Abstract
The development of shrub willow as a bioenergy feedstock contributes to renewable energy portfolios in many countries with temperate climates and marginal croplands. As willow is developed commercially in the US Northeast, there is a need to better understand its impact on water quality and greenhouse gas (GHG) emissions compared to alternative land uses (e.g., corn, hay). We measured the impact of cultivated willow of various ages (2 and 5 years) and management strategies (fertilized vs. unfertilized) compared to corn and hay on water table depth, soil water NO3 − and PO4 3− concentrations, and N2O, CH4, and CO2 fluxes at the soil-atmosphere interface during a drier than normal year in heavy clay soils with marginal agricultural value in upstate New York, USA. Soil water concentrations resulted in higher PO4 3− in willow and higher NO3 − in corn and hay, although willow is unlikely to negatively impact water quality with respect to phosphorus due to shorter periods of hydrologic connectivity in willow and hay than in corn. Gas fluxes varied spatially and temporally with hot moments of CH4 and N2O in corn and hay and seasonally variable CO2 in willow. While CH4 did not vary between fields, N2O was higher in corn and hay, and CO2 in willow, resulting in no net difference between CO2 equivalent (CH4, CO2, and N2O) emissions between fields. Converting marginal cropland on clay soils from corn or hay to willow left overall GHG emissions unaffected, slightly increased PO4 3−, and decreased NO3 − concentrations in soil water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In many countries around the world (e.g., Canada, Sweden, Germany, USA) where temperate climate and marginal cropland due to excessive soil moisture are common, shrub willow (Salix ssp.) has been identified as a potential perennial woody biomass energy crop (US DOE 2011; Dimitriou et al. 2012; Schmidt-Walter and Lamersdorf 2012; Klingebiel and Montgomery 1961). Indeed, willow’s dense roots, high transpiration rates, and tolerance to wet conditions make it well suited to marginal lands with recurring wetness caused by poor internal drainage due to high silt and clay content (Richards et al. 2014; Volk et al. 2006). In a broader context, the development of diverse bioenergy markets for feedstocks such as shrub willow serves to mitigate concerns over energy security, environmental and human health, and rural economic development (ACORE 2014). Introducing a replacement crop (willow) on a large scale on marginal cropland where conventional crops such as hay (perennial) and corn (annual) grow poorly owing to poor drainage or excessive moisture has implications for water quality (nitrogen, N and phosphorus, P), water quantity (soil moisture and water table), and nitrous oxide (N2O), carbon dioxide (CO2), and methane (CH4) emissions (primary greenhouse gases emitted from soils that contribute to the greenhouse effect (IPCC 2013)) at the soil-atmosphere interface.
Like for any other energy crop deployment on a large scale (e.g., hemp, poplar, miscanthus (Oates et al. 2015; van der Werf and Turunen 2007), the environmental impacts of the new crop (in this case willow or Salix ssp.) need to be quantified before management decisions are made to fully implement the said new crop on a commercial scale. This information is essential to farmers in this region and other regions with similar conditions (e.g., Canada, Sweden, Germany,) in order to weigh the relative costs and benefits of shrub willow before making decisions that pose long-term implications for the economic and environmental sustainability of the region (Bressler et al. 2017). However, until now, willow has primarily been studied in an experimental yield trial setting in the US Northeast (Volk et al. 2006). Since the first research projects on shrub willow production were initiated in upstate New York in the mid 1980s, research has primarily focused on yield trials associated with various willow species and crop management practices (planting density, weed management, nutrient management, and cover crops) (Volk et al. 2006).
Several studies documenting the impact of willow biomass crop production on water quantity and water quality have been conducted outside the USA in Sweden (Dimitriou et al. 2012) and Germany (Schmidt-Walter and Lamersdorf 2012). These studies indicated that groundwater recharge was reduced by approximately 40% in willow plots owing to enhanced rainfall interception and evapotranspiration compared to fallow ground reference plots. From a water quality standpoint, Schmidt-Walter and Lamersdorf (2012) indicated that, with the exception of the establishment period during which nitrate (NO3 −) concentrations in groundwater can be high (>10 mg/L) in willow fields, well-managed willow biomass crops can prevent NO3 − leaching. In Sweden, Dimitriou et al. (2012) showed that NO3 − losses from willow were significantly lower than that of reference cereal fields and were affected by soil and climate conditions, plant age (root development), and management techniques. The opposite was observed for phosphate (PO4 3−), with higher concentrations in the groundwater of the willow fields than in cereal fields. This may be attributed to macropore subsurface drainage, a primary pathway for P loss from agricultural systems (King et al. 2015). Shrub willow dense perennial root structure is constantly turning over, increasing the likelihood of macropore development compared to annual crops like corn. Since phosphate and nitrate are the main limiting nutrients in fresh water ecosystems, it is important to investigate the impact of willow on field nutrient loss.
We also know from corn studies that results obtained in one region for one crop are often not directly transferrable to other regions owing to differences in soil, climate, topography, and fertilization (Kladivko et al. 2004; Randall and Goss 2001; Gentry et al. 2000; Skaggs and Van Schilfgaarde 1999). It is therefore critical to conduct experiments to understand the full impact of willow on both water quality and water quantity in the US Northeast considering the recent interest in the development of more shrub willow biomass in this region on a commercial scale.
With respect to greenhouse gas (GHG: N2O, CO2, CH4) emissions, research indicates that GHG emissions at the soil-atmosphere interface vary under different land management techniques (Ruan and Robertson 2013; Kern et al. 2012; Alluvione et al. 2009; Ussiri et al. 2009). Several studies have addressed CO2 emissions in willow (Borjesson 1999; Caputo et al. 2014; Pacaldo et al. 2014). In a study designed to assess the GHG and energy balance of shrub willow production from planting to through harvesting and delivery to the gate of the end user, Caputo et al. (2014) estimated GHG emissions at the soil-atmosphere interface using Intergovernmental Panel on Climate Change (IPCC) 2007 guidelines. Elsewhere, Borjesson (1999) discusses the processes by which perennial energy crops tend to reduce carbon dioxide (CO2) emissions compared to annual energy crops like corn by sequestering carbon via no-till practices, root turnover, and leaf decay, but does not provide direct measurements of CO2 fluxes to the atmosphere in willow fields. Pacaldo et al. (2014) made continuous CO2 flux measurements to capture temporal variation, but did not measure nitrous oxide (N2O) or methane (CH4) that are also important GHGs (IPCC 2013). Pacaldo et al. (2013) found that carbon (C) is sequestered long term in coarse roots and above- and below-ground stool in willow fields, while Hu et al. (2016) showed that fine root turnover only provides short-term carbon storage, increasing soil C and resulting in a net loss of CO2 from the soil. Given the lack of consensus on the impact of willow on CO2 emission at the soil-atmosphere interface relative to other crops, it is important to directly measure CO2 flux in willow compared to conventionally tilled corn and hay.
With respect to N2O and CH4, less information is available on the impact of shrub willow on these GHG fluxes compared to other land uses. Caputo et al. (2014) estimated N2O emissions at the soil-atmosphere interface in willow fields based on leaf litter fall, leaf N content, and fertilization rates rather. We know that in forested systems, increases in soil moisture and temperature tend to increase N2O emissions at the soil-atmosphere interface (McDaniel et al. 2014). In agricultural fields where reactive nitrogen is present in sufficient quantity, temperature and moisture have also been shown to increase rates of denitrification and associated N2O emissions (Butterbach-Bahl et al. 2013; Saggar et al. 2013). Because willow generally leads to a decrease in groundwater recharge and N availability (Dimitriou et al. 2012; Schmidt-Walter and Lamersdorf 2012), it is likely to lead to a decrease in N2O emissions compared to corn or hay. For CH4, it is expected that CH4 fluxes will be highly variable, but overall lower in perennial willow than in tilled corn and hay. Upland perennial energy crops have indeed been shown to act as a permanent CH4 sink in many landscapes (Ruan and Robertson 2013; Kern et al. 2012), while conventionally tilled agricultural fields are generally net CH4 sources (Alluvione et al. 2009; Ussiri et al. 2009). We also know from riparian and forestry studies that soils shift from CH4 sinks to sources based on soil water content and temperature (Vidon et al. 2014; Megonigal and Guenther 2008; Andersen et al. 1998). This sensitivity to temperature and moisture results in highly variable CH4 fluxes that spike during hot moments or short periods of time with disproportionately high biogeochemical reaction rates (Jacinthe et al. 2015; Fisher et al. 2014; Vidon et al. 2010). These hot moments often produce a majority of annual emissions, making it important to measure CH4 fluxes during snowmelt and after storm events (Jacinthe et al. 2015). As with N2O, direct measurements of CH4 emissions in shrub willow fields, especially following storms and snowmelt events, are therefore much needed to better estimate the impact of converting corn and hay fields of marginal value into shrub willow fields. In a broader context, these measurements are also needed because CH4 fluxes at the soil-atmosphere interface have not been studied on broad spatial and temporal scales and may account for part of the 10 Tg/year CH4 missing from global carbon budgets (Bernhardt and Schlesinger 2013; Megonigal and Guenther 2008; Andersen et al. 1998).
Based on this brief review of current research, the objectives of this study are therefore to investigate the impact of shrub willow fields on water quantity, water quality (NO3 −, PO4 3−, chlorine (Cl−), ammonium (NH4 +)), and GHG emissions at the soil-atmosphere interface (CO2, N2O, CH4) relative to corn and hay in clay and clay loam soils of upstate New York in an abnormally dry year. Working in heavy clay and clay loam soils as opposed to typical good farming soils (e.g., loam) is especially important to assess the real impact that shrub willow could have when deployed at the commercial scale, as willow is often used as a replacement crop in places where poor corn or hay yields are observed, owing to poor drainage or excessive soil moisture (i.e., clay and clay loam soils) (Richards et al. 2014; Volk et al. 2006). Conducting this study in a year dryer than normal is also especially timely in the context of climate change as climate predictions suggest that the intensity and frequency of summer droughts is likely to increase in the near future (Milly et al. 2005; Karl and Knight 1998).
2 Materials and Methods
2.1 Site Description
This study was conducted in Cape Vincent, NY (Jefferson County) in Lake Ontario’s watershed (44° 7′ N, 76° 18′ W, elevation ∼85 m) (Fig. 1). Climate in the region is temperate humid continental with an average temperature of 7.9 °C, average humidity of 74.5%, and 576.6 mm of precipitation in 2015; 41% below the long-term average of 989 mm (KNYCAPEV1 2015). Test plots were located in the Cheppewa Creek Watershed of Frontal Saint Lawrence River and the Kents Creek Watershed of Frontal Lake Ontario in the Thousand Islands region. Bedrock in the area is primarily composed of crystalline rocks, gneiss, granite, and marble. Soils are clayey or clay loamy, mainly composed of Chaumont-Galloo-Wilpoint-Guffin and Kingsbury-Covington-Chaumont series (McDowell 1989; Soil Survey Staff 2015). These soils are for the most part classified as marginal cropland USDA Class III soils (susceptibility to erosion, wetness or waterlogging, and shallow depths to bedrock that limit the rooting zone). Although they are the type of soils for which the Department of Energy (DOE) has recommended developing shrub willow, they do not represent ideal soil conditions for willow, which typically grow best in sandy loam to silt or clay loam soils (Abrahamson et al. 2010; US DOE 2011).
Location of the study sites in New York, USA. Field sites are located in Cape Vincent (upper left). The Black River ReEnergy Plant (end user) near Fort Drum military base is also shown (right). Each field site has three clusters of instruments (three static chambers, three negative tension lysimeters, and one well). Clusters are distributed through the fields to account for spatial heterogeneity
Of the five experimental plots used in this study, three sites were planted with shrub willow (Salix ssp.) of varying ages (time since establishment), 5–6 years old (W5) and 2–3 years old fertilized (W2F) and unfertilized (W2). One fertilized corn (C) and one hay (H) field adjacent to willow plots were chosen as control sites. Willows were planted in a double row pattern with approximately 1.83 m alley spacing, 0.76 m row spacing, and 0.61 m plant spacing along the row. Contact and pre-emergent herbicides were applied during site preparation and after planting, respectively, and contact herbicides were applied during the first two growing seasons as needed. No fertilizers were applied to the 5- to 6-year-old willow field (W5) or the unfertilized 2- to 3-year-old (W2) field. At the start of the second growing season, 100 lb of elemental nitrogen was applied per acre (amount recommended by Abrahamson et al. 2010) to the 2- to 3-year-old fertilized field (W2F) in response to a poor growth rate during the first year. After the first year, all willow sites were coppiced (i.e., cut back at a height of 5–10 cm) and then allowed to grow for multiple years (3–4). None of the willow sites were harvested before the study was implemented. In April 2015, the hay field was aerated and 60 lb of elemental nitrogen was applied per acre. It was mowed in mid-June and mid-September. The hay field was a mix of grass (creeping foxtail), legume (white clover, hairy vetch), and other herbaceous plants. The corn field was tilled, planted, and fertilized with 100 lb of elemental nitrogen per acre in mid-June 2015 (amount recommended by Cornell Cooperative Extension) and harvested in mid-September. The three willow fields, the cornfield, and hay field were rain fed with no irrigation.
2.2 Field and Laboratory Measurements
In each field (W2, W2F, W5, H, C), three clusters of instruments were distributed to account for heterogeneity in elevation, drainage, and plant growth within the field (Fig. 1). Each cluster consisted of three static chambers to measure GHG flux from the soil, one well to measure groundwater table depth, and three negative tension lysimeters to collect soil pore water (Fig. 1). A full description of each of these elements is provided below. Within each cluster, the three static chambers and associated lysimeters were spaced approximately 1.5 m apart with at least one chamber and associated lysimeter wedged in between two plants and one chamber and associated lysimeter in between rows to account for the potential impact of differences in root density on measured variables.
Gas samples were collected in each field (W2, W2F, W5, H, C) at each location (3 clusters per field with 3 chambers per cluster—45 chambers total) in fall (November 2014), winter (January 2015), spring (April–May 2015), and summer (July–August 2015) baseflow conditions, as well as during two spring snowmelt (April 8, and May 6, 2015) and storm events in order to capture site responses to such events compared to baseline seasonal measurements. Specifically, a total of 4 storms generating more than 2 cm of precipitation were monitored by taking samples 12–24 h after precipitation ended: June 23, 2015—2.49 cm, August 21, 2015—2.77 cm, September 29–30, 2015—2.00 cm, and October 28, 2015—5.03 cm. Gas samples were collected for all 10 sampling dates in all clusters. The only exception is that samples were not taken in corn or hay on January 24, 2015, due to a layer of ice (>1 cm) across the fields, or on August 21, 2015 in the corn field due to equipment malfunction. Whenever gas samples were collected, we attempted to measure water table depth using groundwater wells and to collect water samples using negative tension lysimeters (see well and lysimeter description below). However, water samples were only successfully collected in April, May, and June 2015 when soil pore water was available. Additionally, soil samples were collected every 6 cm to the depth of 90 cm at all sites (W2, W2F, W5, H, C) in Fall 2014 for textural and soil organic carbon analysis in order to characterize soil characteristic at each location.
For soil analysis, soil samples were placed in plastic bags, returned to the laboratory, and then dried and sifted to separate out the particles that were above 2 mm within 24 h of collection. Loss on ignition was then run on each sample to determine soil organic matter (SOM) (Nelson and Sommers 1982). Soil texture analysis was conducted using the hydrometer method (Nelson and Sommers 1982). Groundwater wells located in each cluster for water table depth measurements consisted of 5.1 cm ID PVC pipe perforated throughout their lengths, and screened down to 90 cm below ground surface. To avoid siltation, a well sock covered each well and a benthonite clay seal near the soil surface prevented surface water infiltration into the well (Vidon and Hill 2004). The negative tension lysimeters used for soil water collection were macrorhizons (Macrorhizon, Wageningen, the Netherlands) with the 0.1-μm porous ceramic cup placed 15–25 cm below the ground surface below each static chamber. After collection, filtered water samples (0.1 μm porous ceramic cup) were transported back to the laboratory on ice in a cooler, and stored in a freezer until analysis for nitrate (NO3 −), ammonium (NH4 +), phosphate (PO4 3−), and chloride (Cl−) with a Bran + luebbe Autoanalyzer 3 High Resolution Digital Colorimeter (SEAL Analytical, Mequon, Wisconsin, USA) using standard methods (Clesceri et al. 1998).
Static chambers for GHG collection were made of white PVC material and consisted of two parts: a bottom section (37 cm height and 27 cm diameter) inserted 5 cm into the ground, and an airtight lid fitted with a gas sampling port installed only during sampling to close the chamber (Jacinthe and Dick 1997). At the time of sampling, soil temperature and soil moisture were recorded approximately 15 cm below ground surface using the temperature probe on a portable HI 9125 pH/ORP meter (Hanna Instruments, Woonsocket, Rhode Island, USA), and a portable TRIME TDR (Time Domain Reflectometry with Intelligent MicroElements) probe for soil moisture (IMKO Micromodultechnick GmbH, Ellingen, Baden-Wurttenberg, Germany). When sampling, the chambers were closed with the lid and 20 ml of headspace gas was extracted through the septa with a syringe after approximately 0, 25, and 50 min. Gas samples were then injected into and stored in 10 mL evacuated glass vials sealed with a butyl rubber septa and aluminum crimp cap (Vidon et al. 2015). Vials were transported back to the lab and stored in cardboard boxes in a cool dark cabinet until CO2, CH4, and N2O were analyzed with a Shimadzu GC-2014 gas chromatograph (Shimadzu Corporation, Kyoto, Japan) equipped with a Porapak Q precolumn (90 cm long) and a Hayesep D analytical column (180 cm long), along with a flame ionization detector for CH4 and CO2 detection, and an electron capture detector for N2O detection. Standard gases from Alltech (Deerfild, Illinois, USA) and P5 ECD grade gas from Airgas (North Division-Northeast Region, Syracuse, New York, USA) were used to develop standard curves, which were used to calculate concentration for CO2, CH4, and N2O. Subsequently, GHG fluxes (F) were computed as:
where dC/dt is the rate of change in GHG concentration inside the chamber (mass GHG m−3 air per min), V is the chamber volume (m3), A is the area circumscribed by the chamber (m2), and k is a unit conversion factor (1440 min/day) (Jacinthe and Lal 2004; Vidon et al. 2014; Gomez et al. 2016). GHG emission fluxes were calculated using linear curves calculated from standard gases. Percent deviation from the mean was also calculated from triplicates analysis of the same sample, every 25 samples (<5% for CO2 and N2O, 35% for CH4). Carbon dioxide equivalents for each gas were calculated using the global warming potential for each gas (IPCC 2013). Winter sampling was carried out following the same protocol, except that snow was removed by hand from each chamber immediately before the airtight lid was placed onto the chamber. The snow was returned into the chamber immediately after sampling (Gomez et al. 2016).
2.3 Data Analysis
GHG fluxes were not normally distributed and presented many outlier points. Outlier values, however, are considered valuable data points in spatially heterogeneous GHG flux data studies because they represent potential hot spots and hot moments, which are spikes in gas emissions (particularly for CH4 and N2O flux) generally caused by high rates of methanogenesis and/or denitrification (Jacinthe et al. 2015; Fisher et al. 2014; Vidon et al. 2014). Therefore, all sample points were kept during statistical analysis. Standard transformations of the data were unsuccessful at normalizing the data set. The non-parametric Kruskal-Wallis test was therefore used to analyze differences between groups (n = 25–90 depending on the analysis). In the rare cases when comparisons were made on a date-by-date basis for sub-groups of chambers or lysimeters and the resulting n number was too low to perform a Kruskal-Wallis test (i.e., n < 15), we simply used descriptive statistics (mean, median, interquartile range (IQR), 95% confidence interval (CI)). All statistical analyses were performed in the Project R software and Minitab. Although GHG data were highly variable both temporally and spatially, we believe that the 10 sampling dates used in this study provided us with adequate data to compare GHG flux between sites (W2, W2F, W5, H, C) during a dryer than normal year. The number of sampling dates was determined seasonally (one for each season to collect baseline samples) and one for each storm event during the warm season. During a wetter year, there would have been more opportunity for hot moments of biogeochemical activity (Vidon et al. 2010) and thus samples would have been collected more frequently. Comparing the impact of a dry year relative to a normal year in terms of precipitation is beyond the scope of this study and would require additional data collection.
3 Results
3.1 Soil Characteristics (Texture, Temperature, Moisture)
Soil texture in the shrub willow fields ranged from clay to clay loam with high clay content at most sites, 26–42% clay at the W2 site, 40–70% clay at the W2F site, and 58–71% clay at the W5 site. The average SOM ranged from 5.9–7.5% in the top 10 cm in the willow fields, dropping to 2–3% SOM at greater depths. Soil in corn was generally classified as clay (44–76% clay) and clay loam (38% clay) with an average SOM of 7.9% in the top 10 cm also dropping off to 2–3% SOM at depth. Hay soil was classified as clay (48–77% clay), with SOM decreasing from an average of 7.4% at the surface to 2.3% at depth. Throughout the course of the study, there was no evidence of erosion in the willow or hay fields, although the corn field exhibited signs of soil erosion (rills, muddy runoff water, deposition fans) during spring and fall storms when the field was bare.
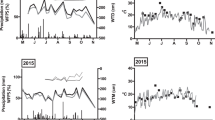
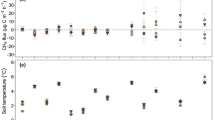
Soil temperature fluctuated seasonally, peaking at the end of July 2015 at 20.81 ± 1.49 °C in willow, 26.22 ± 1.05 °C in corn, and 23.74 ± 1.25 °C in hay. Temperatures dropped below freezing in January and returned to above freezing during snowmelt in mid-April 2015. Soil moisture fluctuated seasonally, with a low moisture during July (willow 22.8 ± 13.0 v/v, corn: 23.5 ± 5.7 v/v, hay 7.2 v/v) and peaking in November (willow 31.9 ± 11.8 v/v, corn: 40.0 ± 8.4 v/v, hay 38.6 ± 5.0 v/v) after fall rain storms and in May after spring snowmelt (willow 32.1 ± 8.1 v/v, corn 41.2 ± 6.8 v/v, Hay: 29.9 ± 2.7 v/v) (Fig. 2). The hay field remained frozen further into the spring than the corn and willow fields, and soil moisture dropped significantly during the summer after the hay field was mowed in June and dry conditions persisted.
Soil temperature and moisture (mean ± standard deviation) for willow, corn, and hay for all dates during the study period (Nov. 2014–Oct. 2015). Soil temperature and moisture data from all three willow fields are averaged together into the willow column. Data was not collected in all fields on January 24, 2015 nor in hay on April 8, 2015 due to frozen soils. Temperature samples were not collected in corn on August 21, 2015 and moisture samples were not collected in corn or hay on August 21, 2015 due to equipment breaking in the field due to dry soils
3.2 Water Quality
When available in spring (April–June) 2015, NO3 − concentrations were consistently higher in corn (mean 2.04 ± 2.07 mg N/L, median 1.45 mg N/L) than in willow fields (mean 0.23 ± 0.48 mg N/L, median 0.047 mg N/L) (Fig. 3), regardless of age (W2 vs. W5) or management practices (W2 vs. W2F) as all willow sites had statistically similar NO3 − concentrations (data not shown). In terms of NH4 +, mean and median NH4 + concentrations were similar between sites (W2, W2F, W5, H, C) during snowmelt, even though higher maximum concentrations were observed in April 2015 in willow (max 0.31 mg N/L) than in corn (max 0.056 mg N/L). NH4 + concentrations were not statistically different among the three willow fields for any of the measurement periods. Hay exhibited significantly higher NH4 + concentrations (mean 0.24 ± 0.46, median 0.085 mg N/L) than willow (mean 0.081 ± 0.13, median 0.032 mg N/L) or corn (mean 0.023 ± 0.01, median 0.024 mg N/L) in May (Kruskal-Wallis, p = 0.003). Fertilization (W2 vs. W2F) or willow age (W2 vs. W5) did not impact NO3 − or NH4 + concentrations.
Box plots (median, 5th, 25th, 75th, and 95th quartiles) illustrating nitrate (NO3 −), ammonium (NH4 +), phosphate (PO4 3−), and chloride (Cl−) concentrations in groundwater in willow (W), corn (C), and hay (H) fields during the April (spring snowmelt), May, and June (post-summer storm event) sampling dates. Black dots indicate the arithmetic mean
No significant differences were observed between sites (W2, W2F, W5, H, C) on any date for PO4 3−. However, phosphate concentrations were measurable in willow (max April 0.054 mg/L, max May 0.024, max June 0.016 mg/L) while at or below the detection limit (<0.005 mg/L) for corn and hay on all dates. Chloride concentrations were significantly lower in willow (median 1.39 mg/L) than in corn in April (median 13.36 mg/L, Kruskal-Wallis, p = 0.0003) and in June (W median 1.41 mg/L C: median 8.32 mg/L, Kruskal-Wallis, p = 0.0027) (Fig. 3). Fertilization (W2 vs. W2F) did not impact PO4 3−, or Cl− concentrations. Willow age (W2 vs. W5) had no impact on PO4 3−.
3.3 Greenhouse Gas Flux
For the willow fields (W2, W2F, W5), mean CO2 fluxes consistently increased with temperature peaking in June 2015 and July 2015. Little variability in CO2 fluxes were observed following time periods often associated with hot moments of GHG emission such as snowmelt (April–May) and post-storm periods (June 23, August 21, September 29–30, and October 28, 2015) (Fig. 4, Table 1). In corn, CO2 emissions also varied seasonally: mean and median CO2 emissions were greater than 0.9 g C/m2/day during the spring and summer months, while less than 0.3 g C/m2/day during the winter months (Fig. 4, Table 1). No clear seasonal pattern of CO2 flux was, however, observed in hay, with high fluxes observed during the summer (August 12, 2015), snowmelt (April 8, May 6, 2015), and following the June 24 storm event (Fig. 4, Table 1). On an annual basis, CO2 flux was significantly higher (Kruskal-Wallis, p = 0.0001) in willow fields (W2, W2F, W5) (mean 2.08 ± 1.93, median 1.84 g C/m2/day) than in corn (mean 1.17 ± 1.23, median 0.99 g C/m2/day), while CO2 flux in hay (mean 1.54 ± 2.70, median 0.92 g C/m2/day) was not statistically different from willow or corn (Fig. 4, Table 1).
With respect to CH4, median fluxes did not consistently vary in willow fields, but outliers increased mean fluxes significantly compared to baseline emissions on select dates (Fig. 4). Mean CH4 fluxes increased significantly (25×) from November 2014 (fall baseline conditions) to April 2015 (snowmelt) (Table 1). After the June 2015 storm event, mean CH4 fluxes increased (2×) compared to July 2015 (summer baseline conditions) (Table 1). In corn, no clear seasonal patterns were observed with mean CH4 fluxes spiking during snowmelt (April 8, 2015 150.99 ± 323.74 mg C/m2/day) and during the middle of the summer (August 12, 2015 47.38 ± 93.87 mg C/m2/day), with no clear underlying seasonal pattern (Fig. 4). Unlike the willow fields, the hay field shifted from being a net consumer of CH4 in November 2014 to being a net producer of CH4 during spring and summer, with fluxes peaking on August 12, 2015 (mean 81.65 ± 138.76, median 12.85 mg C/m2/day) (Fig. 4). When net CH4 emissions were combined across all sampling sates, annual CH4 flux did not vary significantly between sites. However, mean and median emissions were nevertheless higher in corn (mean 34.56 ± 136.61, median 3.45 mg C/m2/day) and hay (mean 34.09 ± 173.02, median 2.40 mg C/m2/day) than in willow (mean 19.69 ± 119.52, median 1.79 mg C/m2/day). No significant differences in CH4 fluxes were observed between willow fields of varying ages or fertilization.
Nitrous oxide fluxes did not vary significantly between sampling dates in the willow fields, and showed little response to snowmelt or storm events (Fig. 5; Table 1). When comparing the willow fields (W2F, W2, and W5), N2O fluxes were significantly higher overall (Kruskal-Wallis, p = 0.0034) in the 2-year-old fertilized willow field than in the 2- or 5-year-old unfertilized fields (data not shown). In corn, N2O fluxes spiked during snowmelt in April 2015 and post-storm in June 2015, dropping to close to zero in November 2014 and July 2015 (Fig. 5, Table 1). In hay, median N2O fluxes spiked in May 2015 (mean 6.62 ± 2.2, median 6.55 mg N/m2/day) during snowmelt rather than during April as seen in willow and corn (Table 1—May data not shown). N2O fluxes in hay did not, however, show a clear response to August and October 2015 storm events (Fig. 5). Interestingly, although the median N2O flux in July 2015 was low (0.30 mg N/m2/day), the mean was high owing to a few extremely high N2O flux values under peak temperature (23.74 ± 1.25 °C) and low soil moisture conditions (7.2 v/v). On an annual basis, N2O emissions were significantly higher (Kruskal-Wallis, p < 0.0001) in corn (mean 7.11 ± 19.30, median 0.62 mg N/m2/day) than in willow (mean 0.71 ± 2.99, median 0.18 mg N/m2/day), while hay (mean 3.45 ± 15.43, median 0.61 mg N/m2/day) was statistically similar to both willow and corn.
Box plots (median, 5th, 25th, 75th, and 95th quartiles, and outliers) illustrating nitrous oxide (N2O) and carbon dioxide equivalent (CO2 eq) flux at the soil-atmosphere interface in willow, corn, and hay. Black dots indicate the arithmetic mean. Shaded areas indicate snowmelt or storm event sampling dates
When converted to CO2-equivalent, GHG emissions were primarily driven by CO2 emissions at all locations, but to a lower extent in hay and corn than in willow (Fig. 6). Indeed, in corn and hay, the net effect of N2O and CH4 on CO2-equivalent flux increased during periods of increased soil wetting after summer storm events and during snowmelt (data not shown). Specifically, N2O was the primary driver of total CO2 equivalent emissions in June in corn (post-storm) and in hay in July (summer seasonal—dry). On an annual basis, CO2 equivalent fluxes did not vary significantly between the willow, corn or hay fields (Fig. 6).
Bar charts (mean ± STD) showing the annual mean carbon dioxide (CO2), methane (CH4), nitrous oxide (N2O), and total greenhouse gas emission (GHG) in g CO2eq /m2/day. W2 2- to 3-year-old unfertilized willow field, W2F 2- to 3-year-old fertilized willow field, W5 5-year-old unfertilized willow field, C corn field, H hay field
4 Discussion
4.1 What Impact Does Willow Have on Field Hydrology and Water Quality?
Previous studies indicate that willow’s dense perennial root systems, high aboveground biomass production, high transpiration rates, and tolerance to waterlogged conditions allow willow to grow well in wet, temperate climates while reducing loss of nutrients through erosion, runoff, and leaching (Volk et al. 2006; Smart et al. 2005; Corseuil and Moreno 2001). These characteristics of willow paired with a dry year in 2015 (precipitation was 41% below the average) resulted in dry wells from July–November 2015 and limited access to soil water. Similar hydrologic conditions were observed in hay, also perennial with dense roots. The 5-year-old willows exhibited signs of water stress as the leaves yellowed and fell prematurely and clay soils became cracked and dry in late summer–early fall 2015. The 2-year-old willows, however, did not exhibit these symptoms, possibly because of their lower water demand than the older, larger willows. Compared to willow and hay, corn soils exhibited higher soil moisture throughout the study with a measurable water table returning in early October after harvest. This is to be expected since mature willow has a longer growing season than corn and a higher evapotranspiration crop coefficient. As climate continues to change and summer droughts are likely to become more common in the near future (Milly et al. 2005; Karl and Knight 1998), willow may therefore reduce soil water availability compared to corn due to their higher water consumption.
With respect to water quality, our results show that willow in upstate New York likely impacts water quality in similar ways in other locales. Indeed, our results are consistent with Dimitriou et al.’s (2012) study (Sweden) finding that NO3 − concentrations (mg N/l) were found to be lower in willow (perennial crop with no tillage or fertilization after initial establishment) than in conventional agricultural fields (e.g., corn-tilled and fertilized annually with elemental N). Nitrate concentrations did not differ between willow fields even though the 2- to 3-year-old fertilized field was fertilized at the beginning of the second growing seasons while the other fields were not. The 2- to 3-year-old field was fertilized during the second growing season because the willow was struggling to outcompete the weeds with very low yields, indicating a nutrient deficiency prior to being fertilized. Thus, seeing no difference in NO3 − concentrations when we sampled was expected. It is likely that differences in NO3 − concentrations between willow, and especially corn, stem from a combination of land management differences (e.g., corn is fertilized every year, while willow fields are generally only fertilized when needed) and fundamental differences between willow (perennial) and corn (annual). Indeed, Kladivko et al. (2004) found that when tilled corn-corn was converted to a no-till corn-soybean rotation, NO3 − decreased quickly after the second year, indicating that changes in land management (reduced N input, no-till) could quickly change N concentrations in the soil.
In terms of PO4 3− concentration in soil water, Dimitriou et al. (2012) found higher PO4 3− concentrations in the groundwater of the willow fields than in cereal fields. Our results are consistent with this finding, showing that willow exhibited higher soil PO4 3− than corn or hay, although these differences were not significant. It is, however, unlikely that willow fields generate higher P losses to streams than corn or hay at the landscape scale. Indeed, from a process standpoint, P is primarily transported through overland flow and subsurface macropores out of agricultural fields (King et al. 2015). The high clay content (26–71%) in the willow fields combined with the absence of artificial drainage, and limited evidence of overland flow or erosion, suggests that if a significant amount of PO4 3− were to be lost from willow systems, it would likely be occurring through soil macropores (Vidon et al. 2012; Vidon and Cuadra 2011). Soil macropores have been shown to act as a preferential flow pathway for solute exports, including phosphorus, in soils with low hydraulic conductivity (Bernhardt and Schlesinger 2013; Vidon et al. 2012; Vidon and Cuadra 2011; Baker et al. 2006). However, the lack of hydrological connectivity between soil water in willow and hay fields and surrounding drainage ditches (soil was dry on most dates) suggest that little PO4 3− was likely lost to local drainages via subsurface flow in the willow or hay fields. Ultimately, although data indicate higher PO4 3− concentrations in soil water in willow than in corn or hay, the lack of hydrological connectivity of willow fields to local water bodies (no water table on most dates) suggests that converting land from corn or hay to willow is unlikely to negatively impact water quality with respect to P, at the landscape scale, at least under the soil and management conditions observed in this study.
4.2 How Does Willow Impact CO2 and CH4 Fluxes at the Soil-Atmosphere Interface?
Our study showed that overall CO2 fluxes at the soil-atmosphere interface were significantly higher in willow than in corn, and similar between willow and hay in the heavy clay soils where our study took place. When expressed on an areal basis for a 12-month period, the mean CO2 flux for the willow sites was 27.84 Mg CO2 ha−1 year−1 (2.08 g C/m2/day), which is close to the low end of the range of the values reported by Pacaldo et al. (2014), namely 28.6–50.6 Mg CO2 eq ha−1 year−1. A possible explanation for the higher CO2 fluxes in willow compared to corn is that fine root turnover and leaf decay in willow may increase CO2 fluxes to the atmosphere (Hu et al. 2016). Indeed, according to Rytter (2001), annual fine root production in willow on clay soils can represent between 30 and 40% of the net primary productivity of shrub willow grown in clay soils, or about 6.5–8.3 Mg ha−1 year−1 (Rytter 2001), whereas in corn, root production typically only represents ∼23% of the net primary productivity (Bolinder et al. 2007). In turn, annual fine root morality was estimated at 6.3–8.3 Mg ha−1 year−1 in that same study (Rytter 2001), suggesting that almost 100% of C stored in fine roots could turn over (converted to CO2) in 1 year. Foliage (leaf litter) in willow stands can also contribute between 2.8–4.2 Mg ha−1 year−1 of biomass every year, with about 46% of this biomass being C (Pacaldo et al. 2013). Another factor is that the more extensive root systems in perennial willow crops may contribute to higher root respiration, which has been shown to contribute between 18 and 33% of the total CO2 flux in willow (Pacaldo et al. 2014). Regardless of actual processes, another important result of our work is that CO2 fluxes in willow followed a distinct seasonal pattern increasing with temperature in the summer months, which is consistent with other CO2 flux studies in riparian zones, willow, forested, or agricultural sites (Gomez et al. 2016; Lutes et al. 2016; Jacinthe et al. 2015; Vidon et al. 2014). As temperatures rise with changing climate conditions, we should therefore expect to see increasing CO2 soil losses in willow landscapes based on our data indicating temperature dependent CO2 increases in willow.
For CH4, each field exhibited both sinks and sources of CH4 on every sampling date with a high degree of spatial variability, as observed by Jacinthe et al. (2015) in riparian and agricultural landscapes. Past studies have also observed that conventionally tilled fields are often small net sources of CH4 (Alluvione et al. 2009; Ussiri et al. 2009), which we see in this study in the cornfield with a median CH4 flux across all sampling dates of 3.45 mg C/m2/day. Although past research suggests that no-till and perennial biomass cropping systems generally behave as CH4 sinks (Ruan and Robertson 2013; Kern et al. 2012), the willow fields in this study were sources of CH4, albeit smaller ones than corn or hay. This result is, however, consistent with the heavy clay soils observed at our sites, as many clay soils often present hydromorphic characteristics typically associated with positive methane fluxes (Turetsky et al. 2014; Morse et al. 2012; Groffman and Pouyat 2009).
In terms of the temporal variability of CH4 fluxes, there was no significant variation between median CH4 fluxes in willow between dates including post-storm events (Table 1). In corn (for snowmelt in April and in June) and hay (for snowmelt in May and in June), clear hot moments of CH4 emission were observed with sites switching from sink (baseline) to source (post-storm event) (Table 1, Fig. 4). Higher CH4 emissions (especially in corn and hay, Fig. 4) were also observed on August 12, 2015, after 1.3 cm of precipitation on August 11 (under the 2 cm threshold). This added moisture and potentially anoxic condition development within soil aggregates near the soil surface, combined with high temperatures on August 12, 2015, may have led to the increase in CH4 fluxes in corn (mean—22.06 °C) and hay (mean—20.92 °C), and to a lesser extent (not significantly different from other dates) in willow (mean—19.5 °C) (Fig. 4). Together, these results are consistent with our current understanding of CH4 biogeochemistry (e.g., Naiman et al. 2005; Hedin et al. 1998; Vidon et al. 2010). With respect to CH4 flux, in particular, there is a need to conduct future studies with continuous measurements to account for spatial variability to better understand soil C cycling dynamics. Indeed, methanogenesis (CH4 production) is extremely sensitive to temperature, and thus varies temporally, with methanogenesis occurring best in the 21–24 °C range (Jacinthe et al. 2015; van Hulzen et al. 1999).
Seasonal changes in CH4 in corn and hay indicated increases in mean fluxes during the summer when temperatures were between 20 and 23 °C and when moisture after storm events were high enough to sustain near saturation, a requirement for methanogenesis to occur effectively (Naiman et al. 2005; Hedin et al. 1998). Such conditions did not present themselves in the willow fields, with saturated conditions only occurring during spring snowmelt when soil temperatures were <3 °C. Indeed, due to the hydrological characteristics of willow (high transpiration and dense roots), saturated conditions did not return after snowmelt, even after several wetting events which failed to produce anoxic conditions (August 21, 2015, 2.77 cm; September 29–30, 2015, 2.00 cm). Soil temperature in willow increased into the ideal range for methanogenesis only during the August 21, 2015 sampling, when the water table was dry and soil moisture was ∼25 v/v. Although 2015 was a particularly dry year compared to the average, this may be the new normal for this region as climate changes (Milly et al. 2005; Karl and Knight 1998). The summer of 2016 resulted in similarly dry conditions in Cape Vincent, NY. Under precipitation conditions closer to the 30-year average for this site, we would have expected to see more frequent periods of peak methanogenesis, leading to higher net CH4 fluxes in all fields.
4.3 How do N2O Fluxes Respond to Changing Field Conditions?
Although research indicates that temperature and moisture increase denitrification rates (Butterbach-Bahl et al. 2013; Saggar et al. 2013), past studies have shown inconsistent impacts of temperature and precipitation on N2O flux (incomplete denitrification) vs. N2 flux (complete denitrification) (Kulkarni et al. 2015; McDaniel et al. 2014; Dijkstra et al. 2012; Cantarel et al. 2011; Carter et al. 2011; Groffman et al. 2011; McHale et al. 1998). Spikes in N2O during wetting events (Keller et al. 2005), particularly after dry periods, have been widely observed and often account for a large proportion of annual emissions (Kim et al. 2010; Hyde et al. 2006; Prieme and Christensen 2001). N2O fluxes did not respond to wetting or temperature changes in willow, with statistically similar net emissions across all seasonal and potential hot moment sampling dates. Past studies (Morse et al. 2012; Orr et al. 2007; Verhoeven et al. 2006; Poe et al. 2003) support the explanation that N2O fluxes did not increase during wetting events in willow due to insufficient NO3 − in the soil, while NO3 − was available in corn and hay resulting in denitrification during wetting events, releasing N2O at the soil-atmosphere interface. In corn, N2O fluxes displayed high emissions during spring snowmelt and after storms and fertilizer application (April–June 2015). This pattern of N2O fluxes was also observed in corn fields by Fisher et al. (2014). In hay, N2O fluxes spiked during wet conditions, but also during dry soil conditions in July (<10% v/v), a phenomenon observed in grasslands by Luo et al. (2013), who found the highest N2O fluxes during warm, dry periods.
Field management differences (till vs. no till, fertilization) may also explain variability in N2O emission between fields, as we saw a significant increase in N2O emissions in the fertilized willow field (W2F) relative to other unfertilized willow fields (W2 and W5) (data not shown), and overall higher N2O fluxes in corn (tilled, high NO3 −) than in willow (no till, low NO3 −). Indeed, Menelik et al. (1994) found that no-till practices resulted in lower N2O emissions than conventional practices due to a higher N uptake, 22% higher in no-till compared to till under conventional fertilization, indicating lower N leaching from the rooting zone. Ruan and Robertson (2013) also reported mean daily N2O emissions under conventional tilling in smooth brome grass 2.8 times those under no-till practices in converted unfertilized soybean. In Ruan and Robertson’s (2013) study, N2O fluxes increased 18- to 55-fold under conventional tillage with mean daily N2O emissions of 4.75 ± 0.63 mg N2O-N/m2/day (47.5 ± 6.3 g N2O-N ha−1 day−1). In our study, we found that daily N2O fluxes averaged across all sampling dates were 10 times higher in tilled corn than in untilled willow, supporting the findings of past studies. Other studies have also shown that fertilization and mineralization induce high N2O fluxes, resulting in higher N2O fluxes from fertilized annual crops than from perennial biomass crops such as willow that are often only fertilized once every 3 to 4 years following harvesting (Hellebrand et al. 2008; Zhu et al. 2013).
5 Tradeoffs and Implications for Management
Our study was conducted on marginal, poorly drained clay soils, where shrub willow is often targeted as a replacement crop for corn or hay in northern temperate climates (Canada, Sweden, Germany, Northeastern USA). The sites chosen for this study represent the type of soils most commonly used to grow willow under temperate conditions (Canada, Sweden, Germany, Northeastern USA) where marginal agricultural land is often associated with excessive soil moisture due to poor drainage. Beyond its broad applicability to temperate landscapes worldwide, this study provides one of the first evaluations of the impact of commercial willow operation on water, N, P, and C cycling relative to corn or hay in North America. Although the fact that 2015 was a drought year (precipitation was 41% below normal) limits our ability to generalize our results, it provides a unique opportunity to start characterizing the impact of willow, relative to corn or hay under our changing climate on poor quality soils, as the intensity and frequency of summer drought is expected to increase in the near future not only in New York, but in many regions around the world. Under these soil and weather conditions, no differences in total GHG fluxes in CO2 equivalent values were observed between willow, corn, and hay. Willow produced more CO2 than corn and hay and contributed to measurable PO4 3− concentrations in soil water, while PO4 3− remained below detection in corn and hay. Corn and hay produced more N2O and contributed to higher NO3 − concentrations in soil water than willow, exhibiting a biogeochemical tradeoff between CO2 emissions in willow, and N2O emissions in corn and hay.
Our study therefore suggests that transitioning land use from corn or hay to willow is unlikely to negatively impact overall GHG emissions at the soil-atmosphere interface, while potentially improving water quality with respect to N, at least for the soils (clay and clay loam), climate (temperate), and management conditions (no till, no annual fertilization) investigated in this study. Before management recommendations are made, it is important to note that other factors should, however, be taken into account. These may include (but are not limited to) cost of transitioning from corn or hay to willow, other ecosystem services provided by willow relative to corn or hay (e.g., habitat for wildlife such as birds, mammals, pollinators), or energy benefits (bioenergy vs. fossil fuel). A companion study addresses some of these issues in an analysis of ecosystem services (Bressler et al. 2017).
References
Abrahamson, L. P., Volk, T. A., Smart, L. B., & Cameron, K. D. (2010). Shrub willow biomass producer’s handbook. State University of New York College of Environmental Science and Forestry. http://www.esf.edu/willow/documents/ProducersHandbook.pdf. Accessed June 28, 2016.
Alluvione, F., Halvorson, A. D., & Del Grosso, S. J. (2009). Nitrogen, tillage, and crop rotation effects on carbon dioxide and methane fluxes from irrigated cropping systems. Journal of Environmental Quality, 38, 2023–2033. doi:10.2134/jeq2008.0517.
American Council on Renewable Energy (ACORE). (2014). Renewable energy for military installations: 2014 industry review. Washington D.C. http://www.acore.org/files/pdfs/Renewable-Energy-for-Military-Installations.pdf. Accessed 1 Jun 2016.
Andersen, B. L., Bidoglio, G., Leip, A., & Rembges, D. (1998). A new method to study simultaneous methane oxidation and methane production in soils. Global Biogeochemical Cycles, 12, 587–594. doi:10.1029/98GB01975.
Baker, N.T., Stone, W.W., Wilson, J.T., Meyer, M.T. (2006). Occurrence and transport of agricultural chemicals in Learly Weber Ditch Basin, Hancock County, Indiana, 2003–04. U.S. Geological Survey scientific investigations report 2006–5251, 44 p. http://pubs.usgs.gov/sir/2006/5251/pdf/sir20065251_web.pdf. Accessed 1 Jun 2016.
Bernhardt, E. S., & Schlesinger, W. H. (2013). Biogeochemistry: an analysis of global change (Third ed.). Elsevier: Boston.
Bolinder, M. A., Janzen, H. H., Gregorich, E. G., Angers, D. A., & VandenBygaart, A. J. (2007). An approach for estimating net primary productivity and annual carbon inputs to soil for common agricultural crops in Canada. Agriculture, Ecosystems, and Environment, 118, 29–42. doi:10.1016/j.agee.2006.05.013.
Borjesson, P. (1999). Environmental effects of energy crop cultivation in Sweden—I: identification and quantification. Biomass and Bioenergy, 16, 137–154. doi:10.1016/S0961-9534(98)00080-4.
Bressler, A., Vidon, P., Volk, T., & Hirsch, P. (2017). Valuation of ecosystem services of commercial shrub willow (Salix spp.) woody biomass crops. Environmental Monitoring and Assessment, 189, 137. doi:10.1007/s10661-017-5841-6.
Butterbach-Bahl, K., Baggs, E. M., Dannenmann, M., Kiese, R., & Zechmeister-Boltenstern, S. (2013). Nitrous oxide emissions from soils: how well do we understand the processes and their controls? Philosophical Transactions of the Royal Society B., 368, 1–13. doi:10.1098/rstb.2013.0122.
Cantarel, A. A. M., Bloor, J. M. G., Deltroy, N., & Soussana, J.-F. (2011). Effects of climate change drivers on nitrous oxide fluxes in an upland temperate grassland. Ecosystems, 14, 223–233. doi:10.1007/s10021-010-9405-7.
Caputo, J., Balogh, S. B., Volk, T. A., Johnson, L., Peuttmann, M., Lippke, B., & Onell, E. (2014). Incorporating uncertainty into a life cycle assessment (LCA) model of short-rotation willow biomass (Salix spp.) crops. Bioenergy Research, 7, 48–59. doi:10.1007/s12155-013-9347-y.
Carter, M. S., Ambus, P., Albert, K. R., Larsen, K. S., Andersson, M., Prieme, A., van der Liden, L., & Beier, C. (2011). Effects of elevated atmospheric CO2, prolonged summer drought and temperature increase on N2O and CH4 fluxes in a temperate heathland. Soil Biology and Biogeochemistry, 43(8), 1660–1670. doi:10.1016/j.landusepol. 2004.01.004.
Clesceri, L. S., Greenberg, A. E., & Eaton, A. D. (1998). Standard methods for the examination of water and waste water. Washington, D.C.: American Public Health Association.
Corseuil, H. X., & Moreno, F. N. (2001). Phytoremediation potential of willow trees for aquifers contaminated with ethanol-blended gasoline. Water Resources, 35(12), 3013–3017. doi:10.1016/S0043-1354(00)00588-1.
Dijkstra, F. A., Prior, S. A., Runion, G. B., Torbert, H. A., Tian, H., Lu, C., & Venterea, R. T. (2012). Effects of elevated carbon dioxide and increased temperature on methane and nitrous oxide fluxes: evidence from field experiments. Frontiers in Ecology and the Environment, 10, 520–527. doi:10.1890/120059.
Dimitriou, I., Mola-Yudego, B., & Aronsson, P. (2012). Impact of willow short rotation coppice on water quality. Bioenergy Research, 5, 537–545. doi:10.1007/s12155-012-9211-5.
Fisher, K., Jacinthe, P. A., Vidon, P., Liu, X., & Baker, M. E. (2014). Nitrous oxide emission from cropland and adjacent riparian buffers in contrasting hydrogeomorphic settings. Journal of Environmental Quality, 43, 338–348. doi:10.2134/jeq2013.06.0223.
Gentry, L. E., David, M. B., Smith-Starks, K. M., & Kovacic, D. A. (2000). Nitrogen fertilizer and herbicide transport from tile drained fields. Journal of Environmental Quality, 29, 232–240. doi:10.2134/jeq2000.00472425002900010030x.
Gomez, J., Vidon, P., Gross, J., Beier, C., Caputo, H., & Mitchell, M. (2016). Estimating greenhouse gas emissions at the soil-atmosphere interface in forested watersheds of the US Northeast. Environmental Monitoring and Assessment, 188(5), 295. doi:10.1007/s10661-016-5297-0.
Groffman, P. M., & Pouyat, R. V. (2009). Methane uptake in urban forests and lawns. Environmental Science and Technology, 43, 5229–5235. doi:10.1021/es803720h.
Groffman, P. M., Hardy, J. P., Fashu-Kanu, S., Driscoll, C. T., Cleavitt, N. L., Fahey, T. J., & Fisk, M. C. (2011). Snow depth, soil freezing and nitrogen cycling in a northern hardwood forest landscape. Biogeochemistry, 102, 223–238. doi:10.1007/s10533-10010-19436-10533.
Hedin, L. O., von Fischer, J. C., Ostrom, N. E., Kennedy, B. P., Brown, M. G., & Robertson, G. P. (1998). Thermodynamic constraints on nitrogen transformations and other biogeochemical processes at soil-stream interfaces. Ecology, 79, 684–703. doi:10.1890/0012-9658(1998)079[0684:TCONAO]2.0.CO;2.
Hellebrand, H. J., Scholz, V., & Jurgen, K. (2008). Fertilizer induced nitrous oxide emissions during energy crop cultivation on loamy sand soils. Atmospheric Environment, 48, 8403–8411. doi:10.1016/j.atmosenv.2008.08.006.
Hu, X., Liu, L., Zhu, B., Du, E., Hu, X., Li, P., Zhou, Z., Ji, C., Zhu, J., Shen, H., & Fang, J. (2016). Asynchronous responses of soil carbon dioxide, nitrous oxide emissions and net nitrogen mineralization to enhanced fine root input. Soil Biology and Biochemistry, 92, 67–78. doi:10.1016/j.soilbio.2015.09.019.
Hyde, B., Hawkins, M., Fanning, A., Noonan, D., Ryan, M., O’Toole, P., & Carton, O. (2006). Nitrous oxide emissions from a fertilized and grazed grassland in the south east of Ireland. Nutrient Cycling in Agroecosystems, 75, 187–200. doi:10.1007/s10705-006-9026-x.
IPCC. (2013). In T. F. Stocker, D. Qin, G. K. Plattner, M. Tignor, S. K. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex, & P. M. Midgley (Eds.), Climate change 2013: The physical science basis: Working group I contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press https://www.ipcc.ch/pdf/assessment-report/ar5/wg1/WGIAR5_SPM_brochure_en.pdf. Accessed 1 June 2016.
Jacinthe, P.-A., & Dick, W. A. (1997). Soil management and nitrous oxide emissions from cultivated fields in southern Ohio. Soil Tillage Research, 41, 221–235. doi:10.1016/S0167-1987(96)01094-X.
Jacinthe, P.-A., & Lal, R. (2004). Effects of soil cover and land-use on the relations flux concentration of trace gases. Soil Science, 169(4), 243–259. doi:10.1097/01.ss.0000126839.58222.0f.
Jacinthe, P.-A., Vidon, P., Fisher, K., Liu, X., & Baker, M. E. (2015). Methane and carbon dioxide fluxes in adjacent cropland and riparian buffers. Journal of Environmental Quality. doi:10.2134/jeq2015.01.0014.
Karl, T. R., & Knight, R. W. (1998). Secular trends of precipitation amount, frequency, and intensity in the United States. Bulletin of the American Meteorological Society, 79, 231–241.
Keller, M., Varner, R., Dias, J. D., & Silva, H. (2005). Soil-atmosphere exchange of nitrous oxide, nitric oxide, methane, and carbon dioxide in logged and undisturbed forest in the Tapajos National Forest, Brazil. Earth Interactions, 9(023), 28. doi:10.5194/bg-8-733-2011.
Kern, J., Hellebrand, H. J., Gommel, M., Ammon, C., & Berg, W. (2012). Effects of climate factors and soil management on the methane flux in soils from annual and perennial energy crops. Biology and Fertility of Soils, 48, 1–8. doi:10.1007/s00374-011-0603-z.
Kim, D.-G., Mishurov, M., & Kiely, G. (2010). Effect of increased N use and dry periods on N2O emission from a fertilized grassland. Nutrient Cycling in Agroecosystems, 88, 397–410. doi:10.1007/s10705-010-9365-5.
King, W. K., Williams, M. R., Macrae, M. L., Fausey, N. R., Frankenberger, J., Smith, D. R., Kleinman, P. J. A., & Brown, L. C. (2015). Phosphorus transport in agricultural subsurface drainage: a review. Journal of Environmental Quality, 44, 467–485. doi:10.2134/jeq2014.04.0163.
Kladivko, E. J., Frankenberger, J. R., Jaynes, D. B., Meek, D. W., Jenkinson, B. J., & Fuasey, N. R. (2004). Nitrate leaching to subsurface drains as affected by drain spacing and changes in crop production system. Journal of Environmental Quality, 33, 1803–1813. doi:10.2134/jeq2004.1803.
Klingebiel, A.A., Montgomery, P.H. (1961). Land-capability classification. U.S. Department of Agriculture: Soil Conservation Service. http://www.nrcs.usda.gov/Internet/FSE_DOCUMENTS/nrcs142p2_052290.pdf. Accessed 11 August 2016.
KNYCAPEV1 (2015) Maddog Farm, 44° 4′ 45″ N, 76° 19′ 4″ W, 265 ft. Weather underground personal weather station. https://www.wunderground.com/personal-weather-station/dashboard?ID=KNYCAPEV1#history. Accessed Nov 2014-Oct 2015.
Kulkarni, M. V., Groffman, P. M., Yavitt, J. B., & Goodale, C. L. (2015). Complex controls of denitrification at ecosystem, landscape and regional scales in northern hardwood forests. Ecological Modeling, 298, 39–52. doi:10.1016/j.ecolmodel.2014.03.010.
Luo, G. J., Keise, R., Wolf, B., & Butterbach-Bahl, K. (2013). Effects of soil temperature and moisture on methane uptake and nitrous oxide emissions across three different ecosystem types. Biogoesciences, 10, 3205–3219. doi:10.5194/bg-10-3205-2013.
Lutes, K., Oelbermann, M., Thevathasan, N. V., & Gordon, A. M. (2016). Effect of nitrogen fertilizer on greenhouse gas emissions in two willow clones (Salix miyabeana, and S. dasyclados) in southern Ontario, Canada. Agroforestry Systems, 90–785. doi:10.1007/s10457-016-9897-z.
McDaniel, M. D., Wagner, R. J., Rollinson, C. R., Kimball, B. A., Kaye, M. W., & Kaye, J. P. (2014). Microclimate and ecological threshold responses in warming and wetting experiment following whole tree harvest. Theoretical and Applied Climatology, 116, 287–299. doi:10.1007/s00704-013-0942-9.
McDowell, L. (1989). Soil survey of Jefferson County, New York. Soil Conservation Service. USDA.
McHale, P. J., Mitchell, M. J., & Bowles, F. P. (1998). Soil warming in a northern hardwood forest: trace gas fluxes and leaf litter decomposition. Canadian Journal of Forest Research, 28, 1365–1372. doi:10.1139/cjfr-28-9-1365.
Megonigal, J. P., & Guenther, A. B. (2008). Methane emissions from upland forest soils and vegetation. Tree Physiology, 28, 491–498. doi:10.1093/treephys/28.4.491.
Menelik, G., Reneau Jr., R. B., & Martens, D. C. (1994). Corn yield and nitrogen uptake as influenced by tillage and applied nitrogen. Journal of Plant Nutrition, 17(6), 911–931.
Milly, P. C. D., Dunne, K. A., & Vecchia, A. V. (2005). Global pattern of trends in streamflow and water availability in a changing climate. Nature, 438, 347–350. doi:10.1038/nature04312.
Morse, J. L., Ardon, M., & Bernhardt, E. S. (2012). Greenhouse gas fluxes in southeastern U.S. coastal plain wetlands under contrasting land uses. Ecological Applications, 22(1), 264–280.
Naiman, R. J., Decamps, H., & McClain, M. E. (2005). Riparia: ecology, conservation, and management of streamside communities. London: Elsevier Academic Press 430 pp.
Nelson, D.W., Sommers L.E. (1982). Total carbon, organic carbon, and organic matter. In: Methods of soil analysis, Part 2, Chemical and microbiological properties agronomy monograph no. 9. 2nd ed. (pp. 539–79).
Oates, L.G., Duncan, D.S., Gelfand, I., Millar, N., Robertson, G.P., Jackson, R.D. (2015). Nitrous oxide emissions during establishment of eight alternative cellusosic bioenergy cropping systems in North Central United States. Global Change Biology Bioenergy, 1–11. doi: 10.1111/gcbb.12268
Orr, C. H., Stanley, E. H., Wilson, K. A., & Finlay, J. C. (2007). Effects of restoration and reflooding on soil denitrification in a leveed Midwestern floodplain. Ecological Applications, 17, 2365–2376. doi:10.1890/06-2113.1.
Pacaldo, R. S., Volk, T. A., & Briggs, R. D. (2013). No significant differences in soil organic carbon contents along a chronosequence of shrub willow biomass crop fields. Biomass and Bioenergy, 58, 136–142. doi:10.1016/j.biombioe.2013.10.018.
Pacaldo, R. S., Volk, T. A., & Briggs, R. D. (2014). Carbon sequestration in fine roots and foliage biomass offsets soil CO2 effluxes along a 19-year chronosequence of shrub willow (Salix x dasyclados) biomass crops. Bioenergy Research, 7, 769–776. doi:10.1007/s12155-014-9416-x.
Poe, A. C., Piehler, M. F., Thompson, S. P., & Pearl, H. W. (2003). Denitrification in a constructed wetland receiving agricultural runoff. Wetlands, 23(4), 817–826. doi:10.1672/0277-5212(2003)023[0817:DIACWR]2.0.CO;2.
Prieme, A., & Christensen, S. (2001). Natural perturbations, drying-wetting and freezing-thawing cycles and the emission of nitrous oxide, carbon dioxide and methane from farmed organic soils. Soil Biology and Biochemistry, 33, 2083–2091. doi:10.1016/S0038-0717(01)00140-7.
Randall, G. W., & Goss, M. J. (2001). Nitrate losses to surface water through subsurface tile drainage. In R. F. Follett & J. L. Hatfield (Eds.), Nitrogen in the environment: sources, problems, and management (pp. 95–122). Amsterdam: Elsevier Science B.V.
Richards, B., Stoof, C. R., Cary, I. J., & Woodbury, P. B. (2014). Reporting on marginal lands for bioenergy feedstock production: a modest proposal. Bioenergy Research, 7, 1060–1062. doi:10.1007/s12155-014-9408-x.
Ruan, L., & Robertson, P. (2013). Initial nitrous oxide, carbon dioxide, and methane costs of converting conservation reserve program grassland to row crops under no-till vs. conventional tillage. Global Change Biology, 19, 2478–2489. doi:10.1111/gcb.12216.
Rytter, R. M. (2001). Biomass production and allocation, including fine-root turnover, and annual N uptake in lysimeter-grown basket willows. Forest Ecology Management, 140, 177–192. doi:10.1016/S0378-1127(00)00319-4.
Saggar, S., Jha, N., Deslippe, J., Bolan, N. S., Luo, J., Giltrap, D. L., Kim, D. G., Zaman, M., & Tillman, R. W. (2013). Denitrification and N2O:N2 production in temperate grasslands: processes, measurements, modeling and mitigating negative impacts. Science of the Total Environment, 465, 173–195. doi:10.1016/j.scitotenv.2012.11.050.
Schmidt-Walter, P., & Lamersdorf, N. P. (2012). Biomass production with willow and poplar short rotation coppices on sensitive areas—the impact on nitrate leaching and groundwater recharge in a drinking water catchment near Hanover, Germany. Bioenergy Research, 5, 546–562. doi:10.1007/s12155-012-9237-8.
Skaggs, R.W., Van Schilfgaarde, J. V. (ed.). (1999). Agricultural drainage. Agronomy Monograph, 38. ASA: Madison, WI.
Smart, L. B., Volk, T. A., Lin, J., Kopp, R. F., Phillips, I. S., Cameron, K. D., White, E. H., & Abrahamson, L. P. (2005). Genetic improvement of shrub willow (Salix spp.) crops for bioenergy and environmental applications in the United States. Unasylva, 221(56), 51–55.
Soil Survey Staff. (2015). Natural Resources Conservation Services. United States Department of Agriculture. Soil Survey Geographic (SSURGO) Database. http://sdmdataaccess.nrcs.usda.gov/. Accessed 2015.
Turetsky, M. R., Kotowska, A., Bubier, J., Dise, N. B., Crill, P., Hornibrook, E. R. C., Minkkinen, K., Moore, T. R., Myers-Smith, I. H., Nykänen, H., Olefeldt, D., Rinne, J., Saarnio, S., Shurpali, N., Tuittila, E. S., Waddington, J. M., White, J. R., Wickland, K. P., & Wilmking, M. (2014). A synthesis of methane emissions from 71 northern, temperate, and subtropical wetlands. Global Change Biology, 20, 2183–2197. doi:10.1111/gcb.12580.
US DOE (U.S. Department of Energy). (2011). U.S. billion-ton update: biomass supply for a bioenergy and bioproducts industry. Perlack R.D., Stokes, B.J. (Leads). ORNL/TM-2011/224. Oak Ridge National Laboratory. (pp. 1–227). Oak Ridge.
Ussiri, D. A., Lal, R., & Jarecki, M. K. (2009). Nitrous oxide and methane emissions from long-term tillage under a continuous corn cropping system in Ohio. Soil & Tillage Research, 104, 247–255. doi:10.1016/j.still.2009.03.001.
van der Werf, H. M. G., & Turunen, L. (2007). The environmental impacts of the production of hemp and flax textile yarn. Industrial Crops and Products, 27, 1–10. doi:10.1016/j.indcrop.2007.05.003.
Van Hulzen, J. B., Segers, R., van Bodegom, P. M., & Leffelaar, P. A. (1999). Temperature effects of soil methane production: an explanation for observed variability. Soil Biology and Biochemistry, 31, 1919–1929. doi:10.1016/S0038-0717(99)00109-1.
Verhoeven, J. T. A., Arheimer, B., Yin, C. Q., & Hefting, M. M. (2006). Regional and global concerns over wetland and water quality. Trends in Ecology and Evolution, 21, 96–103. doi:10.1016/j.tree.2005.11.015.
Vidon, P., & Cuadra, P. E. (2011). Phosphorus dynamics in tile-drain flow during storms in the US Midwest. Agricultural Water Management, 98, 532–540. doi:10.1016/j.agwat.2010.09.010.
Vidon, P. G. F., & Hill, A. R. (2004). Landscape controls on the hydrology of stream riparian zones. Journal of Hydrology, 292, 210–228. doi:10.1016/j.jhydrol.2004.01.005.
Vidon, P., Allan, C., Burns, D., Duval, T., Gurwick, N., Inamdar, S., Lowrance, R., Okay, J., Scott, D., & Sebestyen, S. (2010). Hot spots and hot moments in riparian zones: potential for improved water quality management. Journal of the American Water Resources Association, 46(2), 278–298. doi:10.1111/j.1752-1688.2010.00420.x.
Vidon, P., Hubbard, H., Caudra, P., & Hennessy, M. (2012). Storm phosphorus concentrations and fluxes in artificially drained landscapes of the US Midwest. Agricultural Sciences, 3(4), 474–485. doi:10.4236/as.2012.34056.
Vidon, P. G., Jacinthe, P.-A., Liu, X., Fisher, K., & Baker, M. (2014). Hydrobiogeochemical controls on riparian nutrient and greenhouse gas dynamics: 10 years post-restoration. Journal of the American Water Resources Association, 50(3), 639–652. doi:10.1111/jawr.12201.
Vidon, P. G., Marchese, S., Welsh, M., & McMillan, S. (2015). Short-term spatial and temporal variability in greenhouse gas fluxes in riparian zones. Environmental Monitoring and Assessment, 187, 503. doi:10.1007/s10661-01504717-x.
Volk, T. A., Abrahamson, L. P., Nowak, C. A., Smart, L. B., Tharakan, P. J., & White, E. H. (2006). The development of short-rotation willow in the Northeastern United States for bioenergy and bioproducts, agroforestry and phytoremediation. Biomass and Bioenergy, 30, 715–727. doi:10.1016/j.biombioe.2006.03.001.
Zhu, T., Zhang, J., Yang, W., & Zucong, C. (2013). Effects of organic material amendment and water content on NO, N2O, and N2 emissions in nitrate-rich vegetable soil. Biology and Fertility of Soils, 49, 153–163. doi:10.1007/s00374-012-0711-4.
Acknowledgements
This work was supported by the New York State Research and Development Authority (NYSERDA); the Empire State Development Division of Science, Technology and Innovation (NYSTAR); and through the Agriculture and Food Research Initiative Competitive Grant No. 2012-68005-19703 from the US Department of Agriculture National Institute of Food and Agriculture (USDA NIFA). The authors would like to thank Marty Mason and Celtic Energy Farm for allowing us to install monitoring equipment in their fields during the study period and Justin Heavey for field extension services and access to willow data. Thanks are due to Obed Varughese for help in the field and laboratory.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bressler, A.S., Vidon, P.G. & Volk, T.A. Impact of Shrub Willow (Salix spp.) as a Potential Bioenergy Feedstock on Water Quality and Greenhouse Gas Emissions. Water Air Soil Pollut 228, 170 (2017). https://doi.org/10.1007/s11270-017-3350-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-017-3350-4