Abstract
Sulfide, found in some wastewaters and industrial off-gases, is a toxic and highly corrosive pollutant, especially in wastewater applications. Sulfide removal was studied in a new sulfide-oxidizing reactor (External Silicone Membrane Reactor—ESMR) that employs a tubular silicone rubber membrane for micro-aeration. The chemical and biological sulfide oxidation at pH 8.0, 9.0, and 10.0 were investigated. The applied velocity (V s) in the membrane was also investigated as a system control parameter. The local overall mass transfer coefficient (R) was estimated for the tubular silicone rubber membrane and had an average value of 0.153 m.h−1. Oxygen mass transfer was found to not be influenced by the applied velocity. The sulfide oxidation to sulfate could be partially avoided and the biotic tests showed larger sulfur aggregates deposited in the silicone membrane, which could easily be washed away upon flushing. By contrast, colloidal sulfur formation observed in the chemical oxidation assays was harder to separate from the liquid phase. This study reveals that the ESMR is a suitable reactor design to promote partial sulfide oxidation because it provides an adequate oxygen supply with minimized aeration costs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Hydrogen sulfide is a pollutant present in some wastewaters, anaerobic bioreactor discharges, and industrial off-gases. Because of its characteristics, such as strong odor, corrosive effects, and health hazards, the conversion of hydrogen sulfide in wastewater treatment plants is necessary. Several non-biological alternatives have been developed for sulfide removal from the liquid phase, including chemical oxidation processes, which involve the use of air, ozone, hydrogen peroxide, stripping, and metal precipitation (Lens et al. 1998). The use of these oxidizing agents results in chemical waste generation, high costs, and unwanted sulfate production. An alternative treatment process involves the formation of elemental sulfur, an insoluble intermediate that can be separated from the liquid phase and reused (Janssen et al. 1999; Lohwacharin and Annachhatre 2010; Krishnakumar et al. 2005; Pokasoowan et al. 2009; Xu et al. 2012). Elemental sulfur is obtained by the partial biological or chemical oxidation of sulfide.
Chemical oxidation involves a series of complex reactions and the formation of intermediates such as polysulfide, sulfur, thiosulfate, sulfite, and sulfate. The reactions can be catalyzed by metal ions, and the reaction products depend on conditions such as pH and sulfide/oxygen ratio (Kleinjan et al. 2005). By contrast, biological sulfide oxidation can proceed under aerobic, anoxic, and even anaerobic conditions (Jensen and Webb 1995) and can be performed by microorganisms such as Thiobacillus (Alcantara et al. 2004; Buisman et al. 1990; Buisman et al. 1991; Janssen et al. 1999). The main products of biological conversion are elemental sulfur and sulfate, as shown in Eqs. 1 and 2 (Kuenen 1975).
Partial sulfide oxidation to S0 only occurs under oxygen-limiting conditions or under high-sulfide loading rates. Janssen et al. (1995) have demonstrated that at oxygen concentrations below 0.1 mg.L−1 or at an oxygen/sulfide molar ratio between 0.6 and 1.0, sulfur is the major end-product of sulfide oxidation. Micro-aerobic conditions were studied by Jenicek et al. (2010) in an anaerobic digester that reduced H2S levels in the biogas. Other investigators studied the dissolved oxygen (DO) levels, where a DO level of 0.8 mg.L−1 demonstrated denitrifying sulfide (Chen et al. 2010) and levels between 0.2 and 1.0 mg.L−1 promoted conversion of 90 % of the sulfide to elemental sulfur (Lohwacharin and Annachhatre 2010). Yu et al. (2014) also studied the impacts of micro-aerobic conditions on the microbial functional structure of sulfate-reducing sulfur-producing bioreactors and revealed that sulfur-oxidizing bacteria (SOB) can be greatly stimulated by DO levels of 0.09 mg.L−1; however, under relatively high DO levels (0.33 mg.L−1), sulfate-reducing bacteria (SRB) and SOB were strongly inhibited, whereas heterotrophic microorganisms (especially fermentative microorganisms) were enriched, leading to granular disintegration.
Because the air supply and the reactor configuration are important criteria for the optimization of sulfur formation, several sulfide-oxidizing reactor configurations have been studied, including stirred tank reactors (Buisman et al. 1989; Janssen et al. 1995), expanded granular sludge bed reactors (Janssen et al. 1997; Xu et al. 2012), gas-fed batch reactors (Jensen and Webb 1995), air lift reactors (Lohwacharin and Annachhatre 2010; Moghanloo et al. 2010; Pokasoowan et al. 2009), reverse fluidized loop reactors (Krishnakumar et al. 2005), and floated bed micro-aerobic reactors (Chuang et al. 2005). Directly limiting the oxygen supply to anaerobic sludge digesters has also been studied (Díaz et al. 2011). In most reactor configurations, the oxygen supply is controlled by direct air injection or by spatially separated liquid aeration. However, direct injection generally results in turbulence, which disrupts the aggregates. Thus, strict injection control is required.
By contrast, synthetic membranes have long been used to promote bubbleless aeration in wastewater treatment systems (Suzuki et al. 1994; Wilderer et al. 1985) and have also been studied for the removal of xylene (Debus et al. 1992; Debus et al. 1994), chlorophenols (Wobus et al. 1995), acetate (Casey et al. 1999), organic carbon, and inorganic nitrogen (Yamagiwa et al. 1998). In membrane aeration systems, oxygen transfer is controlled by the partial pressure of oxygen, the flow characteristics of the gas side, membrane thickness, and biofilm formation on the membrane (Brindle and Stephenson 1996).
Conversely, there is a lack of information about the use of silicone membranes for sulfide removal, which constitutes an opportunity to attain micro-aeration conditions for sulfide oxidation to elemental sulfur. For these reasons, we have chosen to explore a tubular silicone rubber membrane to control the air supply. This paper explores the micro-aeration properties of the External Silicone Membrane Reactor (ESMR) and its application for chemical and biological partial sulfide oxidation to elemental sulfur in lab-made wastewater and also in anaerobic reactor effluent. The influence of pH and applied velocity in the membrane was investigated. In addition, the local overall gas–liquid mass transfer coefficient was determined.
2 Materials and Methods
2.1 Experimental Apparatus
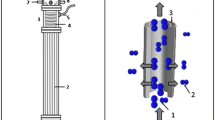
The ESMR consisted of a stirred tank and a tubular silicone rubber membrane to promote micro-aeration and partial sulfide oxidation (Fig. 1a). The reactor was operated in a discontinuous mode (batch reactor) in which lab-made wastewater containing dissolved sulfide was circulated through the silicone tube by a positive displacement pump. The commercial silicone tube (Perfimed, São Paulo—Brazil) had an internal diameter of 8.0 mm, wall thickness of 2.4 mm and length of 200 mm, corresponding to a superficial area of 0.804 dm2. The tank, with a working volume of 1.8 L, was completely filled with liquid to minimize the volatilization of the sulfur compounds. The reactive medium was kept agitated at 90 rpm by a magnetic stirrer. The system control variable was the applied velocity, V s, in the membrane, which was calculated as the ratio of the recirculation flow rate (Q) to the membrane cross-sectional area (Asec) (Eq. 3). The temperature was maintained at 25 ± 1 °C.
a Schematic diagram of the ESMR. The reactive medium (1.8 L) inside the tank (1) was recirculated through the silicon membrane (8.0 × 12.8 × 200 mm) (2) by a positive displacement pump (3). The medium was magnetically stirred at 90 rpm (4). b The resistive structures in silicon membrane based on the resistance in series model are also showed: (a) gas phase boundary layer, (b) silicon membrane wall, (c) liquid phase boundary layer. c Schematic of oxygen concentration profiles through membrane silicon wall
2.2 Experimental Protocol
Sulfide oxidation in the ESMR was studied in batch experiments for three different pH values of 8.0, 9.0 and 10.0, and V s values of 23.7 95.5 and 167.1 m.h−1. Abiotic batch tests (denoted by A) were conducted with lab-made wastewater to assess chemical oxidation, and biotic batch runs were performed with lab-made wastewater (denoted by B1) as well as with effluent from an anaerobic reactor (denoted by B2) treating sulfate-rich wastewater simulating domestic sewage as described by Camiloti et al. (2013).
The lab-made wastewater used in the batch assays was composed of a solution of sodium sulfide (50 mg.L−1), a phosphate buffer system (Na2HPO4 and NaH2PO4) for pH values of 8.0 and 9.0, a borate-boric acid buffer system for pH values of 10.0 and a nutritional solution for autotrophic conditions (Robertson and Kuenen 2006). The lab-made wastewater was flushed with N2 to remove dissolved oxygen.
In the biological tests, activated sludge from a recycled paper mill wastewater treatment plant (São Carlos S/A—Indústria de Papel e Embalagens) was used. To enrich the inoculums in sulfide-oxidizing bacteria and to adapt the sludge for each pH condition (8.0, 9.0, and 10.0), the activated sludge was acclimated by gradually feeding in the corresponding lab-made wastewater. After 4 weeks of cultivation, 180 mL of sludge was added to the ESMR bioreactor, corresponding to a volatile suspended solids (VSS) content of approximately 1500 mg VSS.L−1.
2.3 Analytical Methods
Sulfide was detected colorimetrically in accordance with American Public Health Association—APHA (2005). Elemental sulfur was detected by the method developed by Bartlett and Skoog (1954). The ion sulfate was quantified by an ion chromatograph (Thermo Fisher Scientific, ICS 5000) using an IonPac AS23 analytical column with a carbonate/bicarbonate eluent (4.5 mM Na2CO3/ × 0.8 mM NaHCO3 at 1 mL.min−1). The dissolved oxygen concentration was measured by a Hach HQ40D® portable meter equipped with a luminescent dissolved oxygen sensor (LDO101).
2.4 Calculations
The conversion of sulfide to elemental sulfur was calculated according to the total dissolved sulfide conversion (Eq. 4) and the sulfate formation (Eq. 5). The elemental sulfur formation was calculated by Eq. 6 based on Buisman et al. (1989).
S-TDS is the total dissolved sulfide (mg.L−1), S CONV is the S converted, S t=0 is the initial concentration and S t is the final concentration. S-SO 4 2− CONV is sulfide or other intermediate converted into sulfate (mg.L−1) and Gap-S is the fraction of the initial sulfide concentration that could not be retrieved as sulfide or sulfate (mg.L−1).
2.5 Determination of Membrane Overall Oxygen Transfer Resistance
To describe the oxygen mass flow through the silicone membrane, a resistance in series model was used as described by Coté et al. (1989). The model assumes that the mass transfer resistance is due to the membrane itself as well as to the boundary layers formed in the gas and in the liquid phases at the membrane interface (Fig. 1b). The contribution of each one of the three individual resistance structures can be summed into a local overall mass transfer coefficient (R) as expressed by Eq. 7,
in which K M is the membrane mass transfer coefficient (m.h−1), K G is the gas film mass transfer coefficient (m.h−1), and K L is the liquid film mass transfer coefficient (m.h−1).
As reported previously by Coté et al. (1989), the gas film resistance (1/K G ) has a minor influence compared with the membrane and the liquid film resistances and is thus usually disregarded. The membrane resistance (1/K M ) is a function of its thickness and permeability, and can be expressed according to Eq. 8,
in which τe is the membrane equivalent thickness for hollow tubular membranes (m), P is the membrane oxygen permeability (mol.Pa−1.m−1.s−1), and H is Henry’s law constant (Pa.m3.mol−1).
The membrane equivalent thickness for hollow tubing can be calculated according to Eq. 9,
in which r out is the membrane outer radius (m) and r in is the membrane inner radius (m).
Therefore, the individual contributions of the membrane resistance and the liquid film resistance to the overall oxygen transfer resistance can then be expressed by Eq. 10.
The oxygen mass flux through the membrane can be expressed by a resistance vs. driving force type relation, which is expressed by Eq. 11 (Casey et al. 1999; Côté et al. 1989),
in which J is the oxygen mass flux (kg.m−2.h−1), R is the local overall mass transfer coefficient (m.h−1), p is the oxygen partial pressure in air (N.m−2), H is Henry’s law constant (N.m.kg−1), and C L is the oxygen concentration in the bulk liquid (kg.m−3).
Considering the ESMR as a completely stirred tank and assuming that oxygen transfer through the silicone membrane occurs only radially, Eq. 11 can be rearranged to:
A is the total surface area available for oxygen transfer (m2), and V is the reactor working volume (m3).
Equation 14 can be integrated to obtain
The local overall mass transfer coefficient (R) can then be assessed by temporal profiles of dissolved oxygen concentration using Eq. 15. The profiles were obtained by the dynamic gassing-in method (Atkison and Mavituna 1983). The assays were conducted in triplicate for V s of 23.7 95.5 and 167.1 m.h−1.
The experimental data resulting from the R determinations with different V s were subjected to the one-way ANOVA statistical technique to verify the influence of the operational parameter on mass transfer.
3 Results and Discussion
3.1 Local Overall Mass Transfer Coefficient (R)
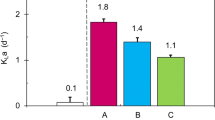
The local overall mass transfer coefficient (R) was assessed to evaluate the silicone membrane’s ability to promote micro-aeration. Table 1 summarizes the R values obtained for each V s. low R values obtained for the oxygen transport through the membrane indicate that micro-aeration was achieved.
The coefficient was studied for each V s to verify variations in the mass transfer. It is expected that the hydraulic conditions inside the silicone membrane would influence the liquid film. The increase in agitation would reduce boundary layer resistance, increasing the oxygen mass transfer.
Although the R values tended to increase with the increase of V s (Fig. 2), this trend was not found to be statistically significant when the data were subjected to a statistical analysis. This analysis permitted the conclusion that the levels of applied velocity tested did not cause significant differences in the R value for the significance level of 5 %. Therefore, the mass transfer process was not significantly influenced in the range of V s values tested.
This result can be explained by assessing the membrane resistance contribution to the overall mass transfer resistance. As documented in the literature, untreated polydimethylsiloxane silicone membranes, such as those used in the present work, have oxygen permeabilities ranging from 2.0110−13 mol.Pa−1.m−1.s−1 (Côté et al. 1989) to 2.08 × 10−13 mol.Pa−1.m−1.s−1 (Houston et al. 2002), and levels can reach up to 3.12 × 10−13 mol.Pa−1.m−1.s−1 (Stern et al. 1987). For Henry’s law constant for oxygen of 77.9 × 103 Pa.m3.mol−1 at 25 °C and a calculated equivalent thickness of 0.003 m (Eq. 6), the membrane resistance (1/K M ) is estimated to range between 1.23 × 105 s.m−1 and 1.92 × 105 s.m−1 according to Eq. 5. These obtained values are of the same order of magnitude as the experimentally assayed overall mass transfer resistance (1/R), which ranges from 0.93 × 105 s.m−1 to 1.12 × 105 s.m−1. Consequently, these values indicate that the membrane resistance was responsible for almost all the overall oxygen transfer resistance.
Tang (1975) investigated oxygen transfer through silicone tubing and reported that for laminar regimes and silicone thicknesses of up to 371 μm, the mass transfer resistance of the liquid film accounted for up to 74 % of the total resistance. However, in the present study, although the Reynolds number was well into the laminar region (Re < 460 for all experiments), the membrane thickness employed was over six times higher. Therefore, in this case, a higher contribution of membrane resistance to the overall oxygen transfer resistance is expected.
Membrane resistance dominance in overall mass transfer in silicone membranes has also been reported for other permeates. Raghunath and Hwang (1992) found that the membrane resistance will dominate for a silicone membrane above 1.16 mm in thickness, and Brookes and Livingston (1995) reported an increase by a factor of 1.5 in the overall mass transfer coefficient for phenol when the membrane thickness was reduced from 0.5 to 0.3 mm. In a similar manner, Chuichulcherm et al. (2001) used silicone-based membranes (from 0.24 to 0.59 mm in thickness) combined with biological sulfide production for the treatment of metal-containing wastewater. They used the resistances-in-series model and compared liquid film resistances with the mass membrane resistance values to show that the membrane resistance was the dominant resistance for H2S transfer.
In summary, the alteration of liquid film thickness by hydraulic condition variation had little or no influence on the mass transfer process. To use V s as a system control parameter, silicone membranes of lower thickness or increased oxygen permeability would be necessary.
3.2 Sulfide Conversion
The performance of chemical and biological sulfide oxidation under micro-aerobic conditions was evaluated in batch assays. Under chemical oxidation conditions (A), 94.1 ± 5.8 % of the total dissolved sulfide (TDS) was removed and under biotic conditions (B1), an average of 94.1 ± 3.1 %, of TDS was removed in the ESMR. When anaerobically treated wastewater was used (B2), an average of 95.6 ± 5.4 % of TDS was removed. Elemental sulfur formation was observed in all experiments and was confirmed by colorimetric analysis. The conversion of sulfide to sulfate and other intermediates (mass balance gap) are shown in Table 2. The conversion data was presented for three different levels of applied velocity and three different pH values (8.0; 9.0, and 10.0).
The chemical oxidation experiments (A), showed low percentages of sulfide conversion to sulfate, except for the test with a pH value of 8.0 and V s of 23.7 m.h−1. The low percentage of sulfide conversion to sulfate denotes the ESMR capacity of micro-aeration and sulfide removal by the conversion to other intermediates, such as elemental sulfur. A colloidal sulfur suspension was developed in the chemical oxidation experiments, as confirmed by colorimetric analysis.
Elemental sulfur formed in the B1 and B2 tests was deposited in the membrane and had a pale yellow color, which is associated with biological sulfur formation (Janssen et al. 1999). This is an important result, since the sulfur attached can be removed and recovered.
The biological tests performed with lab-made wastewater (B1) and anaerobic reactor effluent (B2) presented the highest conversion to sulfate for the batch runs with V s of 167.1 m.h−1 and pH 8.0. Both tests showed an increase in the sulfate formation for V s values above 95.5 m.h−1, indicating that the applied velocity could interfere in the sulfide conversion. Although the applied velocity did not cause significant differences (significance level of 5 %) in the mass transfer process, the increase in the flow could have flushed the sulfur attached to the membrane, which could have reacted with sulfide resulting in polysulfides, sulfate, and other intermediates (Eqs. 16–18).
Besides the type of elemental sulfur aggregates formed, the time for the completion of the reaction (Table 2) was another advantageous property of biological oxidation tests. While B1 and B2 assays took between 4872 and 2448 h, respectively, for the total oxidation of sulfide, the abiotic assays (A) took between 96 and 168 h. The experimental temporal profiles obtained for the chemical and biological (B1) sulfide oxidation assays at pH 9.0 are presented in Fig. 3 along with the fitted rate equations. The advantage of biological sulfide conversion in relation to chemical conversion can be observed in this plot. This result is in agreement with the observed batch duration necessary for total sulfide conversion. The combined chemical and biological oxidation (B1 and B2) took, in average, 36 % less time than chemical oxidation alone.
The B1 and B2 tests showed low percentages for sulfide conversion to sulfate also for pH 10.0. However, in these tests the biomass probably played no role in sulfide conversion. Although the biomass was gradually acclimated to each pH condition, in the tests with pH 10.0 the biomass precipitated after only a few hours, denoting its inactivation. Chemical oxidation is probably the main mechanism involved at this pH. In fact, Chen and Morris (1972) and Millero et al. (1987) reported that sulfide auto-oxidation was accelerated with the increase in pH. Further careful acclimation of the biomass to this extreme pH could be of interest in future studies.
Mixed chemical and biological oxidation in the B1 and B2 tests was probably responsible for the sulfide consumption. While investigating mixed chemical and biological sulfide oxidation at a pH value of 7.0, Wilmot et al. (1988) reported that biological oxidation is responsible for less than 40 % of the total oxidation.
The Gap-S, presented in Table 2, can be understood as the conversion of sulfide to other intermediates, such as thiosulfate, sulfite, polysulfide, and elemental sulfur. Although thiosulfate and sulfite could not be detected, evidence of polysulfide formation was observed. Color alteration in the reactive medium was noted in the chemical and biological sulfide oxidation tests. For pH values of 7.6 to 9.0, a greenish color was observed at the beginning of the experiments, followed by conversion to a whitish color upon total sulfide oxidation. The greenish color is associated with the formation of S4 2− and S5 2−, whereas the whitish color is related to colloidal sulfur suspension formation (Chen and Morris 1972; Buisman et al. 1989 and Janssen et al. 1995). Steudel (1996) studied the stoichiometry of the oxidation of polysulfide ions. The author reported that the final reaction products after the yellow-to-orange color transition, after the disappearance of the polysulfide solution were thiosulfate and sulfur (Eq. 19).
In the experiments in which medium color alteration was observed, a whitish color was noted, indicating total polysulfide oxidation, with elemental sulfur and thiosulfate being possible end products. Krishnakumar et al. (2005) used a reverse fluidized loop reactor treating a sulfide solution and reported that polysulfide was always observed along with a high accumulation of thiosulfate. Thiosulfate, as demonstrated in Eq. 19, most probably originates from the autoxidation of polysulfide anions (Steudel 1996).
The higher values to the sulfide conversion to sulfate for pH values of 8.0 and 9.0 in the B1 and B2 tests can be a result of the thiosulfate and other sulfur intermediates formed by chemical auto-oxidation that were further oxidized to sulfate in biotic conditions. The activation energy of sulfate formation can be decreased by enzymatic reactions promoted by microorganisms, which is in contrast to chemical oxidation that requires elevated activation energy (González-Sánchez and Revah 2007). However, the intermediate formed in both chemical and biological tests were not completely oxidized to sulfate due to the limited oxygen supply. These results were also found by Gonzáles-Sánchez and Revah (2007) and indicate the importance of strict oxygen concentration control.
The reduced sulfate concentrations achieved in the batch assays denote that the micro-aeration promoted by the silicone membrane avoided further oxidation of the sulfur intermediates to sulfate. Although the biotic oxidation tests (B1 and B2) had higher sulfide conversion to sulfate when compared with the chemical oxidation tests and exhibited biomass inactivation at pH values of 10.0, biological oxidation proved advantageous with respect to the type of aggregation of the elemental sulfur formed and the average time for complete sulfide oxidation. The sulfur particles attached to the membrane could be drawn from the system by a change in the hydrodynamic conditions in the membrane, such as increasing the flow inside the membrane and withdrawing sulfur to avoid further oxidation to intermediate and sulfate.
4 Conclusions
The performance of the sulfide oxidation in an ESMR was investigated. Significant silicone membrane potential for micro-aeration and sulfide oxidation was found in all experiments.
The local overall oxygen mass transfer coefficient (R) determined for the silicone membrane had an average value of 0.153 m.h−1 and was not influenced by the applied velocity in the membrane. To use the applied velocity as a system control parameter, silicone membranes of lower thickness or increased oxygen permeability would be necessary.
The elemental sulfur observed in the biotic oxidation experiments was formed into large aggregates that could easily be washed away upon flushing of the membrane. Colloidal sulfur was observed in the chemical oxidation tests. Reduced sulfate concentrations were achieved in the batch assays and denote that the micro-aeration promoted by the silicone membrane avoided further oxidation of the sulfur intermediates to sulfate. Although the biotic oxidation tests had higher sulfide conversion to sulfate, the biological oxidation proved advantageous with respect to the type of aggregation of the elemental sulfur formed and the average time for complete sulfide oxidation.
In summary, the ESMR was able to convert sulfide to sulfur and to avoid complete sulfide oxidation to sulfate. Further investigations of the ESMR combined with an anaerobic reactor are in progress to achieve both sulfate reduction and sulfide oxidation in a single unit.
References
Alcantara, S., Velasco, A., Munoz, A., Cid, J., Revah, S., & Razo-Flores, E. (2004). Hydrogen sulfide oxidation by a microbial consortium in a recirculation reactor system: sulfur formation under oxygen limitation and removal of phenols. Environmental Science and Technology, 38(3), 918–923. doi:10.1021/es034527y.
American Public Health Association, E. A. D. A. W. W. A. W. E. F. (2005). Standard methods for the examination of water and wastewater. Washington, D.C.: APHA-AWWA-WEF.
Atkison, B., & Mavituna, F. (1983). Biochemical engineering and biotechnology handbook. New York: Macmillan Publishers Ltda.
Bartlett, J. K., & Skoog, D. A. (1954). Colorimetric determination of elemental sulfur in hydrocarbons. Analytical Chemistry, 26(6), 1008–1011. doi:10.1021/ac60090a014.
Brindle, K., & Stephenson, T. (1996). The application of membrane biological reactors for the treatment of wastewaters. Biotechnology and Bioengineering, 49(6), 601–610. doi:10.1002/(sici)1097-0290(19960320)49:6<601::aid-bit1>3.0.co;2-s.
Brookes, P. R., & Livingston, A. G. (1995). Aqueous-aqueous extraction of organic pollutants through tubular silicone rubber membranes. Journal of Membrane Science, 104(1–2), 119–137. doi:http://dx.doi.org/10.1016/0376-7388(95)00020-D.
Buisman, C., Post, R., Ijspeert, P., Geraats, G., & Lettinga, G. (1989). Biotechnological process for sulphide removal with sulphur reclamation. Acta Biotechnologica, 9(3), 255–267. doi:10.1002/abio.370090313.
Buisman, C. J. N., Geraats, B. G., Ijspeert, P., & Lettinga, G. (1990). Optimization of sulfur production in a biotechnological sulfide-removing reactor. Biotechnology and Bioengineering, 35(1), 50–56.
Buisman, C. J. N., Ijspeert, P., Hof, A., Janssen, A. J. H., Tenhagen, R., & Lettinga, G. (1991). Kinetic-parameters of a mixed culture oxidizing sulfide and sulfur with oxygen. Biotechnology and Bioengineering, 38(8), 813–820.
Camiloti, P. R., Mockaitis, G., Domingues Rodrigues, J. A., Rissato Zamariolli Damianovic, M. H., Foresti, E., & Zaiat, M. (2013). Innovative anaerobic bioreactor with fixed-structured bed (ABFSB) for simultaneous sulfate reduction and organic matter removal. Journal of Chemical Technology and Biotechnology, 89(7), 1044–1050. doi:10.1002/jctb.4199.
Casey, E., Glennon, B., & Hamer, G. (1999). Oxygen mass transfer characteristics in a membrane-aerated biofilm reactor. Biotechnology and Bioengineering, 62(2), 183–192. doi:10.1002/(sici)1097-0290(19990120)62:2<183::aid-bit8>3.0.co;2-l.
Chen, K. Y., & Morris, J. C. (1972). Kinetics of oxidation of aqueous sulfide by oxygen. Environmental Science and Technology, 6(6), 529–537. doi:10.1021/es60065a008.
Chen, C, Wang, A, Ren, N, Zhao, Q, Liu, L, Adav, SS, et al (2010) Enhancing denitrifying sulfide removal with functional strains under micro-aerobic condition. Process Biochemistry 45(6),1007–1010. doi:10.1016/j.procbio.2010.02.013.
Chuang, S. H., Pai, T. Y., & Horng, R. Y. (2005). Biotreatment of sulfate-rich wastewater in an anaerobic/micro-aerobic bioreactor System. Environmental Technology, 26(9), 993–1002. doi:10.1080/09593332608618487.
Chuichulcherm, S., Nagpal, S., Peeva, L., & Livingston, A. (2001). Treatment of metal-containing wastewaters with a novel extractive membrane reactor using sulfate-reducing bacteria. Journal of Chemical Technology and Biotechnology, 76(1), 61–68. doi:10.1002/1097-4660(200101)76:1<61::aid-jctb357>3.0.co;2-o.
Côté, P, Bersillon, J-L, & Huyard, A (1989) Bubble-free aeration using membranes: mass transfer analysis. Journal of Membrane Science, 47(1–2), 91–106. doi:10.1016/S0376-7388(00)80862-5.
Díaz, I, Pérez, SI, Ferrero, EM, & Fdz-Polanco, M (2011) Effect of oxygen dosing point and mixing on the microaerobic removal of hydrogen sulphide in sludge digesters. Bioresource Technology 102(4), 3768–3775. doi:10.1016/j.biortech.2010.12.016.
González-Sánchez, A, & Revah, S (2007) The effect of chemical oxidation on the biological sulfide oxidation by an alkaliphilic sulfoxidizing bacterial consortium. Enzyme and Microbial Technology 40(2), 292–298. doi:10.1016/j.enzmictec.2006.04.017.
Houston, KS, Weinkauf, DH, & Stewart, FF (2002) Gas transport characteristics of plasma treated poly(dimethylsiloxane) and polyphosphazene membrane materials. Journal of Membrane Science 205(1–2), 103–112. doi:10.1016/S0376-7388(02)00068-6.
Janssen, A. J. H., Sleyster, R., Vanderkaa, C., Jochemsen, A., Bontsema, J., & Lettinga, G. (1995). Biological sulfide oxidation in a fed-batch reactor. Biotechnology and Bioengineering, 47(3), 327–333.
Janssen, A. J. H., Ma, S. C., Lens, P., & Lettinga, G. (1997). Performance of a sulfide-oxidizing expanded-bed reactor supplied with dissolved oxygen. Biotechnology and Bioengineering, 53(1), 32–40.
Janssen, A. J. H., Lettinga, G., & de Keizer, A. (1999). Removal of hydrogen sulphide from wastewater and waste gases by biological conversion to elemental sulphur—colloidal and interfacial aspects of biologically produced sulphur particles. Colloids and Surfaces a-Physicochemical and Engineering Aspects, 151(1–2), 389–397.
Jenicek, P. K., Bindzar, J., & Zabranska, J. (2010). Advantages of anaerobic digestion of sludge in microaerobic conditions. Water Science and Technology, 62(2), 7.
Jensen, A. B., & Webb, C. (1995). Treatment of h2s-containing gases—a review of microbiological alternatives. Enzyme and Microbial Technology, 17(1), 2–10.
Kleinjan, W. E., de Keizer, A., & Janssen, A. J. H. (2005). Kinetics of the chemical oxidation of polysulfide anions in aqueous solution. Water Research, 39(17), 4093–4100. doi:10.1016/j.watres.2005.08.006.
Krishnakumar, B, Majumdar, S, Manilal, VB, & Haridas, A (2005) Treatment of sulphide containing wastewater with sulphur recovery in a novel reverse fluidized loop reactor (RFLR). Water Research 39(4):639–647. doi:10.1016/j.watres.2004.11.015.
Kuenen, J. G. (1975). Colourless sulfur bacteria and their role in the sulfur cycle. Plant and Soil, 43(1–3), 49–76. doi:10.1007/bf01928476.
Lens, P. N. L., Visser, A., Janssen, A. J. H., Pol, L. W. H., & Lettinga, G. (1998). Biotechnological treatment of sulfate-rich wastewaters. Critical Reviews in Environmental Science and Technology, 28(1), 41–88.
Lohwacharin, J., & Annachhatre, A. P. (2010). Biological sulfide oxidation in an airlift bioreactor. Bioresource Technology, 101(7), 2114–2120. doi:10.1016/j.biortech.2009.10.093.
Millero, F. J., Hubinger, S., Fernandez, M., & Garnett, S. (1987). Oxidation of H2S in seawater as a function of temperature, pH, and ionic strength. Environmental Science and Technology, 21(5), 439–443. doi:10.1021/es00159a003.
Moghanloo, G. M. M., Fatehifar, E., Saedy, S., Aghaeifar, Z., & Abbasnezhad, H. (2010). Biological oxidation of hydrogen sulfide in mineral media using a biofilm airlift suspension reactor. Bioresource Technology, 101(21), 8330–8335. doi:10.1016/j.biortech.2010.05.093.
Pokasoowan, C, Kanitchaidecha, W, K C BK, & Annachhatre, AP (2009) Investigation on laboratory and pilot-scale airlift sulfide oxidation reactor under varying sulfide loading rate. Journal of Environmental Science and Health, Part A 44(1), 87–98. doi:10.1080/10934520802515426.
Raghunath B, & Hwang ST (1992) General treatment of liquid-phase boundary layer resistance in the pervaporation of dilute aqueous organics through tubular membranes. Journal Membrane Science, 75(1–2):29–46. doi:10.1016/0376-7388(92)80004-4.
Robertson, L, & Kuenen, JG (2006) The Genus Thiobacillus. In: M Dworkin, S Falkow, E Rosenberg, K-H Schleifer, & E Stackebrandt (Eds) The Prokaryotes (pp. 812–827): Springer New York.
Stern, S. A., Shah, V. M., & Hardy, B. J. (1987). Structure-permeability relationships in silicone polymers. Journal of Polymer Science Part B: Polymer Physics, 25(6), 1263–1298. doi:10.1002/polb.1987.090250607.
Steudel, R. (1996). Mechanism for the formation of elemental sulfur from aqueous sulfide in chemical and microbiological desulfurization processes. Industrial and Engineering Chemistry Research, 35(4), 1417–1423. doi:10.1021/ie950558t.
Suzuki, Y., Miyahara, S., & Takeishi, K. (1994). Oxygen supply method using gas-permeable film for wastewater treatment. Water Science and Technology, 28(7), 8.
Tang, T. S. (1975). Mass transfer of dissolved bases through membrane tubing. Iowa City: University of Iowa.
Wilderer, PA, Bräutigam, J, & Sekoulov, I (1985) Application of gas permeable membranes for auxiliary oxygenation of sequencing batch reactors. Conservation & Recycling, 8(1–2), 181–192. doi:10.1016/0361-3658(85)90035-9.
Wilmot, P. D., Cadee, K., Katinic, J. J., & Kavanagh, B. V. (1988). Kinetics of sulfide oxidation by dissolved oxygen. Journal Water Pollution Control Federation, 60(7), 1264–1270.
Wobus, A, Ulrich, S, & Röske, I (1995) Degradation of chlorophenols by biofilms on semi-permeable membranes in two types of fixed bed reactors. Water Science Technology 32(8), 205–212. doi:10.1016/0273-1223(96)00027-3.
Xu, X-J, Chen, C, Wang, A-J, Fang, N, Yuan, Y, Ren, N-Q, et al (2012) Enhanced elementary sulfur recovery in integrated sulfate-reducing, sulfur-producing reactor under micro-aerobic condition. Bioresource Technology, 116(0), 517–521. doi:10.1016/j.biortech.2012.03.095.
Yamagiwa K, Yoshida M, Ito A, & Ohkawa A (1998) A new oxygen supply method for simultaneous organic carbon removal and nitrification by a one-stage biofilm process. Water Science Technology, 37(4–5), 117–124. doi:10.1016/S0273-1223(98)00093-6.
Yu H, Chen C, Ma J, Xu X, Fan R, & Wang A (2014) Microbial community functional structure in response to micro-aerobic conditions in sulfate-reducing sulfur-producing bioreactor. Journal of Environmental Sciences, 26(5), 1099–1107. doi:10.1016/S1001-0742(13)60589-6.
Acknowledgments
The authors thank the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) process no. 2009/15984-0.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Declaration
The authors also declare that this research did not involve human participants and/or animals.
Rights and permissions
About this article
Cite this article
Camiloti, P.R., Oliveira, G.H.D. & Zaiat, M. Sulfur Recovery from Wastewater Using a Micro-aerobic External Silicone Membrane Reactor (ESMR). Water Air Soil Pollut 227, 31 (2016). https://doi.org/10.1007/s11270-015-2721-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-015-2721-y