–857.
Kennette, D., Hendershot, W., Tomlin, A., & Sauvé, S. (2002). Uptake of trace metals by the earthworm Lumbricus terrestris L. in urban contaminated soils. Applied Soil Ecology, 19, 191–198.
Laidlaw, M. A. S., Zahran, S., Mielke, H. W., Taylor, M. P., & Filippelli, G. M. (2012). Re-suspension of lead contaminated urban soil as a dominant source of atmospheric lead in Birmingham, Chicago, Detroit and Pittsburgh, USA. Atmospheric Environment, 49, 302–310.
Łaszczyca, P., Augustyniak, M., Babczyńska, A., Bednarska, K., Kafel, A., Migula, P., Wilczek, G., & Witas, I. (2004). Profiles of enzymatic activity in earthworms from zinc, lead and cadmium polluted areas near Olkusz (Poland). Environment International, 30, 901–910.
Lévêque, T., Capowiez, Y., Schrek, E., Mombo, S., Mazzia, C., Foucault, Y., & Dumat, C. (2015). Effects of historic metal(oid) pollution on earthworm communities. Science of the Total Environment, 511, 738–746.
Li, M., Liu, Z., Xu, Y., Cui, Y., Li, D., & Kong, Z. (2009). Comparative effects of cd and Pb on biochemical response and DNA damage in the earthworm Eisenia fetida (Annelida, Oligochaeta). Chemosphere, 74, 621–625.
Luo, W., Verwij, R. A., & van Gestel, C. A. (2014). Determining the bioavailability and toxicity of lead contamination to earthworms requires using a combination of physicochemical and biological methods. Environmental Pollution, 185, 1–9.
McGeer, J. C., Brix, K. V., Skeaff, J. M., DeForest, D. K., Brighman, S. I., Adams, W. J., & Green, A. (2003). Inverse relationship between bioconcentration factor and exposure concentration for metals: Implications for hazard assessment in the aquatic environment. Environmental Toxicology and Chemistry, 22, 1017–1037.
Nahmani, J., Hodson, M. E., & Black, S. (2007). Effects of metals on life cycle parameters of the earthworm Eisenia fetida exposed to field-contaminated, metal-polluted soils. Environmental Pollution, 149(1), 44–58.
Nahmani, J., Hodson, M. E., Devin, S., & Black, S. (2009). Uptake kinetics of metals by the earthworm Eisenia fetida exposed to field-contaminated soils. Environmental Pollution, 157, 2622–2628.
Nannoni, F., Protano, G., & Riccobono, F. (2011). Uptake and bioaccumulation of heavy elements by two earthworm species from a smelter contaminated area in northern Kosovo. Soil Biology and Biochemistry, 43, 2359–2367.
Neuhauser, E., Loehr, R., Milligan, D., & Malecki, M. (1985). Toxicity of metals to the earthworm Eisenia fetida. Biology and Fertility of Soils, 1(3), 149–152.
OECD guideline for testing chemicals 207. Earthworm acute toxicity test. Adopted: 4 April 1984.
Reimann, C., Flem, B., Fabian, K., Birke, M., Ladenberger, A., Négrel, P., Demetriades, A., & Hoogewerff, J. (2012). The GEMAS Project Team 2012. Lead and lead isotopes in agricultural soils in Europe – The continental perspective. Applied Geochemistry, 27, 532–542.
Reinecke, A. J., Reinecke, S. A., & Maboeta, M. S. (2001). Cocoon production and viability as endpoints in toxicity testing of heavy metals with three earthworm species. Pedobiologia, 45, 61–68.
Römbke, J., Jänsch, S., & Didden, W. (2005). The use of earthworms in ecological soil classification and assessment concepts. Ecotoxicology and Environmental Safety, 62, 249–265.
Sanchez-Hernandez, J. C. (2006). Earthworm biomarkers in ecological risk assessment. Reviews in Environmental Contamination Toxicology, 188, 85–126.
Sinha, R. K., Herat, S., Bharambe, G., & Brahambhatt, A. (2010). Vermistabilization of sewage sludge (biosolids) by earthworms: Converting a potential biohazard destined for landfill disposal into a pathogen-free, nutritive and safe biofertilizer for farms. Waste Management and Research, 28(10), 872–881.
Spurgeon, D. J., & Hopkin, S. P. (1995). Extrapolation of the laboratory-based OECD earthworm toxicity test to metal-contaminated field sites. Ecotoxicology, 4, 190–205.
Spurgeon, D. J., & Hopkin, S. P. (1996). Effects of metal-contaminated soils on the growth, sexual development and early cocoon production of the earthworm Eisenia fetida, with particular reference to zinc. Ecotoxicology and Environmental Safety, 35, 86–95.
Spurgeon, D., & Hopkin, S. (1999). Comparisons of metal accumulation and excretion kinetics in earthworms (Eisenia fetida) exposed to contaminated field and laboratory soils. Applied Soil Ecology, 11, 227–243.
Spurgeon, D. J., Hopkin, S. P., & Jones, D. T. (1994). Effects of cadmium, copper, lead and zinc on growth, reproduction and survival of the earthworm Eisenia fetida (Savigny): Assessing the environmental impact of point-source metal contamination in terrestrial ecosystems. Environmental Pollution, 84, 123–130.
Spurgeon, D. J., Stürzenbaum, S., Svendsen, C., Hankard, P. K., Morgan, A. J., Weeks, J. M., & Kille, P. (2004a). Toxicological, cellular and gene expression responses in earthworms exposed to copper and cadmium. Comparative Biochemistry and Physiology C, 8, 11–21.
Spurgeon, D. J., Svendsen, C., Kille, P., Morgan, A. J., & Weeks, J. M. (2004b). Responses of earthworms (Lumbricus rubellus) to copper and cadmium as determined by measurement of juvenile traits in a specifically designed test system. Ecotoxicology and Environmental Safety, 57, 54–64.
Spurgeon, D. J., Svendsen, C., Lister, L. J., Hankard, P. K., & Kille, P. (2005). Earthworm responses to cd and cu under fluctuating environmental conditions: A comparison with results from laboratory exposures. Environmental Pollution, 136, 443–452.
Spurgeon, D. J., Lister, L., Kille, P., Pereira, G. M., Wright, J., & Svendsen, C. (2011). Toxicokinetic studies reveal variability in earthworm pollutant handling. Pedobiologia, 54, 217–222.
Wang, K., Qiao, Y., Zhang, H., Yue, S., Li, H., Ji, X., & Liu, L. (2018). Bioaccumulation of heavy metals in earthworms from field contaminated soil in a subtropical area of China. Ecotoxicology and Environmental Safety, 148, 876–883.
Wu, B., Liu, Z., Xu, Y., Li, D., & Li, M. (2012). Combined toxicity of cadmium and lead on the earthworm Eisenia fetida (Annelida, Oligochaeta). Ecotoxicology and Environmental Safety, 81, 122–126.
Žaltauskaitė, J., & Sodienė, I. (2010). Effects of total cadmium and lead concentrations in soil on the growth, reproduction and survival of earthworm Eisenia fetida. Ekologija, 56(1–2), 10–16.
Žaltauskaitė, J., & Sodienė, I. (2014). Effects of cadmium and lead on the life-cycle parameters of juvenile earthworm Eisenia fetida. Ecotoxicology and Environmental Safety, 103, 9–16.
Zhang, Y., Shen, G., Yu, Y., & Zhu, H. (2009). The hormetic effect of cadmium on the activity of antioxidant enzymes in the earthworm Eisenia fetida. Environmental Pollution, 157, 3064–3068.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Žaltauskaitė, J., Kniuipytė, I. & Kugelytė, R. Lead Impact on the Earthworm Eisenia fetida and Earthworm Recovery after Exposure. Water Air Soil Pollut 231, 49 (2020). https://doi.org/10.1007/s11270-020-4428-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-4428-y