Abstract
An erythromycin-degrading bacterium was isolated from the activated sludge of a sewage treatment plant (STP). Based on the morphological and physiological characteristics, the isolated strain was identified and named as Pseudomonas sp. ERY-E. In an inorganic salt medium inoculated at 1 % (v/v) of ERY-E strain containing 50 mg/L of erythromycin (ERY), the removal efficiency of ERY as high as 83.7 % was obtained under the optimum conditions with temperature of 30 °C, pH of 7.0, and 10 mg/L of yeast as the external carbon source. Subsequently, the ERY-E strain was used for bioaugmenting a biological aerated filter (BAF) to treat surface water containing low-concentration ERY. The influence of hydraulic retention time (HRT) and air-liquid ratio (A/L) on the performance of BAF was investigated. The average removal efficiencies of ERY and permanganate index (CODMn) were about 60.6 and 26.1 % in bioaugmented system (BAF2) and 26.9 and 26.0 % in unbioaugmented system (BAF1), respectively, under the optimum conditions with HRT of 4.0 h and A/L of 4:1 at steady state. Due to the stable removal of CODMn in BAF2 as compared with BAF1, it can be concluded that the introduction of ERY-E strain could collaborate with the indigenous microorganisms to attain a better ERY removal efficiency. As a result, the bioaugmented BAF method can be considered as an alternative technology for the treatment of surface water containing low-concentration emerging pollutants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The presence of antibiotics in the environment has been drawn much attention due to their intrinsic bioactivity, chronic toxicity, antibiotic resistance, etc. (Gao et al. 2012a; González-Pleiter et al. 2013; Zhang et al. 2011). A growing number of studies reported that various antibiotics were found in the environmental waters, soils, and sediments at low concentrations (i.e., ng/L or μg/L for liquid phases and ng/g or μg/g for solid phases) (Gao et al. 2012b; Gao et al. 2015; Li et al. 2012; Michael et al. 2013; Yan et al. 2013). Erythromycin (ERY) is a macrolide antibiotic used in both veterinary and human medicine with a broad activity against a number of bacterial infections via inhibiting the aminoacyl translocation (Tenson et al. 2003). It has been reported that ERY is frequently detected in the wastewater effluent (Fatta-Kassinos et al. 2011). As a result numerous research studies were performed to assess the occurrence and overall removal of ERY in wastewater treatment systems. However, many studies indicated that ERY was recalcitrant during the biological treatment processes and the ERY removal efficiency was extremely low (Göbel et al. 2005; Yang et al. 2011). Finally, a considerable amount of ERY residue was released into the aquatic environment, contaminating the untreated drinking water sources (Focazio et al. 2008). In USA, ERY has been identified as one of the priority contaminants in the Drinking Water Contaminant Candidate List released by US Environmental Protection Agency (USEPA 2010). A recent published nationwide cohort survey in Denmark showed that an increasing probability of infantile hypertrophic pyloric stenosis in infants was associated with a long-term exposure to macrolide antibiotics, especially ERY (Lund et al. 2014). Therefore, as the wastewater effluent containing ERY residue was discharged, it is urgent and crucial to investigate the further removal of ERY from the contaminated surface water.
Recently, several studies have been focused on the toxic impact and removal of ERY in activated sludge systems (Louvet et al. 2010; Pala-Ozkok and Orhon 2013; Pala-Ozkok et al. 2014). However, there is a lack of information describing the removal of ERY in surface water treatment, especially applying bioaugmentation with functional bacteria, which has been shown to be an efficient and cost-effective technology for water treatment containing recalcitrant compounds (Bai et al. 2011; Payne et al. 2011). Furthermore, a biological aerated filter (BAF) is regarded as a novel and flexible fixed-film bioreactor at various stages of water treatment process. It can maintain high amounts of active biomass and hydraulic retention time (HRT), which are essential for the efficient degradation of recalcitrant compounds (Feng et al. 2012). In this context, the main objective of this study was to assess the bioaugmentation of BAF for the removal of low-concentration ERY in surface water using ERY-degrading strain isolated from activated sludge in a sewage treatment plant (STP). The effect of operating parameters including HRT and air-liquid ratio (A/L) on BAF performance was studied. This study aims to investigate the availability and feasibility of bioaugmentation technology in the treatment of surface water containing trace emerging pollutants in a BAF.
2 Materials and Methods
2.1 Chemicals
ERY (>98 % purity) was purchased from Acros Organics Company (Geel, Belgium). HPLC-grade methanol and acetonitrile were obtained from Sigma-Aldrich Chemical Company (St. Louis, MO, USA). Stock solution of ERY was prepared in methanol and stored at 4 °C prior to use. Other chemicals used were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Ultrapure water was produced using a Millipore Direct-Q device (Millipore, USA).
2.2 Isolation and Identification of ERY-Degrading Bacteria
Activated sludge was collected from the secondary sedimentation tank of a STP in Shanghai in China. The methods for isolation and identification of the ERY-degrading strain were referred to the general procedures reported in the literatures (Dong and Cai 2001; Shen and Chen 2007). Two grams of the sludge sample were added to 100 mL inorganic salt media (g/L) including K2HPO4 4.35, KH2PO4 1.7, NH4C1 2.1, FeSO4·7H2O 0.01, MgSO4 0.2, MnSO4 0.05, and CaC12·2H2O 0.03, pH 6.8~7.0, with 1 mg/L of ERY. The culture was incubated in the dark at 30 °C and shaken at 160 rpm in a rotary shaker (Shanghai DuKe Automation Equipment Co., Ltd.). The isolates were domesticated using a gradient method. For each cultivation cycle, 5 % of the isolates were picked for incubation with a concentration gradient of 10, 20, 40, 60, 80, and 100 mg/L of ERY. The culture medium was diluted with sterile normal saline by gradient in the range of 10−1~10−6. Subsequently, each 1 mL of the diluted medium was streaked onto solid culture media (g/L) containing peptone 10.0, beef extract 3.0, NaCl 5.0, and agar 17.0, pH 7.2. According to the morphological characteristics (i.e., size, shape, color, etc.) of the colonies, the most frequently detected colonies were inoculated into enrichment culture media (g/L) including peptone 10.0, beef extract 3.0, and NaCl 5.0, pH 7.4, and cultured at 30 °C for 24 h. Then, the enriched cells were harvested by centrifuging at a speed of 8000 rpm for 15 min, washed three times with 30 mL sterile normal saline, and transferred to 100 mL inorganic salt media with 50 mg/L of ERY. After cultivation for 5 days, the residual ERY in the inorganic salt media was measured. The strain with the highest removal efficiency of ERY was inoculated onto an enrichment culture media slant and stored at 4 °C.
For identification of ERY-degrading strain, the bacteria were inoculated onto enrichment culture media at 30 °C for 24 h until reaching the logarithmic phase of growth. The bacterial cells were harvested, and total DNA was extracted using a SK1201-UNIQ-10 DNA extraction kit (Sangon Biotech, China). PCR amplification of the 16S rDNA gene of the bacteria was performed with 27F and 1492R primer pairs. The PCR products were separated by agarose (1 %) gel electrophoresis, and the target DNA fragments were cut with a blade and purified with a UNIQ-10 purification kit (Sangon Biotech, China). The purified DNA fragments were cloned into plasmids according to the manufacturer’s protocol (Sangon Biotech, China). Positive clones were selected randomly for sequencing, which was conducted by Shanghai Invitrogen Biotechnology Co., Ltd., China. The obtained sequences were aligned and a phylogenetic tree was constructed with the neighbor joining method with MEGA 4.0 software (Tamura et al. 2007). The originated 16S rDNA gene sequence was deposited in the National Center for Biotechnology Information (NCBI) database under an accession number of JN226401.
Additionally, for observation of ERY-degrading strain, the isolated bacteria were cultured in enrichment culture media at 30 °C for 24 h. Then, the suspensions were centrifuged at 8000 rpm for 10 min. The supernatant was decanted and the precipitates were fixed with glutaraldehyde (2.5 %) and washed with phosphate buffer solution (pH 7.2). Afterward, the bacteria were processed by gradient dehydration using ethanol and subsequently lyophilized. Finally, the prepared samples were sent to Analytical Center, Donghua University, for morphological observation by a scanning electron microscope (SEM, JEOL JSM-5600, Japan).
2.3 Degradation Characteristics of ERY-Degrading Strain
The ERY-E strain cells were inoculated at 1 % (v/v, OD600 = 0.01) into 250-mL flasks containing 100 mL inorganic salt media and 50 mg/L of ERY each. The pH of the obtained solution was adjusted to 7.0 and incubated in the dark at 30 °C and shaken at 160 rpm in a rotary shaker. After 5 days, the residual ERY was measured. The impact of temperature (15–35 °C) and pH (5.0–9.0) on ERY degradation by ERY-E strains was investigated, and then the optimum conditions were determined. Also, the effect of external carbon sources on ERY degradation was studied by supplying with glucose, beef extract, and yeast of 10 mg/L separately under the optimized conditions as mentioned above.
2.4 BAF Configuration and Bioaugmentation Operation
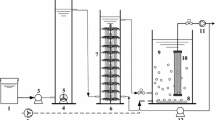
The schematic of the experimental setup is shown in Fig. 1. The two identical lab-scale BAFs were both made of plexiglass with a height of 150 cm and an inner diameter of 10 cm, which were filled with ceramic granular media with a depth of 100 cm. The ceramic granular media were obtained from a ceramics factory (Pingxiang, Jiangxi Province, China). The physical properties were summarized as follows: particle size of 2~4 mm, specific surface area of 3.99 m2/g, bulk density of 1.18 g/cm3, and total porosity of 47.2 %. After compaction, the packing density and porosity of the ceramic granular media in the columns are about 692 kg/m3 and 43.6 %, respectively. At the bottom, 10 cm of the column was filled with graded pebbles in the size of 10 cm. Aeration pipes with a pore diameter of 2 mm were installed on the surface of the graded gracel layer. The air was introduced into the columns using an air compressor, and the flow rate was controlled with an airflow meter. Raw influent was pumped to the BAFs from inlet in an up-flow mode by peristaltic pumps.
Schematic diagram of the BAFs (a) and SEM micrographs of ceramic granular media before (b) and after inoculation (c). BAF2 was bioaugmented with ERY-degrading strains, while BAF1 was not. (1) ceramic granular media; (2) feed tank; (3) peristaltic pump; (5) liquid flow meter; (6) graded pebbles; (7) air compressor; (8) airflow meter; (9) effluent port
At the beginning, two columns were seeded with the original activated sludge (16.3 g, dry weight) and domesticated with raw influent collected from a scenic lake in Donghua University, which was supplemented with 50 μg/L of ERY similar to the actual concentration present in the aquatic compartments. The raw influent quality was as follows: CODMn of 5.8~8.3 mg/L, turbidity of 15~28 NTU, pH of 6.5~7.8, and temperature of 14.8~19.6 °C. After 7 days, the column 2 (BAF2) was inoculated with the isolated ERY-E strain at the exponential phase at about 3 % of the column volume. The inoculation period was 14 days and then the stable operation began. The morphological difference before and after inoculation was presented in Fig. 1b, c. The steady running conditions were as follows: HRT of 4.0 h, A/L of 4:1, and total flow rate of 3 L/h. The dissolved oxygen (DO) in each column was controlled at around 3~5 mg/L.
2.5 Analytical Methods
Prior to measurement of ERY, the samples were firstly filtered through a 0.45-μm cellulose membrane. ERY concentrations were analyzed using an Agilent 1100 high-performance liquid chromatography equipped with a multi-wavelength UV detector. The analytes were separated by means of an XDB-C18 reverse-phase column (Eclipse, 150 mm × 4.6 mm, particle size 5 μm). Acetonitrile and K2HPO4 solution (pH 7.0 using 0.1 mol/L phosphoric acid) (v/v = 35:65) were used as the mobile phases in isocratic mode at a flow rate of 0.6 mL/min. The detection wavelength was set at 210 nm and the injection volume was 20 μL. The reported ERY concentrations were estimated using the external standard method.
The CODMn was analyzed using standard method (Ministry of Environmental Protection of People’s Republic of China 2006). The pH and DO were measured by a pH meter (Mettler Toledo S220-K, Switzerland) and a DO meter (Mettler Toledo SG6-FK2, Switzerland), respectively.
3 Results
3.1 Identification of ERY-Degrading Strain
One strain of ERY-degrading bacterium named as ERY-E was isolated from the activated sludge in a STP with the selective media. As shown in Fig. 2, the morphological characteristics of ERY-E strain colonies are 1.5~2.0 mm in diameter, circular, opaque, smooth surface, glossy edge, and milk white color. The cells are rod-shaped and the size is 0.5~1.5 μm in length. Biochemical assays were carried out to identify the strain. The results showed that Gram staining, glucose fermentation, methyl red, hydrogen sulfide production, citrate, and gelatin tests were negative and glucose production, starch hydrolysis, urease, catalase, oxidase, and V-P tests were positive. Additionally, the 16S rDNA gene sequence of the ERY-E strain was submitted to GenBank under an accession number JN226401. The phylogenetic tree constructed by neighbor joining method is shown in Fig. 3, indicating that ERY-E strain fell into the genus of Pseudomonas.
3.2 Factors Affecting the Degradation Capability of ERY-E Strain
Effect of temperature, initial pH, and external carbon source on ERY degradation by ERY-E strain was investigated. As shown in Fig. 4a, the ERY degradation rate increased as the temperature was raised from 15 to 30 °C. Nevertheless, the degradation dropped dramatically when the temperature was increased above 30 °C. The highest ERY degradation rate of 66.3 % was obtained at 30 °C. Furthermore, Fig. 4a clearly showed that the OD600 value decreased by 34 % as the temperature was increased from 30 to 35 °C. Therefore, the optimum temperature of 30 °C was chosen for the subsequent tests.
The effect of initial pH on the ERY degradation by ERY-E strain was also studied. It can be seen from Fig. 4b that the best ERY degradation as high as ~71 % was observed when the pH was in the range of 7.0~7.5. The cell biomass (OD600) as well as ERY degradation declined as the pH was out of this range. Therefore, the pH of 7.0 was regarded as the optimum value for ERY degradation by ERY-E strain.
Several studies reported that adding a second carbon source could potentially enhance degradation of the recalcitrant compounds (Fischer and Majewsky 2014; Tao et al. 2007). Herein, three external carbon sources including glucose, beef extract, and yeast were used to investigate their abilities to promote ERY degradation by ERY-E strain. As shown in Fig. 4c, the ERY removal rate was improved by 8.4, 12.9, and 18.3 % as glucose, beef extract, and yeast were added at a concentration of 10 mg/L, respectively. Accordingly, the OD600 value was increased as an external carbon source was added (data not shown).
3.3 Application of ERY-E Strain for Bioaugmentation in Lab-Scale BAFs
Generally, the presence of ERY in surface water is relatively low (Silva et al. 2011; Yan et al. 2013). Therefore, a concentration of 50 μg/L was added for the bioaugmentation experiments. As shown in Fig. 5, the bioaugmented system (BAF2) performed better ERY removal rate than the unbioaugmented system (BAF1) under the same operating conditions. The average removal efficiency of ERY in BAF2 was 60.6 %, while that in BAF1 was only 26.9 %. Nevertheless, the average removal efficiency of CODMn was about 26.0 % for both BAF1 and BAF2. The results indicated that the introduction of ERY-E strain did not compete with the indigenous biomass in BAF2, resulting in an improved ERY removal and a comparable removal efficiency of CODMn as compared with that in BAF1.
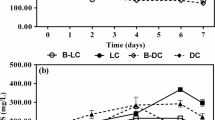
HRT is a key factor influencing the biological water treatment. Three different HRTs (i.e., 4.0, 2.4, and 1.5 h) were performed to investigate its impact on the removal of ERY and CODMn. A 7-day monitoring study was conducted for each HRT condition, respectively. As demonstrated in Fig. 6, the ERY removal rate decreased gradually as HRT reduced. The average removal efficiencies were 25.2, 20.6, and 13.4 % in BAF1 and 55.6, 50.5, and 39.6 % in BAF2, corresponding to HRT of 4.0, 2.4, and 1.5 h, respectively. Also, the removal of CODMn decreased slightly as HRT was reduced. The average removal efficiencies of CODMn were 25.7, 25.0, and 20.2 % in BAF1 and 25.2, 23.3, and 19.2 % in BAF2 under HRT of 4.0, 2.4, and 1.5 h, respectively.
A proper A/L is essential for the normal running in biological water treatment. Three different A/L (i.e., 2:1, 4:1, and 6:1) were tested to study its effect on the removal of ERY and CODMn at a HRT of 4.0 h as determined above. Under each A/L, a 7-day monitoring was performed and the effluent samples were measured daily. As shown in Fig. 7, the removal of ERY showed a small increase as A/L increased from 2:1 to 4:1. The average removal efficiencies of ERY were 16.7 and 22.0 % in BAF1, compared with 50.7 and 56.0 % in BAF2 under A/L of 2:1 and 4:1, respectively. Under an A/L of 6:1, the ERY removal decreased slightly in contrast with A/L of 4:1, such that the average removal efficiencies were 20.6 and 50.8 % in BAF1 and BAF2, respectively. Differently, the removal of CODMn showed a gradual increase as A/L ranged from 2:1 to 6:1, although the extent was not significant. Its average removal efficiencies were 19.2, 23.7, and 25.1 % in BAF1 with A/L of 2:1, 4:1, and 6:1, corresponding to 18.3, 22.8, and 23.1 % in BAF2.
4 Discussion
4.1 ERY-E Strain Analysis
Based on the morphological characterization and phylogenetic analysis, it can be obtained that ERY-E was mostly closed to a number of Pseudomonas isolates, including Pseudomonas putida strain 75 (100 % sequence similarity), Pseudomonas sp. RA-18 (100 % sequence similarity), and putida strain BAB-535 (100 % sequence similarity), etc. Pseudomonas spp. were metabolic versatile and were able to use a wide range of chemical compounds as carbon sources (Wasi et al. 2013). An ERY esterase was isolated and purified from an ERY-resistant Pseudomonas sp. previously (Kim et al. 2002). Such an enzyme is able to inactivate ERY and participates in the hydrolysis of the lactone ring of ERY (Barthélémy et al. 1984). Later on, a continuing study investigated the mineralization of ERY (Kim et al. 2004). This study suggested that ERY A is partially or completely mineralized by the sediment microbial populations containing ERY-resistant Pseudomonas sp. Our study was consistent with these previous experiments and indicated a positive link between ERY removal and Pseudomonas-related bacteria.
4.2 Optimization of ERY Degradation by ERY-E Strain
Usually, higher temperature can accelerate the bacterial biochemical reaction rates and then enhance the metabolic activity for pollutant degradation. However, as the temperature is higher than a certain value, such as 30 °C in this case, the enzymes responsible for pollutant degradation will probably be deactivated, resulting in the retardation of bacterial metabolites and the reduction of cell biomass, as indicated in Fig. 4a. Similar phenomenon was also observed in other studies (Xu et al. 2014; Yoon et al. 2003). Also, pH value is another key factor affecting the degrading performance of microorganisms. As evidently shown in Fig. 4b, the ERY removal efficiency decreased significantly as the pH was not optimal. Besides, the addition of an external carbon source was determined to increase the ERY removal (Fig. 4c). The possible explanation is that an external carbon source may promote the bacterial growth (Tao et al. 2007) and potentially trigger the secretion of more catabolic enzymes for ERY degradation.
4.3 Assessment of BAF Performance Bioaugmented with ERY-E Strain
As mentioned above, the ERY removal was significantly enhanced in the bioaugmented BAF system at steady state. This may be attributed to the addition of ERY-E bacteria, which acclimate to the biological system promptly and function immediately. Moreover, the removal of CODMn was not affected as ERY-E strain was introduced as demonstrated in Fig. 5. Several previous studies reported bioaugmentation failures by inoculating a single functional strain into the biological system concerning the acclimation of the inoculated strains, competition between the introduced species and the indigenous microorganisms, etc. (Bouchez et al. 2000, 2009). However, our results indicated that bioaugmentation of a BAF with ERY-E inoculation for efficient ERY degradation was feasible, without affecting the performance of the indigenous microorganisms as demonstrated by the stable CODMn removal rate above.
Normally, HRT and A/L are both important parameters for BAF operation. Shorter HRT means greater hydraulic loading, resulting in stronger shearing force. Although decreasing HRT can enhance the transfer rate of organic substances and dissolved oxygen (Wu et al. 2011), microorganisms in BAFs are supposed to be substantially affected. Higher hydraulic loading led to stronger scouring on the medium surface, causing a decrease in the biomass responsible for ERY and COD degradation. Overall, it can be observed that the influence of HRT on CODMn removal in both BAF1 and BAF2 was not significant under HRT of 4.0 and 2.4 h. However, a HRT of 4.0 h was selected in order to ensure high removal of ERY.
In addition, as indicated in Fig. 7, when A/L rose from 2:1 to 6:1, the DO concentration increased accordingly, resulting in an increase in the removal of CODMn. These results agree with other studies on the removal of organic matters in BAFs (Li et al. 2010; Liu et al. 2008; Wu et al. 2011). However, overhigh A/L will generate strong air shearing forces scouring the microorganisms including ERY-E strains on the medium surface, resulting in loss of biomass. The results showed that the best ERY removal of 56.0 % was achieved in BAF2 with A/L of 4:1.
5 Conclusion
In this study, we had isolated a strain of ERY-degrading bacterium named as ERY-E from activated sludge in a STP, which was finally identified as Pseudomonas sp. Based on the analyzed results, it can be observed that the removal efficiency of ERY as high as 83.7 % was obtained under the optimum conditions with temperature of 30 °C and pH of 7.0, as well as an external carbon source addition of yeast. Our experimental results showed that bioaugmentation with the ERY-E strain was found to be an efficient and feasible way to enhance the ERY removal in a BAF. The average removal efficiency of ERY was increased from 26.9 to 60.6 % as the ERY-E strain was inoculated at the operation conditions with HRT of 4.0 h and A/L of 4:1, while CODMn removal remained stable at around 26 %, indicating that the introduction of ERY-E strain could collaborate with other indigenous microorganisms to achieve a favorable ERY removal efficiency. Based on the above results, it can be concluded that bioaugmented BAF is a potentially alternative technology for the treatment of surface water containing low-concentration emerging pollutants.
References
Bai, Y. H., Sun, Q. H., Sun, R. H., Wen, D. H., & Tang, X. Y. (2011). Bioaugmentation and adsorption treatment of coking wastewater containing pyridine and quinoline using zeolite-biological aerated filters. Environmental Science and Technology, 45(5), 1940–1948.
Barthélémy, P., Autisser, D., Gerbaud, G., & Courvalin, P. (1984). Enzymatic hydrolysis of erythromycin by Escherichia coli. A new mechanism of resistance. Journal of Antibiotics, 37(12), 1692–1696.
Bouchez, T., Patureau, D., Dabert, P., Juretschko, S., Doré, J., Delgenès, P., Moletta, R., & Wagner, M. (2000). Ecological study of a bioaugmentation failure. Environmental Microbiology, 2(2), 179–190.
Bouchez, T., Patureau, D., Delgenès, J. P., & Moletta, R. (2009). Successful bacterial incorporation into activated sludge flocs using alginate. Bioresource Technology, 100(2), 1031–1032.
Dong, X. Z., & Cai, M. Y. (2001). Handbook of Common Bacteria Identification System. Beijing: Science Press.
Fatta-Kassinos, D., Meric, S., & Nikolaou, A. (2011). Pharmaceutical residues in environmental waters and wastewater: current state of knowledge and future research. Analytical and Bioanalytical Chemistry, 399(1), 251–275.
Feng, Y., Yu, Y. Z., Qiu, L. P., Zhang, J. W., & Gao, L. L. (2012). The characteristics and application of grain-slag media in a biological aerated filter (BAF). Journal of Industrial and Engineering Chemistry, 18(3), 1051–1057.
Fischer, K., & Majewsky, M. (2014). Cometabolic degradation of organic wastewater micropollutants by activated sludge and sludge-inherent microorganisms. Applied Microbiology and Biotechnology, 98(15), 6583–6597.
Focazio, M. J., Kolpin, D. W., Barnes, K. K., Furlong, E. T., Meyer, M. T., Zaugg, S. D., Barber, L. B., & Thurman, M. E. (2008). A national reconnaissance for pharmaceuticals and other organic wastewater contaminants in the United States - II) Untreated drinking water sources. Science of the Total Environment, 402(2-3), 201–216.
Gao, P., Munir, M., & Xagoraraki, I. (2012a). Correlation of tetracycline and sulfonamide antibiotics with corresponding resistance genes and resistant bacteria in a conventional municipal wastewater treatment plant. Science of the Total Environment, 421–422, 173–183.
Gao, P., Ding, Y. J., Li, H., & Xagoraraki, I. (2012b). Occurrence of pharmaceuticals in a municipal wastewater treatment plant: Mass balance and removal processes. Chemosphere, 88(1), 17–24.
Gao, P., He, S., Huang, S. L., Li, K. Z., Liu, Z. H., Xue, G., & Sun, W. M. (2015). Impacts of coexisting antibiotics, antibacterial residues, and heavy metals on the occurrence of erythromycin resistance genes in urban wastewater. Applied Microbiology and Biotechnology, 99, 3971–3980.
Göbel, A., Thomsen, A., McArdell, C. S., Joss, A., & Giger, W. (2005). Occurrence and sorption behavior of sulfonamide, macrolides, and trimethoprim in activated sludge treatment. Environmental Science and Technology, 39(11), 3981–3989.
González-Pleiter, M., Gonzalo, S., Rodea-Palomares, I., Leganés, F., Rosal, R., Boltes, K., Marco, E., & Fernández-Piñas, F. (2013). Toxicity of five antibiotics and their mixtures towards photosynthetic aquatic organisms: Implications for environmental risk assessment. Water Research, 47(6), 2050–2064.
Kim, Y. H., Cha, C. J., & Cerniglia, C. E. (2002). Purification and characterization of an erythromycin esterase from an erythromycin-resistant Pseudomonas sp. FEMS Microbiology Letters, 210(2), 239–244.
Kim, Y. H., Pak, K., Pothuluri, J. V., & Cerniglia, C. E. (2004). Mineralization of erythromycin A in aquaculture sediments. FEMS Microbiology Letters, 234(1), 169–175.
Li, S. P., Cui, J. J., Zhang, Q. L., Fu, J., Lian, J. F., & Li, C. (2010). Performance of blast furnace dust clay sodium silicate ceramic particles (BCSCP) for brewery wastewater treatment in a biological aerated filter. Desalination, 258(1-3), 12–18.
Li, W. H., Shi, Y. L., Gao, L. H., Liu, J. M., & Cai, Y. Q. (2012). Occurrence of antibiotics in water, sediments, aquatic plants, and animals from Baiyangdian Lake in North China. Chemosphere, 89(11), 1307–1315.
Liu, F., Zhao, C. C., Zhao, D. F., & Liu, G. H. (2008). Tertiary treatment of textile wastewater with combined media biological aerated filter (CMBAF) at different hydraulic loadings and dissolved oxygen concentrations. Journal of Hazardous Materials, 160(1), 161–167.
Louvet, J. N., Heluin, Y., Attik, G., Dumas, D., Potier, O., & Pons, M. N. (2010). Assessment of erythromycin toxicity on activated sludge via batch experiments and microscopic techniques (epifluorescence and CLSM). Process Biochemistry, 45(11), 1787–1794.
Lund, M., Pasternak, B., Davidsen, R. B., Feenstra, B., Krogh, C., Diaz, L. J., Wohlfahrt, J., & Melbye, M. (2014). Use of macrolides in mother and child and risk of infantile hypertrophic pyloric stenosis: nationwide cohort study. BMJ, 348, 1908–1917.
Michael, I., Rizzo, L., McAcdell, C. S., Manaia, C. M., Merlin, C., Schwartz, T., Dagot, C., & Fatta-Kassinos, D. (2013). Urban wastewater treatment plants as hotspots for the release of antibiotics in the environment: A review. Water Research, 47(3), 957–995.
Ministry of Environmental Protection of People’s Republic of China. (2006). Monitoring and analysis method of water and wastewater (4th ed.). Beijing: China Environmental Science Press (in Chinese).
Pala-Ozkok, I., & Orhon, D. (2013). Chronic effect of erythromycin on substrate biodegradation kinetics of activated sludge. Biochemical Engineering Journal, 81, 29–39.
Pala-Ozkok, I., Rehman, A., Ubay-Cokgor, E., Jonas, D., & Orhon, D. (2014). Pyrosequencing reveals the inhibitory impact of chronic exposure to erythromycin on activated sludge bacterial community structure. Biochemical Engineering Journal, 90, 195–205.
Payne, R. B., May, H. D., & Sowers, K. R. (2011). Enhanced reductive dechlorination of polychlorinated biphenyl impacted sediment by bioaugmentation with a dehalorespiring bacterium. Environmental Science and Technology, 45(20), 8772–8779.
Shen, P., & Chen, X. D. (2007). Microbiology Experiment (4th ed.). Beijing: Higher Education Press.
Silva, B. F. D., Jelic, A., López-Serna, R., Mozeto, A. A., Petrovic, M., & Barceló, D. (2011). Occurrence and distribution of pharmaceuticals in surface water, suspended solids and sediments of the Ebro river basin, Spain. Chemosphere, 85(5), 1331–1339.
Tamura, K., Dudley, J., Nei, M., & Kumar, S. (2007). MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Molecular Biology and Evolution, 24(8), 1596–1599.
Tao, X. Q., Lu, G. N., Dang, Z., Yang, C., & Yi, X. Y. (2007). A phenanthrene-degrading strain Sphingomonas sp. GY2B isolated from contaminated soils. Process Biochemistry, 42(3), 401–408.
Tenson, T., Lovmar, M., & Ehrenberg, M. (2003). The mechanism of action of macrolides, lincosamides and streptogramin B reveals the nascent peptide exit path in the ribosome. Journal of Molecular Biology, 330(5), 1005–1014.
USEPA (2010) Drinking Water Contaminant Candidate List 3 - CCL. http://water.epa.gov/scitech/drinkingwater/dws/ccl/ccl3.cfm
Wasi, S., Tabrez, S., & Ahmad, M. (2013). Use of Pseudomonas spp. for the bioremediation of environmental pollutants: a review. Environmental Monitoring and Assessment, 185(10), 8147–8155.
Wu, S. Q., Yue, Q. Y., Qi, Y. F., Gao, B. Y., Han, S. X., & Yue, M. (2011). Preparation of ultra-lightweight sludge ceramics (ULSC) and application for pharmaceutical advanced wastewater treatment in a biological aerobic filter (BAF). Bioresource Technology, 102(3), 2296–2300.
Xu, B. J., Gao, P., Liu, Z. H., Xue, G., Liu, Y. N., & Wu, F. (2014). Influence of cosubstrates on iopromide degradation by Pseudomonas sp. I-24. Water, Air, and Soil Pollution, 225(2), 1–8.
Yan, C. X., Yang, Y., Zhou, J. L., Liu, M., Nie, M. H., Shi, H., & Gu, L. J. (2013). Antibiotics in the surface water of the Yangtze Estuary: Occurrence, distribution and risk assessment. Environmental Pollution, 175, 22–29.
Yang, X., Flowers, R. C., Weinberg, H. S., & Singer, P. C. (2011). Occurrence and removal of pharmaceuticals and personal care products (PPCPs) in an advanced wastewater reclamation plant. Water Research, 45(16), 5218–5228.
Yoon, J. H., Oh, H. M., Yoon, B. D., Kang, K. H., & Park, Y. H. (2003). Paenibacillus kribbensis sp. nov. and Paenibacillus terrae ap. nov., bioflocculants for efficient harvesting of algal cells. International Journal of Systematic and Evolutionary Microbiology, 53(1), 295–301.
Zhang, Q. C., Lambert, G., Liao, D., Kim, H., Robin, K., Tung, C., Pourmand, N., & Austin, R. H. (2011). Acceleration of emergence of bacterial antibiotic resistance in connected microenvironments. Science, 333(6050), 1764–1767.
Acknowledgments
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (51208086, 51178093), the Shanghai Pujiang Program (13PJ1400100), the DHU Distinguished Young Professor Program, and the Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Gao, P., Wei, X., Gu, C. et al. Isolation and Characterization of an Erythromycin-Degrading Strain and Application for Bioaugmentation in a Biological Aerated Filter. Water Air Soil Pollut 226, 190 (2015). https://doi.org/10.1007/s11270-015-2449-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-015-2449-8