Abstract
Metal cations could enhance the sorption of tetracyclines but sometimes the effects are negligible. It is still not clear how these metals produce different effects. In this study, the sorption of chlortetracycline (CTC), tetracycline (TC), and oxytetracycline (OTC) was performed in the presence of Cd (II) to reveal the unknown mechanisms with two river sediments. It is found that Cd (II) could enhance the sorption of TCs on sediment SS, while it is negligible on sediment SY. For different tetracyclines, the enhancement effect by Cd (II) was more significant for CTC, while it is inferior for OTC and TC. Sorption isotherms of Cd (II) under strong and weak background electrolyte and pH decrease of sorption solutions indicate specific sorption is major on SY and cation exchange is significant on SS. Consequently, specific sorption is unfavorable for the enhanced sorption of TCs in the presence of Cd (II) because it is not favorable for the sorption of Cd-TCs by complexation and cation exchange. By the theoretical calculations, it is found that the significant enhancement of CTC is due to the higher electron affinity of Cd-CTC complex than the others to the surface groups. In conclusion, TCs sorption will not be affected by Cd (II) on sediments or soils with strong specific sorption characters of Cd (II).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Tetracyclines (TCs) are frequently detected in surface waters due to their tremendous production, usage, and emission in China and worldwide (Zhang et al. 2015; Wang et al. 2014). They not only produce eco-toxicity and broom resistant bacteria but also result in food and groundwater contamination (Fernández-Calviño et al. 2015; Huang et al. 2015; Xu et al. 2015). The sorption-desorption of TCs has been well studied on soils and sediments, which is mainly influenced by the physicochemical characters of soils/sediments and contaminants (Sassman and Lee 2005; Zhao et al. 2012), such as pH, ionic strength, cation exchange capacity (CEC), and organic matter content (OMC). Recently, TCs and highly toxic metals (e.g., cadmium and lead) are frequently found together in the river sediments and some contaminated soils (Bu et al. 2013; Chang and Ren 2015; Chen et al. 2015; Meng et al. 2015; Zhu et al. 2013). It has drawn great attention of environment researchers, due to the increasing possibility of interrelated migration and eco-toxicity.
Cd (II) is one of the most dangerous toxic metals and many studies have studied its co-sorption with TCs on soils and sediments (Wan et al. 2010; Zhao et al. 2013). However, Cd (II) has different effects on the sorption of TCs and none of the authors provided reasons for the different effects on the two sediments. For instance, it is found that 10 mg/L Cd (II) could enhance the sorption of TC on a cinnamon soil even in the presence of 112 times excess of Ca (II), which is generally abundant in soils (Wan et al. 2010). Ca (II) could enhance the TCs sorption on montmorillonite (Parolo et al. 2012). Both works attribute the sorption mechanism to the bridging effect between soil particles and TCs. However, Cd (II) could not enhance the sorption of TC on a soil named BS (Zhao et al. 2013). The phenomenon also happens on the co-sorption of TC with Cu (II) (Zhao et al. 2011; Zhang et al. 2011). However, none of the physicochemical properties of these soils and sediments can explain the significant or not significant enhancement effect. Thus, it is quite meaningful to discover the underlying reasons because it would help us to predict the sorption and migration of TCs as affected by Cd (II). In terms of TCs, tetracycline (TC) was generally adopted as the representative of TCs to evaluate the sorption characters of sediments and soils. However, such evaluation was incomplete because oxytetracycline (OTC) and chlortetracycline (CTC) are well proved to behave differently from TC and their abundance is also similar with TC (Li et al. 2015). Thus, it is desirable to test their sorption characters in the presence of Cd (II) on river sediments.
Invoked by the unknown mechanisms and incomplete evaluation of TCs, the objectives of this study were to evaluate the sorption of TCs (TC, OTC, and CTC) in the presence of Cd (II) accurately and identify key factors that influence the enhancement effect of Cd (II) on the sorption of TCs. As a result, we collected two sediments from rivers in Beijing (Yongding River and Shahe River) and batch sorption studies were performed. By potentiometric titrations and quantum chemical calculations, the interactions were studied between Cd (II) and TCs. By the analysis of sediment properties and the evaluation of specific sorption and cation exchange sorption of sediments, the key factors were proposed to explain the different effects of Cd (II) on the sorption of TCs.
2 Material and Methods
2.1 Materials and Reagents
Tetracycline (TC, Det.purity 98.0 %), oxytetracycline (OTC, Det.purity 97.5 %), and chlortetracycline (CTC, Det.purity 92.0 %) were purchased from Dr. Ehrenstorfer, Germany. Citric acid monohydrate (99.8 %) and cadmium dinitrate (Cd (NO3)2 · 4H2O, 99 %) were from Aladdin, China. Other analytical reagents were from Sinopharm Chemical Reagent. Ultra-pure water was made in our lab (Aquapro International, USA).
2.2 Sediment Sampling and Properties
Two sediments were sampled from rivers in Beijing. One is from Shahe River in Changping District and denoted as SS. The other is from Yongding River in Fengtai District and denoted as SY. All samples were air-dried and then gently ground to pass through a 60-mesh sieve, and stored at room temperature for further applications.
The contents of mercury and arsenic were measured with atomic fluorescence spectrometry (AFS-933, Beijing Titan instruments) and the other metals were measured with ICP-Mass (Agilent 7700E). Sediment pH was measured with a pH electrode (Inlab science Pro, Mettler Toledo) according to National Standards of China (NY/T 1121.2-2006). Organic matter content was measured with potassium dichromate oxidation spectrophotometric method (HJ 615–2011). The characteristics of the river sediment samples are listed in Table 1.
2.3 Potentiometric Titration of TCs with Cd (II)
The interactions of Cd (II) with TCs (TC, OTC, and CTC) were studied by potentiometric titrations that were carried out using a potentiometric titrator (SE700, Mettler-Toledo, Switzerland). The binary system solution was titrated to pH 3 using 1 % HNO3. The 0.2 mM TCs were then slowly titrated to pH 9 using NaOH. Three concentrations of NaOH (0.100, 0.0200, and 0.00500 M) were used to make sure the titration points distribute uniformly in the studied pH range.
The experiment was carried out at 20 ± 1 °C. The equilibrium was defined as a potential drift <0.03 mV per 8 s. During the titrations, the titrated solution was put in a polyethylene beaker and the pH was measured in real time with a Mettler-Toledo Inlab® Science Pro composite electrode. All the titrations were carried out with 0.01 M NaNO3 as background electrolyte and the precipitation of metal ions was not observed in the examined pH range.
2.4 Cd (II) Sorption on Two Sediments
25.0 mL Cd (II) solution containing 0.01 M NaNO3 or 1 M NaNO3 was added to each triangular flask containing 0.500 g sediment, with Cd (II) concentrations about 3.0, 5.0, 10.0, 15.0, and 20.0 mg/L. A control for each concentration was also made to calculate the amount of Cd (II) adsorbed. The control contained Cd (II) in 25 mL of NaNO3 solution without sediments. Thus, the samples were shaken for 24 h at 25 °C, centrifuged at 3500 rpm for 5 min, and then filtered through a 0.45-μm syringe filter (PES, 13 mm, Bojin). The pH of supernatants were measured and then acidified with 50 μL HNO3 (65 wt.%) for analysis. The content of Cd (II) in the supernatant was determined by the PerkinElmer PinAAcle 900T atomic absorbance spectrometer. All experiments were carried out two times and the mean value was presented.
2.5 TCs Sorption in the Presence and Absence of Cd (II)
In each polystyrene triangular flask, 0.500 g sediment and 25.0 mL antibiotic solution were mixed and kept at 25 °C in a thermostat shaker (DLHR-Q200) with a speed of 150 rpm. 0.01 M NaNO3 was used as background electrolyte in all experiments. The solution contains 3, 5, 10, 15, and 20 mg/L antibiotic and 0 or 10 mg/L Cd (II) for TCs sorption in the absence and presence of Cd (II), respectively. In the absence of Cd (II), the sorption solution pH is about 8.1 and 8.0 for SS and SY; in the presence of Cd (II), the sorption solution pH is 7.9 and 7.5. A preliminary sorption experiment indicates that 24 h was enough to attain sorption equilibrium. Thus, the samples were continuously shaken for 24 h (Teixidó et al. 2012; Pils and Laird 2007). Control solutions were treated the same way as above to eliminate the adsorption of containers. After sorption, the adsorption solutions were centrifuged at a speed of 3500 r/min for 5 min. The supernatants were collected and filtered through 0.22 μm filter and stabilized with 3 M HNO3 (50 μL) for HPLC analysis.
2.6 Determination of TCs
TCs in the supernatants were analyzed by high performance liquid chromatography (Agilent 1260). The column was Zorbax Eclipse-C18 reverse phase column (4.6 mm × 250 mm, 5 μm). Mobile phase A consisted of 0.01 M citric acid and 50 μM EDTA, B was acetonitrile, and C was methanol. Isocratic elution (72 % A, 20 % B and 8 % C) was performed with a flow rate of 0.8 mL/min. TCs were monitored at 365 nm with a variable wavelength detector (UV). The injection volume was 10 μL. In order to test free TCs accurately in supernatants accurately, the LOD were measured and all experiment points were designed to keep real TCs concentrations above the quantitative LOD (Table S1).
2.7 Theoretical Calculations
The theoretical calculations were performed in Beijing Institute of Technology. For convenience, Cd-TC, Cd-OTC, and Cd-CTC were defined as the complexes of Cd (II) with \( {\mathrm{TCs}}^{2-} \). B3LYP method was used for configuration optimization and frequency analysis of complexes. LanL2DZ and 3-21 g were applied for Cd (II) and other non-metal elements, respectively (Zhao et al. 2013; Scalmani and Frisch 2010). Solvent effect has been considered in all optimizations by using a PCM model with water as solvent. Connolly molecular area (CAA) and Connolly solvent excluded volume (CSV) were calculated by Chem3D after structure optimization with water as probe. Primary configurations of TC, OTC, and CTC were adopted from European Pharmacopoeia (Fifth Edition). However, the conformation was unclear for the amide group Fig. S1 (A) and (B). After comparison, structure (A) is found to have lower energy and further calculations were based on structure (A).
3 Results and Discussion
3.1 Sorption Isotherms of TCs in the Presence and Absence of Cd (II)
Centrifuge tubes were generally used to evaluate TCs sorption on soils and sediments in order to minimize handling and transfer errors (Wan et al. 2010; Sassman and Lee 2005; Jia et al. 2008; Zhang et al. 2011). However, it is found that sediments accumulated at the bottom immediately even agitated strongly in our thermostat shaker (Fig. S2). As underestimation of TCs’ sorption may occur, TCs sorption was measured with both centrifuge tubes and triangular flasks as containers. It is found that the amount of TC adsorbed is dramatically higher in triangular flasks than that in centrifuge tubes (Fig. S3). Meanwhile, the error bars of TC adsorbed with triangular flasks as container are smaller than those with centrifuge tubes. In the triangular flasks, both sediments are homogeneously dispersed in the sorption solution. This indicates that the incomplete sorption of TC was due to the inhomogeneous agitation of the sorption solution and deposition of sediments. One possibility to overcome this problem is to use triangular flasks as containers instead of centrifuge tubes and in the following experiment triangular flasks were used for convenience.
Sediment properties were also obtained to avoid the background interference to the sorption of TCs and Cd (II) (Table 1). All of the sediment properties, including pH, CEC, OM, metal, and metal oxide contents, would affect the sorption of TCs and Cd (II). Large contents of metals would interfere with the sorption of TCs and Cd (II). Therefore, typical heavy metals were also measured (Table 1). All of the metals (Pb, Cd, Cu, and Hg) and their total amount (∼0.1 g/kg) are much less than Cd (II) (20 g/kg) added in the experiment. Therefore, they will not interfere with the evaluation of sorption of Cd (II) and TCs. The background level of TCs was also determined in the two sediments. In the test, 25 mL 0.01 M NaNO3 was added to each triangular flask containing 0.500 g sediment and shaken for 24 h. The supernatants were measured and no TCs could be detected in either SY or SS under current method. After all of these studies, adsorption isotherms were obtained for TC, CTC, and OTC on sediments (SS and SY) with 10 mg/L and without Cd (II) (Fig. 1).
In the absence of Cd (II), the quantity of TCs adsorbed on the two sediments follows the order of CTC > TC > OTC, which is consistent with a very early work (Sassman and Lee 2005). In the presence of Cd (II), the quantity of TCs adsorbed also follows the same order. Meanwhile, enhanced sorption of TCs were found on sediment SS, while the enhancement effect is negligible on sediment SY. The sorption isotherms of TCs were fitted with Freundlich model and the fitting parameters were listed in Table S2. Clearly, the K f on SS and the K f on SY are almost the same with each other for the same antibiotic in the absence of Cd (II), while the introduction of Cd (II) makes a difference between the two values. Generally, the higher CEC of SS indicates higher sorption of TCs than SY. This is probably due to the factors other than CEC. In order to quantify the enhancement effect of Cd (II) on the sorption of TCs, the enhancement factor (EF) was defined as the ratio of the Freundlich adsorption coefficient (K f ) in the presence of Cd (II) to that in the absence of Cd (II). Clearly, for the EF values on SS, CTC > TC > OTC. The sorption difference must be determined by molecular properties of TCs on the same sorbent.
3.2 The Origin of Sorption Differences of TCs
The sorption differences of TC, OTC, and CTC are observed by several groups, though they have similar structure, pKa, and solubility. It is reported that the steric hindrance between hydroxyl group at C5 and protonated dimethyl amino group makes the sorption of OTC smaller than TC (Avisar et al. 2010). However, hydroxyl group does not exist in CTC and TC at C5, but the sorption of CTC is greater than TC. Steric hindrance, therefore, cannot be used to explain the higher sorption of CTC than TC. In one of our previous studies, we calculated molecular polarities of CTC, OTC, and TC and found that steric hindrance and molecular polarity together determine the sorption of TCs on sediments (Li et al. 2015).
Potentiometric titration was performed for TC, OTC, and CTC with Cd (II) to study the interaction between Cd (II) and TCs. The titration curves of TCs with and without Cd (II) are shown in Fig. 2 and the molar ratio of TCs with Cd (II) is one. In the absence of Cd (II), no turbidity or precipitation was observed among the pH range. However, in the presence of Cd (II), once the pH is approaching 8.0, significant turbidity appeared for the CTC solution. It is reported CTC is particularly prone to undergo isomerization by the bond cleavage at C11-C11a under alkali conditions (Waller et al. 1952). The turbidity is probably due to the precipitation of Cd (II) with isochlorotetracycline. Because the complexation process of Cd (II) with TCs is accompanied by the replacement of H+, leading to a decrease of the solution pH, the titration curves showed that Cd-TCs binary systems needed more NaOH than TCs alone to obtain the same solution pH value. The order of NaOH amount to obtain the same pH is CTC > OTC > TC and the NaOH amount is almost the same for Cd-TC, Cd-OTC, and Cd-CTC (Fig. 2). All results indicate that Cd (II) could complex with all TCs in our experiment.
The complexation was also modeled with quantum chemical calculations to discover the sorption differences of TCs. Before the calculations, the major species and preferred conformations and configurations were determined for TCs. It is reported that the major ratios are usually 1:1 and 1:2 for Cd (II) to TC (Jezowska-Bojczuk et al. 1993; Ghandour et al. 1992). However, the latter mode would weaken the sorption of TCs because cation exchange sites were buried in the complexes. Therefore, only the 1:1 binding mode is considered for the sorption of Cd (II) and TCs.
For the dissociation state, the titration results indicated that only TCs2 ‐ could complex with Cd (II) when the pH is over 7.0 (Ghandour et al. 1992; Zhao et al. 2013). The solution pH is larger than 7.5 in our experiment. Consequently, the following modeling is focus on complexes between TCs2 ‐ and Cd (II). For the conformation of TCs, it is well proved that TC could either adopt an extended conformation (conformation A), where the dimethylamino group lies below the plane spanned by the BCD ring system, or a twisted conformation (conformation B), where the dimethylamino group lies above the BCD ring system in order to relieve the steric crowding between N4 and O12a Gulbis and Everett (1976). Therefore, TCs mainly adopt conformation A in our experiment.
In terms of binding sites, the exact binding site has not been reported for TC, OTC, and CTC with Cd (II) in the sorption state. It is well accepted that O11-O12, O2-O3, and N4-O12a could be donor sites for TCs in conformation A (Ghandour et al. 1992; Jezowska-Bojczuk et al. 1993). N4-O3 was also reported to be the major binding site for Mg (II)-TC complex in conformation B at basic solutions (Wessels et al. 1998). (1), (2), (3), and (4) are used to stand for the four binding sites above, respectively. The ΔG was calculated to identify the preferred binding site and conformation (Eqs. 1 and 2).
Where, i is from (1) to (4), G j is the Gibbs free energy of TC, OTC or CTC, respectively and \( {\mathrm{G}}_{\mathrm{C}{\mathrm{d}}^{2+}} \) is the Gibbs free energy of Cd (II).
The dipole moment (DM), Connolly molecular area (CMA) and Connolly solvent excluded volume (CSV), and E LUMO were also calculated. Because it is very hard to calculate the Gibbs free energy of surface reactions, − E LUMO was obtained from the optimized structures, which could reflect the electron affinity of complexes to the surface groups on sediments. The Gibbs free energy and complex properties are summarized in Table 2.
Comparing ΔG1 of different complex, the complex has the lowest Gibbs free energy change when Cd (II) binds TCs at O2-O3 site. Therefore, it can be predicted that the bridging of Cd (II) happens at O2-O3 for the sorption TCs on sediments. This is different from several works, in which O11-O12 is proved to be the binding site (Jezowska-Bojczuk et al. 1993; Wessels et al. 1998). It is probably because most of these complexes are in the form of 1:2 (metal:ligand) and the conjugated BCD ring could stabilize the complexes by binding at O11-O12 in solution. Except for Cd-OTC, all Cd-TCs complexes have the negative Gibbs free energy change, indicating the strong complexation between Cd (II) and TCs. This is coincident with results from potentiometric titrations.
After the prediction of binding site, the other properties were compared. ΔG2 of Cd-CTC is comparable with Cd-TC but lower than Cd-OTC; CMA, CSV and − E LUMO of Cd-CTC is larger than Cd-OTC and Cd-TC. The larger electron affinity of Cd-CTC could be used to explain the significant enhanced sorption of CTC on SS. However, further studies are needed to know whether the larger CMA and CSV of Cd-CTC contribute to the enhancement effect.
3.3 The Mechanism of TCs Sorption in the Presence of Cd (II)
In the presence of Cd (II), the sorption is significantly enhanced for TCs on SS, which is also observed on a cinnamon soil (Wan et al. 2010). For sediment SY, the enhancement is not significant, which is similar with a black soil (Zhao et al. 2013). Obviously, the enhancement effect could be either significant or negligible, depending on different sediments and soils. However, any physicochemical properties cannot be used to explain the significant or not significant enhancement effect confidently.
In theory, the more free Cd (II) in solution or Cd (II) adsorbed would be favorable for the sorption of TCs because it is favorable for the bridging effect of Cd (II) between sediments and TCs (Jia et al. 2008; Zhang et al. 2011). If the free Cd (II) is more in the sorption solution of SY, then it could explain why the enhancement effect was negligible. Consequently, free Cd (II) and Cd (II) adsorbed were monitored in sorption process with different initial TCs in the presence of 10 mg/L Cd (II) (Table 3). Unexpectedly, the Cd (II) adsorbed on SS is more than those on SY. Therefore, the sorption mechanisms of Cd (II) and sediment properties were further investigated.
For Cd (II), the sorption mechanisms include cation exchange (Fig. 3e) and specific sorption (or chemisorption, Fig. 3d) (Schindler et al. 1976). Cation exchange happens in the diffusion layer, while specific adsorption happens in the Stern layer of sediment-water interface. In theory, only cation exchange sorption of Cd (II) is favorable for the complexation (Fig. 3b) and sorption of Cd-TCs complexes (Fig. 3c). The difference of sorption mechanisms may explain the different effects of Cd (II) on the sorption of TCs on sediment SS and SY.
The physicochemical properties of SS and SY were compared to evaluate the above hypothesis. As is shown in Table 1, cation exchange capacity (CEC) is higher in SS than in SY, while organic matter content (OMC) is opposite. Clearly, the CEC data supports the above hypothesis. OMC cannot be correlated with cation exchange or specific sorption directly. Because organic matter contains negative carboxylic groups, phenolic group, hydroxyl groups, etc. Some of them are favor for cation exchange sorption and some of them are favorable for specific sorption. For the content of metal oxide, it is well proved that specific adsorption is positively correlated with oxides of ferrum, manganese (Phillips 1999), and aluminum when pH >7.0 on sediments and soils (Floroiu et al. 2001). While the content of MnO2, Fe2O3, and Al2O3 of SY is higher than that of SS (Table 1). In general, more physicochemical properties support that specific sorption of Cd (II) is predominant on SY and minor in SS but direct evidence must be provided. In the paragraphs ahead, the specific sorption and cation exchange properties of Cd (II) were evaluated on both SS and SY.
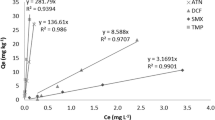
It is reported that the specific sorption of Cd (II) could be negligible when the background electrolyte is more than 1 M (Zou et al. 2012). Accordingly, the sorption isotherms of Cd (II) were measured on SS and SY with both 0.01 M and 1 M NaNO3 as background electrolyte (Fig. 4). The solution pH was also measured (insets of Fig. 4).
Obviously, the sorption of Cd (II) on SY is stronger than on SS in the presence of 0.01 M NaNO3. Once we use 1 M NaNO3 to mask cation exchange effect, the sorption of Cd (II) on SS significantly decreased while the sorption change is nearly negligible on SY. As for the pH, deceases were observed in all cases (insets of Fig. 4), which proves the release of protons due to the reactions Cd (II) with the hydroxyl groups on sediments. Generally, the pH drop would increase the TCs sorption (Li et al. 2011; Yanping Zhao et al. 2011; Parolo et al. 2012). However, the enhancement effect was not significant for SY. At this moment, it is concluded that the cation exchange, relating with OM content and CEC, is favorable for the enhanced sorption of TCs in the presence of Cd (II) on sediments; while, specific adsorption, relating with MnO2, Fe2O3, and Al2O3 content, is unfavorable for the bridging effect of Cd (II) and thus no significant enhancement on the sorption of TCs. This conclusion can also be used to explain the different effects of Cu (II) on two soils (Jia et al. 2008).
4 Conclusions
Many studies have focused on the evaluation of Cd-TC combined contamination with sorption methods. However, TC cannot be the representative of TCs as the quite different physical and chemical properties. It is necessary to evaluate the combined sorption of Cd and other TCs, such as CTC and OTC. Meanwhile, it is still not clear that how cadmium and complex properties of Cd-TCs will affect the sorption of TCs on sediments.
With two river sediments, batch sorption studies were carried out to discuss the effect of Cd (II) on the sorption of TCs. By comparing the sorption isotherms, it is found that centrifuge tubes may underestimate the sorption amount and the experiments should be designed and performed carefully. By potentiometric titrations and calculations, Cd (II) is proved to form complexes with all TCs and it is predicted that the bridging effect of Cd (II) mainly via O2-O3 groups for the sorption of TCs on sediments. Among the TCs investigated, Cd (II) could significantly enhance the sorption of CTC on sediment SS, which is due to the higher electron affinity of Cd-CTC to the sediment surface than the other complexes. Meanwhile, it is found that Cd (II) could significantly enhance the sorption of TCs on sediment SS while it is negligible on sediment SY. By the analysis of sediment properties and evaluating the specific and cation exchange sorption of Cd (II) on both sediments, it is found that sediment SY are rich in MnO2, Fe2O3, and Al2O3, which would make SY adsorb Cd (II) via the mechanism of specific adsorption and thus diminished the bridging effect of Cd (II). In contrast, the higher CEC make the cation exchange sorption of Cd (II) more significant on SS, which is favorable for the enhanced sorption of TCs. In view of pollution prevention and control, it is necessary to pay close attention to the combined contamination of Cd (II) and TCs and especially those sediments or soils with strong specific sorption characters.
References
Avisar, D., Primor, O., Gozlan, I., & Mamane, H. (2010). Sorption of sulfonamides and tetracyclines to montmorillonite clay. Water, Air, & Soil Pollution, 209(1–4), 439–450.
Bu, Q., Wang, B., Huang, J., Deng, S., & Yu, G. (2013). Pharmaceuticals and personal care products in the aquatic environment in China: a review. Journal of Hazardous Materials, 262, 189–211. doi:10.1016/j.jhazmat.2013.08.040.
Chang, B.-V., & Ren, Y.-L. (2015). Biodegradation of three tetracyclines in river sediment. Ecological Engineering, 75, 272–277. doi:10.1016/j.ecoleng.2014.11.039.
Chen, Z., Sun, L., Luo, A., He, Y., Zhang, Y., & Li, Y. (2015). Photoluminescent materials for highly toxic metals sensing: from downconversion to upconversion. Trends in Environmental Analytical Chemistry, 6–7, 1–9. doi:10.1016/j.teac.2015.04.001.
Fernández-Calviño, D., Bermúdez-Couso, A., Arias-Estévez, M., Nóvoa-Muñoz, J. C., Fernández-Sanjurjo, M. J., Álvarez-Rodríguez, E., et al. (2015). Competitive adsorption/desorption of tetracycline, oxytetracycline and chlortetracycline on two acid soils: stirred flow chamber experiments. Chemosphere, 134, 361–366. doi:10.1016/j.chemosphere.2015.04.098.
Floroiu, R. M., Davis, A. P., & Torrents, A. (2001). Cadmium adsorption on aluminum oxide in the presence of polyacrylic acid. Environmental Science & Technology, 35(2), 348–353.
Ghandour, M. A., Azab, H. A., Hassan, A., & Ali, A. M. (1992). Potentiometric studies on the complexes of tetracycline (TC) and oxytetracyclin (OTC) with some metal ions. Chemical Monthly, 123(1–2), 51–58. doi:10.1007/BF01045296.
Gulbis, J., & Everett, G. W., Jr. (1976). Metal binding characteristics of tetracycline derivatives in DMSO solution. Tetrahedron, 32(8), 913–917. doi:10.1016/0040-4020(76)85048-X.
Huang, B., Li, Z., Huang, J., Chen, G., Nie, X., Ma, W., et al. (2015). Aging effect on the leaching behavior of heavy metals (Cu, Zn, and Cd) in red paddy soil. Environmental Science and Pollution Research, 22(15), 11467–11477. doi:10.1007/s11356-015-4386-x.
Jezowska-Bojczuk, M., Lambs, L., Kozlowski, H., & Berthon, G. (1993). Metal ion-tetracycline interactions in biological fluids. 10. Structural investigations on copper (II) complexes of tetracycline, oxytetracycline, chlortetracycline, 4-(dedimethylamino) tetracycline, and 6-desoxy-6-demethyltetracycline and discussion of their binding modes. Inorganic Chemistry, 32(4), 428–437. doi:10.1021/ic00056a015.
Jia, D.-A., Zhou, D.-M., Wang, Y.-J., Zhu, H.-W., & Chen, J.-L. (2008). Adsorption and cosorption of Cu (II) and tetracycline on two soils with different characteristics. Geoderma, 146(1–2), 224–230. doi:10.1016/j.geoderma.2008.05.023.
Li, T., Di, Z., Yang, X., & Sparks, D. L. (2011). Effects of dissolved organic matter from the rhizosphere of the hyperaccumulator Sedum alfredii on sorption of zinc and cadmium by different soils. Journal of Hazardous Materials, 192(3), 1616–1622.
Li, Y., Pan, T., Miao, D., Chen, Z., & Tao, Y. (2015). Sorption–desorption of typical tetracyclines on different soils: environment hazards analysis with partition coefficients and hysteresis index. Environmental Engineering Science, 32(10), 865–871. doi:10.1089/ees.2014.0325.
Meng, B., Liu, J., Li, Y., & Shi, X. (2015). Speciation and ecological risk assessment of heavy metals in surfacial sediments of Lianshui River in Beijing. Journal of Agro-Environment Science, 34(5), 964–972 (in Chinese).
Parolo, M. E., Avena, M. J., Pettinari, G. R., & Baschini, M. T. (2012). Influence of Ca2+ on tetracycline adsorption on montmorillonite. Journal of Colloid and Interface Science, 368(1), 420–426. doi:10.1016/j.jcis.2011.10.079.
Phillips, I. R. (1999). Copper, lead, cadmium, and zinc sorption by waterlogged and air-dry soil. Journal of Soil Contamination, 8(3), 343–364. doi:10.1080/10588339991339379.
Pils, J. R. V., & Laird, D. A. (2007). Sorption of tetracycline and chlortetracycline on K- and Ca-saturated soil clays, humic substances, and clay-humic complexes. Environmental Science & Technology, 41(6), 1928–1933.
Sassman, S. A., & Lee, L. S. (2005). Sorption of three tetracyclines by several soils: assessing the role of pH and cation exchange. Environmental Science & Technology, 39(19), 7452–7459. doi:10.1021/es0480217.
Scalmani, G., & Frisch, M. J. (2010). Continuous surface charge polarizable continuum models of solvation. I. General formalism. The Journal of Chemical Physics, 132(11), 114110. doi:10.1063/1.3359469.
Schindler, P. W., Fürst, B., Dick, R., & Wolf, P. U. (1976). Ligand properties of surface silanol groups. I. surface complex formation with Fe3+, Cu2+, Cd2+, and Pb2+. Journal of Colloid and Interface Science, 55, 469–475.
Teixidó, M., Granados, M., Prat, M. D., & Beltrán, J. L. (2012). Sorption of tetracyclines onto natural soils: data analysis and prediction. Environmental Science and Pollution Research, 19(8), 3087–3095.
Waller, C. W., Hutchings, B. L., Broschard, R. W., Goldman, A. A., Stein, W. J., Wolf, C. F., et al. (1952). Degradation of aureomycin. VII.1 aureomycin and anhydroaureomycin. Journal of the American Chemical Society, 74(19), 4981–4982. doi:10.1021/ja01139a539.
Wan, Y., Bao, Y., & Zhou, Q. (2010). Simultaneous adsorption and desorption of cadmium and tetracycline on cinnamon soil. Chemosphere, 80(7), 807–812. doi:10.1016/j.chemosphere.2010.04.066.
Wang, D., Sui, Q., Zhao, W., Lv, S., Qiu, Z., & Yu, G. (2014). Pharmaceutical and personal care products in the surface water of China: a review. Chinese Science Bulletin, 59, 743–751 (in Chinese).
Wessels, J. M., Ford, W. E., Szymczak, W., & Schneider, S. (1998). The complexation of tetracycline and anhydrotetracycline with Mg2+ and Ca2+: a spectroscopic study. Journal of Physical Chemistry B, 102(46), 9323–9331. doi:10.1021/jp9824050.
Xu, G., Liu, J., Pei, S., Gao, M., Hu, G., & Kong, X. (2015). Sediment properties and trace metal pollution assessment in surface sediments of the Laizhou Bay, China. Environmental Science and Pollution Research, 22(15), 11634–11647. doi:10.1007/s11356-015-4393-y.
Zhang, Z., Sun, K., Gao, B., Zhang, G., Liu, X., & Zhao, Y. (2011). Adsorption of tetracycline on soil and sediment: effects of pH and the presence of Cu (II). Journal of Hazardous Materials, 190(1–3), 856–862. doi:10.1016/j.jhazmat.2011.04.017.
Zhang, Q.-Q., Ying, G.-G., Pan, C.-G., Liu, Y.-S., & Zhao, J.-L. (2015). Comprehensive evaluation of antibiotics emission and fate in the river basins of China: source analysis, multimedia modeling, and linkage to bacterial resistance. Environmental Science & Technology, 49(11), 6772–6782. doi:10.1021/acs.est.5b00729.
Zhao, Y., Jinju, G., Xiaorong, W., Xueyuan, G., & Shixiang, G. (2011). Tetracycline adsorption on kaolinite: pH, metal cations and humic acid effects. Ecotoxicology, 20(5), 1141–1147. doi:10.1007/s10646-011-0665-6.
Zhao, Y., Gu, X., Gao, S., Geng, J., & Wang, X. (2012). Adsorption of tetracycline (TC) onto montmorillonite: cations and humic acid effects. Geoderma, 183(3), 12–18.
Zhao, Y., Tan, Y., Guo, Y., Gu, X., Wang, X., & Zhang, Y. (2013). Interactions of tetracycline with Cd (II), Cu (II) and Pb (II) and their cosorption behavior in soils. Environmental Pollution, 180(3), 206–213.
Zhu, G., Guo, Q., Chen, T., Marc, P., Yang, J., Zhang, H., et al. (2013). Pollution characteristics and risk assessment of heavy metals in the sediments of Nansha River in Beijing. Chinese Journal of Ecology, 32(8), 2148–2153 (in Chinese).
Zou, X., Zhang, C., Ning, J., Wei, L., & Yang, S. (2012). Behaviors of copper ions different in concentration in sorption-desorption by soils—and existence of weak-specific—adsorption state. Acta Pedologica Sinica, 49(5), 892–900.
Acknowledgments
This research was supported by Natural Science Foundation of Beijing Municipality (Grant No. 8142020).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
Table S1 Calibration curves for TCs. Table S2 Freundlich parameters for the sorption isotherms of TCs as affected by Cd (II). Fig. S1 possible configurations of TCs. Fig. S2 sediment dispersions in triangular flasks and containers. Fig. S3 TC sorption isotherms in triangular flasks and centrifuge tubes. Fig. S4 FTIR spectra of sediments and sediments after sorption of TC and Cd (II). (DOC 2133 kb)
Rights and permissions
About this article
Cite this article
Chen, Z., Li, G., Sun, L. et al. Tetracyclines Sorption in the Presence of Cadmium on River Sediments: the Effects of Sorption Mechanism and Complex Properties. Water Air Soil Pollut 227, 283 (2016). https://doi.org/10.1007/s11270-016-2982-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-016-2982-0