Abstract
A sensitive, high-throughput, and cost-effective method for screening bacterial pathogens in the environment was developed. A variety of environmental samples, including aerosols, soil of various types (sand, sand/clay mix, and clay), wastewater, and vegetable surface (modeled by tomato), were concomitantly spiked with Salmonella enterica and/or Pseudomonas aeruginosa to determine recovery rates and limits of detection. The various matrices were first enriched with a general pre-enrichment broth in a dilution series and then enumerated by most probable number (MPN) estimation using quantitative PCR for rapid screening of amplicon presence. Soil and aerosols were then tested in non-spiked environmental samples, as these matrices are prone to large experimental variation. Limit of detection in the various soil types was 1–3 colony-forming units (CFU) g−1; on vegetable surface, 5 CFU per tomato; in treated wastewater, 5 CFU L−1; and in aerosols, >300 CFU mL−1. Our method accurately identified S. enterica in non-spiked environmental soil samples within a day, while traditional methods took 4 to 5 days and required sorting through biochemically and morphologically similar species. Likewise, our method successfully identified P. aeruginosa in non-spiked aerosols generated by a domestic wastewater treatment system. The obtained results suggest that the developed method presents a broad approach for the rapid, efficient, and reliable detection of relatively low densities of pathogenic organisms in challenging environmental samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Activities such as dairy farming, treatment, and application of wastewater or urban runoff necessitates real-time monitoring of pathogens in environmental matrices. However, standard methods (originally developed for food, clinical, or water) require indicators and/or pathogens detection methods that rely on labor- and time-intensive culturing techniques. For example, detection of Salmonella enterica, according to the FDA (2011), requires initial processing, nonspecific and specific enrichment steps, then culture and isolation, and lastly biochemical testing of the numerous isolates on three types of selective media, amounting to at least 4 days. This rigorous detection scheme is not amenable to the rapid and high-volume screening of multiple environmental samples. Because of these shortcomings, a large body of research has been dedicated to finding rapid and high-throughput screening techniques for the presence of disease-causing organisms in different samples (Girones et al. 2010; Noble and Weisberg 2005; Straub et al. 2005). One promising and rapidly evolving avenue is biosensors capable of interacting with biological molecules at the nanometer scale (Vikesland and Wigginton 2010). However, the suitability of these assays to environmental samples has not been established (Vikesland and Wigginton 2010), and the high cost of these technologies currently prevents their large scale application (Qu et al. 2013).
For the past 20 years the most popular alternative to culture-based detection of pathogens has been molecular-based assays, such as quantitative polymerase chain reaction (qPCR), due to their speed, reliability, and sensitivity (Ishii et al. 2013; Straub et al. 2005; Yeung et al. 2006). However, even molecular-based assays are unreliable when it comes to detection of microorganisms in environmental samples such as wastewater, soil, vegetable surfaces, or aerosols. The assays can be affected by the presence of free nucleic acids from nonviable cells (Bae and Wuertz 2009), co-extracted inhibitory materials (Schriewer et al. 2011), or simply debris from inevitable impurities in the extracted nucleic acids material (Girones et al. 2010), resulting in over- or under-estimation of bacterial densities. Pathogen detection in aerosols, for example, poses a unique challenge: The sampling is often performed using the settle-plate technique or utilizing a vacuum to suck air into a liquid impinger or onto an agar plate (Napoli et al. 2012). However, all air detection methods have their limitations (Chinivasagam and Blackall 2005; Park et al. 2014) as there are a variety of different designs each needing calibration for each microorganism (Deloge-Abarkan et al. 2007; Landman et al. 2013; Ryan et al. 2014) and for each nutrient medium used; hence, the results are variable and can be difficult to interpret (Napoli et al. 2012).
To improve the detection levels such that they reflect low pathogen concentrations (especially ones with low infective doses) in the environment, some additional steps are required. Such steps could be immuno-magnetic separation (Warren et al. 2007), pre-amplification of the target DNA (Ishii et al. 2013), or nonspecific enrichment (Edel and Kampelmacher 1973), aiming to increase the likelihood of detecting (but not directly enumerating) viable pathogens (Krämer et al. 2011; Malorny et al. 2004). The combination of nonspecific enrichment followed by DNA extraction and qPCR analysis coupled with most probable number (MPN) estimation was found to be a useful tool to increase the likelihood of pathogen detection when initial concentrations are low (Krämer et al. 2011; Russo et al. 2014; Wright et al. 2007). However, several shortcomings precluded the application of the proposed method to more challenging environmental matrices such as soil or aerosols. The processing schemes presented were directed at specific food matrices, i.e., meat (Krämer et al. 2011), oysters (Wright et al. 2007), or vegetables freshly cut and eaten raw (Russo et al. 2014). These food matrices naturally lack the overwhelming bacterial diversity found in soil or wastewater; thus, pathogen enrichment and detection should be simpler. In addition, the previously presented methods applied enrichment schemes that were aimed at specific pathogens (Krämer et al. 2011; Russo et al. 2014; Wright et al. 2007). In fact, different enrichments were employed if more than one pathogen was targeted (Russo et al. 2014).
This is the first study to comprehensively assess pathogen concentrations in a variety of difficult environmental matrices using an evolved MPN type enrichment coupled with qPCR, with a particular emphasis on soil types and aerosols. The model pathogens S. enterica and Pseudomonas aeruginosa were chosen to be tested in this study as there are no published studies as of yet applying the MPN enrichment for their simultaneous detection and yet they are leading causes of illness, have high survival potential in the environment, and are considered difficult to detect accurately (CDC 2014; Doyle and Erickson 2008).
2 Materials and Methods
2.1 Overview
The general outline for the method is a serial dilution scheme of the sample in pre-enrichment broth followed by qPCR to detect the presence of amplicons in each dilution, as opposed to standard methods requiring isolation and biochemical tests (Fig. 1). The pattern of present/absent results obtained by qPCR is then converted to quantitative data with an MPN table (Blodgett 2010) or an MPN calculator (Curiale 2004).
Comparison of standard and hybrid detection strategies for pathogens. I MPN-qPCR method: (A) Enrichment using general, low nutrient media in dilution series for adaptability to most probable number (MPN) estimation followed by (B) DNA extraction, and (C) qPCR performed as a qualitative present/absent test providing immediate results. II Standard methods for pathogens detection (inspired by EPA 1682): (A) Enrichment using selective media, followed by (B) bacterial isolation on solid media, then (C) colony picking and biochemical validation. The (D) DNA of validated colonies is extracted, and (E) used as template in qPCR reaction to validate the pathogen
2.2 Bacterial Spike Preparation
Bacterial cultures of S. enterica serovar Abony (NCTC 6017) and P. aeruginosa (ACTC 47085D-5) were grown overnight at 200 rpm and 37 °C in Luria-Bertani (LB) broth (Becton Dickinson (BD), Franklin Lakes, NJ). The cultures were diluted in sterile phosphate-buffered saline (PBS) to spiking solutions containing approximately 100 to 104 colony-forming units (CFU) mL−1. The precise concentrations were confirmed by viable counts.
2.3 Traditional S. enterica and P. aeruginosa Culturing
We compared the developed method with the standard methods (Rice et al. 2012). Briefly, S. enterica were cultivated from all matrices tested (spiked and non-spiked) by nonspecific enrichment in buffered peptone water (BPW; BD), followed by enrichment in Rappaport-Vassiliadis R-10 medium (BD) at 41 °C and inoculation on SS agar (Hi-Media, Mumbai, India). Suspected colonies were tested biochemically with Hy-Enterotest (Hy-Labs, Rehovot, Israel) according to the manufacturer’s instructions. Suspected strains were further tested for the presence of the invA encoding gene by qPCR amplification as previously described (Benami et al. 2013).
For P. aeruginosa detection, enriched and non-enriched samples were inoculated on Pseudomonas agar plates (Hi-Media) and visualized under ultraviolet (UV) light (254 nm) for fluorescence, according to manufacturer’s instructions.
2.4 Soil Preparation
Three soil types with varying proportions of sand were obtained: sand (100 %), sand/clay (50 % each), and silt/clay (<10 % sand) classified according to standard methods (USDA, 1975). The samples were air-dried and 5 g was weighed into 50 mL sterile plastic tubes (Corning, Corning, NY). Deionized water (2.5 mL) was applied to each sample to ensure that the spiked bacteria would not desiccate. The bacteria were spiked in triplicate onto the soil surface to final concentrations of 100, 101, and 102 CFU g−1 (verified by plating), while non-spiked samples were used as negative control. The volume in the tubes was adjusted to 25 mL with the addition of 0.1 % (v/v) Tween-80 (Sigma, St. Louis, MO) in BPW (pH 7.2) and incubated in 37 °C at 150 rpm for 30 min and then allowed to settle for 30 min.
2.5 Quantification with MPN Based Enrichment Method
2.5.1 Soil
After mixing well, the samples were diluted in a 12 well plate (Greiner, Frickenhausen, Germany) containing BPW enrichment medium. Soil was diluted to 100, 10−1, and 10−2 g in 5 mL BPW, each in triplicates. The dilution plates were sealed with an aluminum adhesive seal (Excel Scientific, Victorville, CA) and incubated at 37 °C for 16 h at 150 rpm. Then, the DNA from each well was extracted and used as template for qPCR amplification (Benami et al. 2013). The MPN counts were based on the multiplex qPCR unequivocally demonstrating P. aeruginosa or S. enterica amplicons. Concentrations were then estimated based on MPN charts (Blodgett 2010).
To test our suggested method on environmental samples, we sampled loess soil and amended 4 g with 1 g of poultry manure (n = 25) collected from a composting poultry manure pile (30.87 N, 34.80 E). The samples were processed within 24 h of sampling as described above, except for an addition of 10-fold dilution (to 10−3 g in BPW) to account for possibly high pathogen concentrations.
2.5.2 Water
Hundred liter samples of potable as well as secondary and tertiary treated wastewater (TWW) were obtained from an agricultural research station. The TWW (n = 3 for each water type) were spiked with approximately 100 to 103 CFU L−1 of S. enterica in serial dilutions (determined by viable counts). Non-spiked TWW samples (n = 3 for each water type) were used as negative controls. The TWW samples were processed as previously described (Benami et al. 2013). A sample from the concentrate (9 mL) was mixed with 10 mL BPW, diluted, and incubated for 16 h at 37 °C and 200 rpm.
2.5.3 Vegetable Surface
Tomatoes were used as the model vegetable for this study. S. enterica was spiked at approximately 100 to 103 CFU in serial dilutions (verified by viable counts) onto the surfaces of five tomatoes in triplicate (n = 3 for each spiking level). The tomatoes were then washed with 50 mL PBS with 0.01 % Tween-80. The wash was collected and mixed with 50 mL BPW and the mixture was incubated for 16 h at 37 °C and 200 rpm.
2.5.4 Aerosols
One milliliter of 103, 104, and 105 CFU mL−1 of P. aeruginosa was spiked into an aerosol generator (A79-LIFE-Eagle Nebulizer, Keene Medical Products, Enfield, NH) containing 9 mL PBS + 20 % (v/v) glycerol solution and allowed to emit aerosols over 1 h (n = 3). All experiments were performed inside a closed and sterilized (by UV and ethanol disinfection) laminar flow biological hood (ADS model OPTIMALE 12, Cedex, France) with an average temperature of 24.2 ± 5.1 °C and relative humidity 45.8 ± 10.2 % over the duration of these experiments. To collect aerosol samples from the air the BioSampler® impinger (SKC, 225–9595, Eighty Four, PA) was chosen as the active sampling technique following published evaluations of various techniques (Haas et al. 2010; Hogan et al. 2005). The aerosol samples were each collected into a liquid impinger containing 20 mL of sterile PBS collection fluid and operated for 1 h at a constant suction rate of 12.5 L min−1 detected by a flowmeter (Dwyer Instruments Inc., DW-806, Michigan City, IN). The BioSampler® inlet was positioned at 0.1 m away from the nebulizer and was intended to collect the maximum amount of aerosols being emitted from the nebulizer during the 1-h of sampling. The obtained liquid impinger solution was diluted to 100, 10−1, 10−2, and 10−3 mL in BPW, each in triplicates, corresponding to an MPN unit (Blodgett 2010). In addition, the liquid was inoculated onto agar plates selective for Pseudomonas spp. as previously described (Chinivasagam and Blackall 2005; Griffin et al. 2011; Terzieva et al. 1996).
To test our suggested method on environmental aerosol samples, the BioSampler® impinger was positioned 0.3 m away from domestic WW treatment systems (Gross et al. 2008) to collect aerosol emissions (n = 9) as described above. Selective agar plates for Pseudomonas spp. were also placed 0.3 m away from the systems as the standard “settle-plate technique” (n = 9). All samplings were performed between 6 and 8 a.m. when the relative humidity (RH) and temperature were closest to the ideal bioaerosol survival range (approximately 75 % RH and 12 °C), while solar radiation was low (data not shown). A fan was set up behind each system to direct the airflow in one direction and mimic a “windy day” (∼3 m s−1 airflow up to 1 m away). The airflow was detected by the anemometer option on a Kimo AMI300 (Multifunction Data Logger, North Yorkshire, UK).
After collection, the liquid impinger samples were stored at 4 °C and subsamples (<1 mL) were analyzed within 24 h by applying the qPCR + MPN after enrichment technique as described above.
2.5.5 DNA Extraction
Two hundred microliters of aliquots of each enriched sample was transferred to a 96-well microtiter plate (Greiner) and spun for 1 min in a mini plate spinner (MPS-1000, Labnet, Edison, NJ). The supernatant was then removed, and DNA crudely extracted as previously described (Liu et al. 2002; Spilker et al. 2004). The resulting DNA solution was used as the template for qPCR analyses.
2.5.6 Quantitative (q) PCR
The primers and probes used in this study target the genes invA and regA of S. enterica and P. aeruginosa, respectively (Shannon et al. 2007). For easy reference, the sequences and the fluorophores/quenchers used are listed in Table 1. A single qPCR contained 10 μL TrueBluemaster mix (Thermo-Scientific, Waltham, MA), 200 nM each of primers and TaqMan probes (Metabion, Hanover, Germany), 5 μL DNA solution, and water (Sigma) to a final volume of 20 μL. Amplification was performed in a CFX-1000 thermocycler (Bio-Rad). The reaction mix was activated at 95 °C for 15 min and then subjected to 40 cycles of 95 °C for 10 s, 60 °C for 30 s, and acquisition.
The results were analyzed with CFX Manager software (v 2.1, Bio-Rad) on default settings. Fluorescence signals were interpreted as presence/absence of the targeted amplicon. Control reactions without template (lysis buffer with water instead of sample, n = 3) and serially diluted genomic DNA as positive control (ranging from 2.5 × 103 to 2.5 gene copies per reaction, n = 3) were included in each reaction set. All positive template controls (n = 9) had a threshold cycle (Ct) of <35 and relative fluorescence units (RFU) of >150. None of the no-template controls (n = 9) met these criteria, validating these two parameters as present/absent delineators.
2.6 Statistical Analyses
The effect of soil type on the presence/absence amplicons was tested by logistic regression. Linear model regression analysis was used to correlate Ct value with initial bacterial density. Correlation coefficients associated with p < 0.05 were considered significant. Analyses were carried out in R (R Development Core Team 2008).
3 Results
3.1 Spiking Experiments
The objective of the spiking experiments was to assess the method accuracy in various environmental matrices and assess the limit of detection (LOD), i.e., the lowest amount of target that the method can reliably detect (Armbruster and Pry 2008). Therefore, we tested four matrices (water, soil, air, and vegetable surfaces) applying at least two target concentrations of the model pathogens.
3.2 Soil
We employed a three-dilution MPN format of 100, 10−1, and 10−2 g soil in three replicates (Fig. 1), resulting in a possible range of quantification between 0.3 and 110 CFU g−1 (Blodgett 2010). This range was chosen to target low levels of pathogens that may be present in environmental samples. Indeed, the method was highly sensitive to both S. enterica and P. aeruginosa (Table S1), detecting the bacteria in all spiked soil samples, with concentrations as low as 1–3 CFU g−1 soil (Table 2).
Significant (p < 0.050) correlations between the initial bacterial density in the spiked clay soil and the Ct value of the multiplex qPCR assay were noted for P. aeruginosa and S. enterica. However, the correlation coefficient R 2 was 0.89 for the P. aeruginosa but was lower (R 2 = 0.31) for S. enterica (Fig. 2). This trend was observed for all soil types (Table S2, Table S3) and could be due to a number of factors, such as metabolic differences between the two species, generation time, or susceptibility to physico-chemical characteristics of the sample (Dunbar et al. 1997). However, it should be noted that for both species, in samples in which the spiked bacteria were diluted to an initial quantity of less than 1 CFU g−1 (i.e., below the method LOD), Ct increased to the threshold level of 35, at which the samples were no longer considered positive (Tables S2 and S4). This provided empirical validation for the suggested absent/present criterion.
Comparing qPCR threshold cycle (Ct) and log initial inoculum (CFU g−1) of Salmonella enterica and Pseudomonas aeruginosa spiked in clay soil and then detected with the MPN-qPCR procedure. In preparation for the test, a pathogen/soil mixture (200 CFU g−1) was serially diluted until fractional values (depicted by the negative log) were obtained in order to test the qPCR response over a broad range of values that extend into the barely detectable range. Regression coefficients: S. enterica = 0.31, p > 0.05, P. aeruginosa = 0.89, p < 0.05
The culture-based technique (FDA 2011) was applied on spiked clay soil and was equal in sensitivity to the MPN-qPCR technique (LOD = 1 CFU g−1; Table 2). However, at least 4 days were needed to screen and confidently identify the putative pathogen as false positives with biochemical characteristics similar to those of S. enterica obfuscated the detection (Dusch and Altwegg 1995).
After validating the method on spiked samples in the lab, a field study was undertaken. A total of 25 compost-amended soil samples were analyzed concurrently by the proposed MPN-qPCR and by the conventional culture-based method. S. enterica was detected in only one sample by the MPN-qPCR method, and the results were obtained within a day from the start of processing. Importantly, the first dilution level (containing 1 g of incubated soil) did not produce detectable DNA replication, while higher dilutions replicated well and correlated with the initial concentration (Table S5). This highlights the unique importance of pre-enrichment dilutions for environmental samples since the inhibitors were diluted, while the target organisms are enriched. S. enterica isolates were obtained from the same sample by the culture-based method and confirmed with biochemical (Hy-Enterotest) and molecular methods (Benami et al. 2013). No other sample was detected as positive by either method. However, 19 of the 25 samples contained interfering organisms that were morphologically similar to S. enterica, thereby obfuscating and lengthening the detection effort (Table S5).
P. aeruginosa was not detected by either method in the environmental soil samples. However, by the culture-based method, nonspecific growth was noted on all selective agar plates and was verified to not be P. aeruginosa.
3.3 Aerosols
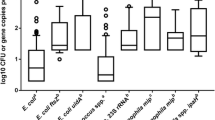
Validation of the MPN method for aerosols was first achieved with controlled laboratory experiments combining an aerosol generator (compressor nebulizer [A79-LIFE-Eagle Nebulizer NBB02]) and a vacuum-based collection method with an impinger (SKC Biosampler®). We employed four dilutions in the MPN format 100, 10−1, 10−2, and 10−3 CFU mL−1 in three replicates (Fig. 1), resulting in a possible range of concentrations between 3.6 and 105 CFU mL−1 (Blodgett 2010). The results are shown in Table S4 and were variable. We note that the recovery of P. aeruginosa was approximately two orders of magnitude lower than the inoculated concentrations (Fig. 3 and Table S4); thus, the LOD was calculated to be ∼300 cells mL−1 (Table 2).
Spread plate and MPN-qPCR quantification techniques comparison after spiking an aerosol generator with 103, 104, and 105 CFU mL−1 of P. aeruginosa and aerosolized for 1 h (n = 3). In environmental samples, the comparison is from P. aeruginosa aerosolized from domestic wastewater treatment systems (n = 9). Error bars refer to the 10th and 95th percentiles of the tested data. Red line indicates mean, solid line the median, and dashed line is the original average of the spiking amount in CFU mL−1
No P. aeruginosa colonies grew on plates inoculated directly from the liquid impinger spiked with 103 and 104 CFU mL−1 after 1 h of aerosolization (data not shown). Only 1–5 colonies grew on the spread plates inoculated from the liquid impingement spiked with 105 CFU mL−1 (data not shown). The LOD was calculated to be ∼105 CFU mL−1.
After the initial laboratory validation, the method was applied to field samples to test for the presence of aerosolized bacteria in the vicinity of a vertical flow constructed wetland treating domestic greywater (Gross et al. 2008). No P. aeruginosa colonies were recovered from the impinger liquid inoculated after collecting air for 1 h (data not shown). Detected concentrations of P. aeruginosa in the greywater aerosols were on average approximately 101 CFU for both settle plate and MPN-qPCR methods; however, the MPN-qPCR method detected up to 2-logs more P. aeruginosa in several samples (Fig. 3). However, the difference between the amounts measured by either method was not significant (p > 0.05).
3.4 Water and Tomato Surface Samples
The detection of S. enterica in tomato wash and water filtrates was performed in the MPN-qPCR developed method and, concomitantly, by culture-based techniques. The spiked samples were detected as positive by both basic enrichment-qPCR (100 % recovery) and the culture-based techniques (90 and 85 % recovery for tomato and water, respectively; Table 2). The non-spiked samples were negative in all methods.
4 Discussion
In this study, we modified the MPN-qPCR method by synthesizing several key aspects previously reported (Krämer et al. 2011; Russo et al. 2014) and adapting them to environmental uses, which are currently lacking. The developed method was applied as an a priori simultaneous screen of two pathogens of major concern and the protocol was adapted for rapid, high-throughput application (Fig. 1). The unequivocal demonstration of multiplex detection in this study indicates that the protocol can be expanded to include a broad number of pathogens simply by adding qPCR reagents.
The detection of pathogens in aerosols is challenging both technically and bacteriologically. Reliable methods for collecting and testing aerosols and even the reported units of measurement are debated (Agranovski 2007; Chinivasagam and Blackall 2005; Dabisch et al. 2012; Park et al. 2014). In addition, aerosolization and collection methods impose stress, resulting in damaged or viable but non-culturable (VBNC) bacteria (Rule et al. 2007; Terzieva et al. 1996). The handful of studies tested recovery rates and LOD of aerosolized bacteria, all reporting low recoveries (Ryan et al. 2014; Simon et al. 2011), often four to five orders of magnitude lower than spiking concentrations, and high LOD (Landman et al. 2013; Ryan et al. 2014). Here, we spiked an aerosol generator with relatively low concentrations of the tested cells ranging from 103 to 105 CFU mL−1 and could not detect P. aeruginosa in the collected air via the standard culture-dependent methods (plating onto selective agar) but only via the MPN-qPCR method after enrichment (Fig. 3). We suggest that the MPN-qPCR approach is a valuable tool to minimize false negatives and to screen for low pathogen concentrations in challenging matrices. It should also be noted that the choice of pathogens selected for this study was based on their epidemiological importance, yet it is probable that some adjustments will allow tailoring of the technique to any pathogen(s) of interest.
A previous report demonstrated a theoretical basis for matching the threshold cycle (Ct) of S. enterica culture assayed at log-phase with the initial density (Krämer et al. 2011). Here, we demonstrated a strong correlation between the Ct value and the initial bacterial density for P. aeruginosa, yet for S. enterica, this trend was not conclusive (Fig. 2, Tables S2 and S3). In general, generating standard curves (i.e., relying on Ct values) from environmental matrices are less reliable for quantification than utilizing an MPN-type approach because different instruments, reagents, and incubation conditions may affect the results (Töwe et al. 2010). This observation, coupled with instances of overestimation of P. aeruginosa in soil (Table S1), indicates that the proposed protocol provides a rapid and sensitive screening procedure, but may not be an exact quantification method.
The strategy proposed here might be more reliable than direct testing of environmental matrices due to the removal of inhibitors by dilution (Malorny et al. 2004) and nonviable bacteria by the pre-enrichment step (Heaton and Jones 2008). This observation is corroborated by the more variable and less sensitive results obtained from non-enriched samples of water, soil, and tomato wash (Table 2). However, the standard culture-based technique could be essentially as sensitive as the enriched qPCR method, but three major disadvantages were identified: (i) the prolonged exposure to putatively pathogenic isolates (Lazcka et al. 2007), (ii) the amount of time and resources needed for each sample (Straub et al. 2005), and (iii) the difficulty in obtaining a definitive answer by biochemical tests (Delgado et al. 2013).
5 Conclusion
Reliable and timely monitoring of pathogens of field samples is important for public health and environmental monitoring. The suggested method offers an accurate, inexpensive, high-throughput, and rapid alternative for screening of viable pathogens from various environmental samples. Further adaptation of this method to additional pathogens such as Legionella pneumophila, Staphylococcus aureus, and Campylobacter jejuni is recommended.
References
Agranovski, I. E. (2007). Personal sampler for viable airborne microorganisms: main development stages. CLEAN–Soil, Air, Water, 35(1), 111–117. doi:10.1002/clen.200600020.
Armbruster, D. A., & Pry, T. (2008). Limit of blank, limit of detection and limit of quantitation. The Clinical Biochemist Reviews, 29(Suppl 1), S49–S52. Accessed 3 November 2013.
Bae, S., & Wuertz, S. (2009). Discrimination of viable and dead fecal bacteroidales bacteria by quantitative PCR with propidium monoazide. Applied and Environmental Microbiology, 75(9), 2940–2944. doi:10.1128/AEM.01333-08.
Benami, M., Gross, A., Herzberg, M., Orlofsky, E., Vonshak, A., & Gillor, O. (2013). Assessment of pathogenic bacteria in treated graywater and irrigated soils. The Science of the Total Environment, 458–460, 298–302. doi:10.1016/j.scitotenv.2013.04.023.
Blodgett, R. (2010). Laboratory methods–BAM Appendix 2: most probable number from serial dilutions. WebContent. http://www.fda.gov/Food/FoodScienceResearch/LaboratoryMethods/ucm109656.htm. Accessed 14 October 2013
CDC. (2014). CDC - Pseudomonas aeruginosa in Healthcare Settings - HAI. http://www.cdc.gov/hai/organisms/pseudomonas.html. Accessed 30 June 2014
Chinivasagam, H. N., & Blackall, P. J. (2005). Investigation and application of methods for enumerating heterotrophs and Escherichia coli in the air within piggery sheds. Journal of Applied Microbiology, 98(5), 1137–1145. doi:10.1111/j.1365-2672.2005.02560.x.
Curiale, M. (2004). MPN Calculator. http://www.i2workout.com/mcuriale/mpn/. Accessed 5 August 2013
Dabisch, P., Bower, K., Dorsey, B., Wronka, L. (2012). Recovery efficiencies for Burkholderia thailandensis from various aerosol sampling media. Frontiers in Cellular and Infection Microbiology, 2. doi:10.3389/fcimb.2012.00078
Delgado, G., Souza, V., Morales, R., Cerritos, R., González-González, A., Méndez, J. L., et al. (2013). Genetic characterization of atypical Citrobacter freundii. PLoS One, 8(9), e74120. doi:10.1371/journal.pone.0074120.
Deloge-Abarkan, M., Ha, T.-L., Robine, E., Zmirou-Navier, D., & Mathieu, L. (2007). Detection of airborne Legionella while showering using liquid impingement and fluorescent in situ hybridization (FISH). Journal of Environmental Monitoring, 9(1), 91–97. doi:10.1039/B610737K.
Doyle, M. P., & Erickson, M. C. (2008). Summer meeting 2007 – the problems with fresh produce: an overview. Journal of Applied Microbiology, 105(2), 317–330.
Dunbar, J., White, S., & Forney, L. (1997). Genetic diversity through the looking glass: effect of enrichment bias. Applied and Environmental Microbiology, 63(4), 1326–1331. Accessed 22 October 2013.
Dusch, H., & Altwegg, M. (1995). Evaluation of five new plating media for isolation of Salmonella species. Journal of Clinical Microbiology, 33(4), 802–804.
Edel, W., & Kampelmacher, E. H. (1973). Comparative studies on the isolation of “sublethally injured” salmonellae in nine European laboratories. Bulletin of the World Health Organization, 48(2), 167–174. Accessed 21 October 2013.
FDA. (2011). In W. H. Andrews, A. Jacobson, & T. Hammack (Eds.), Bacteriological analytical manual (BAM), chapter 5, Salmonella. http://www.fda.gov/Food/ScienceResearch/LaboratoryMethods/BacteriologicalAnalyticalManualBAM/ucm070149.htm. Accessed 27 February 2013.
Girones, R., Ferrús, M. A., Alonso, J. L., Rodriguez-Manzano, J., Calgua, B., de Abreu Corrêa, A., et al. (2010). Molecular detection of pathogens in water–the pros and cons of molecular techniques. Water Research, 44(15), 4325–4339. doi:10.1016/j.watres.2010.06.030.
Griffin, D. W., Gonzalez, C., Teigell, N., Petrosky, T., Northup, D. E., & Lyles, M. (2011). Observations on the use of membrane filtration and liquid impingement to collect airborne microorganisms in various atmospheric environments. Aerobiologia, 27(1), 25–35. doi:10.1007/s10453-010-9173-z.
Gross, A., Sklarz, M. Y., Yakirevich, A., & Soares, M. I. M. (2008). Small scale recirculating vertical flow constructed wetland (RVFCW) for the treatment and reuse of wastewater. Water Science & Technology, 58(2), 487. doi:10.2166/wst.2008.367.
Haas, D., Unteregger, M., Habib, J., Galler, H., Marth, E., & Reinthaler, F. F. (2010). Exposure to bioaerosol from sewage systems. Water, Air, and Soil Pollution, 207(1–4), 49–56. doi:10.1007/s11270-009-0118-5.
Heaton, J. C., & Jones, K. (2008). Microbial contamination of fruit and vegetables and the behaviour of enteropathogens in the phyllosphere: a review. Journal of Applied Microbiology, 104(3), 613–626. doi:10.1111/j.1365-2672.2007.03587.x.
Hogan, C. J., Kettleson, E. M., Lee, M.-H., Ramaswami, B., Angenent, L. T., & Biswas, P. (2005). Sampling methodologies and dosage assessment techniques for submicrometre and ultrafine virus aerosol particles. Journal of Applied Microbiology, 99(6), 1422–1434. doi:10.1111/j.1365-2672.2005.02720.x.
Ishii, S., Segawa, T., & Okabe, S. (2013). Simultaneous quantification of multiple food- and waterborne pathogens by use of microfluidic quantitative PCR. Applied and Environmental Microbiology, 79(9), 2891–2898. doi:10.1128/AEM.00205-13.
Krämer, N., Löfström, C., Vigre, H., Hoorfar, J., Bunge, C., & Malorny, B. (2011). A novel strategy to obtain quantitative data for modelling: combined enrichment and real-time PCR for enumeration of salmonellae from pig carcasses. International Journal of Food Microbiology, 145(Supplement 1), S86–S95. doi:10.1016/j.ijfoodmicro.2010.08.026.
Landman, W. J. M., Feberwee, A., & van Eck, J. H. H. (2013). The effect of the air sampling method on the recovery of Mycoplasma gallisepticum from experimentally produced aerosols. Veterinary Quarterly, 33(2), 54–59. doi:10.1080/01652176.2013.799302.
Lazcka, O., Campo, F. J. D., & Muñoz, F. X. (2007). Pathogen detection: a perspective of traditional methods and biosensors. Biosensors and Bioelectronics, 22(7), 1205–1217. doi:10.1016/j.bios.2006.06.036.
Liu, L., Coenye, T., Burns, J. L., Whitby, P. W., Stull, T. L., & LiPuma, J. J. (2002). Ribosomal DNA-directed PCR for identification of Achromobacter (Alcaligenes) xylosoxidans recovered from sputum samples from cystic fibrosis patients. Journal of Clinical Microbiology, 40(4), 1210–1213. doi:10.1128/JCM.40.4.1210-1213.2002.
Malorny, B., Paccassoni, E., Fach, P., Bunge, C., Martin, A., & Helmuth, R. (2004). Diagnostic real-time PCR for detection of Salmonella in food. Applied and Environmental Microbiology, 70(12), 7046–7052. doi:10.1128/AEM.70.12.7046-7052.2004.
Napoli, C., Marcotrigiano, V., & Montagna, M. (2012). Air sampling procedures to evaluate microbial contamination: a comparison between active and passive methods in operating theatres. BMC Public Health, 12(1), 594. doi:10.1186/1471-2458-12-594.
Noble, R. T., & Weisberg, S. B. (2005). A review of technologies for rapid detection of bacteria in recreational waters. Journal of Water and Health, 3(4), 381–392.
Park, C. W., Park, J.-W., Lee, S. H., & Hwang, J. (2014). Real-time monitoring of bioaerosols via cell-lysis by air ion and ATP bioluminescence detection. Biosensors and Bioelectronics, 52, 379–383. doi:10.1016/j.bios.2013.09.015.
Qu, X., Alvarez, P. J. J., & Li, Q. (2013). Applications of nanotechnology in water and wastewater treatment. Water Research, 47(12), 3931–3946. doi:10.1016/j.watres.2012.09.058.
R Development Core Team. (2008). R: a language and environement for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. http://www.R-project.org. Accessed 2 May 2015
Rice, E. W., Baird, R. B., Eaton, A. D., Clesceri, L. S., American Public Health Association, American Water Works Association, & Water Environment Federation. (2012). Standard methods for examination of water and wastewater 2012. Washington, DC: American Public Health Assn.
Rule, A. M., Kesavan, J., Schwab, K. J., & Buckley, T. J. (2007). Application of flow cytometry for the assessment of preservation and recovery efficiency of bioaerosol samplers spiked with Pantoea agglomerans. Environmental Science & Technology, 41(7), 2467–2472. doi:10.1021/es062394l.
Russo, P., Botticella, G., Capozzi, V., Massa, S., Spano, G., & Beneduce, L. (2014). A fast, reliable, and sensitive method for detection and quantification of Listeria monocytogenes and Escherichia coli O157:H7 in ready-to-eat fresh-cut products by MPN-qPCR. BioMed Research International. doi:10.1155/2014/608296.
Ryan, S. P., Lee, S. D., Calfee, M. W., Wood, J. P., McDonald, S., Clayton, M., et al. (2014). Effect of inoculation method on the determination of decontamination efficacy against Bacillus spores. World Journal of Microbiology and Biotechnology, 30(10), 2609–2623. doi:10.1007/s11274-014-1684-2.
Schriewer, A., Wehlmann, A., & Wuertz, S. (2011). Improving qPCR efficiency in environmental samples by selective removal of humic acids with DAX-8. Journal of Microbiological Methods, 85(1), 16–21. doi:10.1016/j.mimet.2010.12.027.
Shannon, K. E., Lee, D.-Y., Trevors, J. T., & Beaudette, L. A. (2007). Application of real-time quantitative PCR for the detection of selected bacterial pathogens during municipal wastewater treatment. Science of The Total Environment, 382(1), 121–129.
Simon, X., Duquenne, P., Koehler, V., Piernot, C., Coulais, C., & Faure, M. (2011). Aerosolisation of Escherichia coli and associated endotoxin using an improved bubbling bioaerosol generator. Journal of Aerosol Science, 42(8), 517–531. doi:10.1016/j.jaerosci.2011.05.002.
Spilker, T., Coenye, T., Vandamme, P., & LiPuma, J. J. (2004). PCR-based assay for differentiation of Pseudomonas aeruginosa from other Pseudomonas species recovered from cystic fibrosis patients. Journal of Clinical Microbiology, 42(5), 2074–2079. doi:10.1128/JCM.42.5.2074-2079.2004.
Straub, T. M., Dockendorff, B. P., Quiñonez-Díaz, M. D., Valdez, C. O., Shutthanandan, J. I., Tarasevich, B. J., et al. (2005). Automated methods for multiplexed pathogen detection. Journal of Microbiological Methods, 62(3), 303–316. doi:10.1016/j.mimet.2005.04.012.
Terzieva, S., Donnelly, J., Ulevicius, V., Grinshpun, S. A., Willeke, K., Stelma, G. N., & Brenner, K. P. (1996). Comparison of methods for detection and enumeration of airborne microorganisms collected by liquid impingement. Applied and Environmental Microbiology, 62(7), 2264–2272.
Töwe, S., Kleineidam, K., & Schloter, M. (2010). Differences in amplification efficiency of standard curves in quantitative real-time PCR assays and consequences for gene quantification in environmental samples. Journal of Microbiological Methods, 82(3), 338–341. doi:10.1016/j.mimet.2010.07.005.
USDA, Soil Survey Staff. (1975). Soil taxonomy: a basic system of soil classification for making and interpreting soil surveys. US Dept of Agric Handb 436. Washington, D.C.: US Govt Print Off.
Vikesland, P. J., & Wigginton, K. R. (2010). Nanomaterial enabled biosensors for pathogen monitoring—a review. Environmental Science & Technology, 44(10), 3656–3669. doi:10.1021/es903704z.
Warren, B. R., Yuk, H.-G., & Schneider, K. R. (2007). Detection of salmonella by flow-through immunocapture real-time PCR in selected foods within 8 hours. Journal of Food Protection, 70(4), 1002–1006.
Wright, A. C., Garrido, V., Debuex, G., Farrell-Evans, M., Mudbidri, A. A., & Otwell, W. S. (2007). Evaluation of postharvest-processed oysters by using PCR-based most-probable-number enumeration of Vibrio vulnificus bacteria. Applied and Environmental Microbiology, 73(22), 7477–7481. doi:10.1128/AEM.01118-07.
Yeung, S.-W., Lee, T. M.-H., Cai, H., & Hsing, I.-M. (2006). A DNA biochip for on-the-spot multiplexed pathogen identification. Nucleic Acids Research, 34(18), e118. doi:10.1093/nar/gkl702.
Acknowledgments
This research was supported by Research Grant No. CP-9033-09 from The US–Israel Binational Agricultural Research and Development Fund. EO and MB were supported by the Israeli Water Authority graduate fellowships (87227611 and 874130, respectively) and the Kreitman School for Graduate Studies. MB was also supported by the Zuck Maccabi Fund. MD was supported by a fellowship from Kraft food group and the MIT International Science and Technology Initiatives (MIT-MISTI). We acknowledge Ahuva Vonshak, Omar Bawab, and Wa’d Odeh for invaluable assistance.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Materials
Below is the link to the electronic supplementary material.
ESM 1
Table S1. MPN index of soil samples spiked with Salmonella enterica and Pseudomonas aeruginosa at concentrations covering three orders of magnitude and detected by qPCR preceded by pre-enrichment. Table S2. Average threshold cycle (Ct) and ending relative fluoresence units (RFU) obtained from qPCR assays of three soil types spiked with Salmonella enterica at concentrations covering three orders of magnitude and pre-enriched in a dilution series amenable to MPN quantification. Table S3. Average threshold cycle (Ct) and ending relative fluoresence units (RFU) obtained from qPCR assays of three soil types spiked with Pseudomonas aeruginosa at concentrations covering three orders of magnitude and pre-enriched in a dilution series amenable to MPN quantification. Table S4. MPN index of liquid impinger collected air samples after spiked Pseudomonas aeruginosa into nebulizer and allowed to aerosolize the bacteria after 1 h. Concentrations covering three orders of magnitude and detected by qPCR preceded by pre-enrichment. Table S5. Results from MPN-qPCR targeting Salmonella enterica in environmental soil samples amended with composted poultry manure. (PDF 140 kb)
Rights and permissions
About this article
Cite this article
Orlofsky, E., Benami, M., Gross, A. et al. Rapid MPN-Qpcr Screening for Pathogens in Air, Soil, Water, and Agricultural Produce. Water Air Soil Pollut 226, 303 (2015). https://doi.org/10.1007/s11270-015-2560-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-015-2560-x