Abstract
Biochar, a by-product resulting from the pyrolysis of biomass, is considered to be an anthropogenic carbonaceous sorbent. Despite a worldwide increase in the application of biochar on agricultural fields to improve crop productivity over the past few decades, there have been few studies on their influences on the sorption of environmental contaminants. In a field-based study at two experimental sites in Denmark, we investigated the effect of birch wood-derived biochar (Skogans kol) on the sorption of phenanthrene in soils with different properties. The soil sorption coefficient, K d (L kg−1), of phenanthrene was measured on sandy loam and loamy sand soils which have received from zero up to 100 t ha−1 of biochar. Results show that birch wood biochar had a higher K d compared to soils. Furthermore, the application of birch wood biochar enhanced the sorption of phenanthrene in agricultural soils, and the enhancement effect increased with an increasing biochar application rate. Aging, repeated application, and higher clay content suppressed the biochar enhancement effect on the sorption of phenanthrene. Phenanthrene K d was found to be strongly and positively correlated with both total and non-complexed organic carbon, while negatively correlated with clay content. The results also revealed that biochar–mineral interactions play an important role in the sorption of phenanthrene in biochar-amended soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Biochar, a carbon-rich by-product of the pyrolysis of biomass (Kookana et al. 2011), has gained widespread popularity in the last few years as a soil amendment. The carbonaceous structure of biochar is mainly amorphous with localized crystalline and highly conjugated aromatic regions (Graber et al. 2011). Biochar possesses a wide range of physical and chemical characteristics, and its high specific surface area (SSA), aromaticity, microporosity, pH, cation exchange capacity (CEC), and surface heterogeneity have given it an extraordinary capacity for the sorption of chemicals (Kookana et al. 2011). In addition, the organic substances produced during the pyrolysis process (Lin et al. 2011) impart a high sorption capacity to biochar, which is far greater than those of humic substances and soil organic matter (Sheng et al. 2005; Zhang et al. 2006). A broad range of studies quantifying the sorption of numerous chemicals onto biochar based on the sorption coefficient K d (L kg−1) and organic carbon normalized sorption coefficient K oc (L kg−1) can be found in literature. For example, Kleineidam et al. (1999) investigated the sorption of phenanthrene and found exceptionally high K oc values for charcoal and coal. Sun et al. (2013) working with different biochars produced from different feedstocks and at different temperatures observed vast variations in phenanthrene K d and K oc between biochars. Zhang et al. (2010) examined phenanthrene sorption on different soils with freshly spiked biochar and concluded that the biochar applications may modify the soil’s sorption potential of hydrophobic contaminants, and that the enhancement should be more pronounced for soils with low initial organic carbon contents. Spokas et al. (2009), working with a biochar produced from sawdust at 500 °C, noted that on a mass basis biochar was a less effective organic sorbent for two herbicides (atrazine and acetochlor) compared to other forms of soil organic carbon. Clearly, not all types of biochar have the same sorption properties.
Due to the kinetics of physical and biogeochemical interactions of biochar in soil, the properties of biochar may change over time (Martin et al. 2012), a process commonly referred to as aging. Aging may have a substantial effect on the sorption capacity of biochar. For example, the sorption of environmental constituents such as natural organic matter can block micropores in biochar, thereby restricting the sorption of organic contaminants over time (Pignatello et al. 2006). Kookana et al. (2011) reported that the true sorption capacity of biochar for organic molecules can be suppressed by soil minerals with time. Furthermore, pores in biochar particles can themselves become occluded and inaccessible over time and thereby lose the ability to trap molecules (Graber et al. 2011). Of the mechanisms discussed above, two or more are also likely to occur at the same time, which makes sorption in biochar-amended soil a rather complicated process. In addition to aging, repeated application of biochar to a field may also cause different structural and chemical changes to soil and the biochar itself, thereby controlling the sorption characteristics. Despite the common practice of biochar reapplication on agricultural fields, a limited number of studies have focused on the effect of reapplication on sorption properties. For example, Quilliam et al. (2012) documented that the reapplication of biochar had a significant effect on soil quality. However, to the authors’ knowledge, no studies have evaluated in detail the effect of reapplication of biochar on the soil sorption of phenanthrene.
Phenanthrene is a polycyclic aromatic hydrocarbon (PAH) with a molecular structure distinctly characterized by three fused benzene rings and it is widely used as a model organic contaminant in various studies. It typically exhibits strong hydrophobicity, very low water solubility (0.6 mg L−1), and high octanol/water distribution coefficients (K ow) (log (K ow) = 4.57). PAHs are commonly found in soils and sediments. Atmospheric deposition, the use of compost and fertilizers, and the application of effluent from public sewage treatment plants are among its potential sources (Soares et al. 2013). The presence of these compounds in the environment has raised concerns because of associated health risks due to their carcinogenic and mutagenic behavior (Shin et al. 2008). Owing to their strong affinity for geosorbents, sorption is considered to be one of the most important mechanisms in soil that can potentially control the fate and transport of PAHs (Zhang et al. 2010). It has been observed that organic matter governs the sorption of phenanthrene in soil (Ahangar 2010). Cornelissen and Gustafsson (2005) showed that amorphous organic carbon may greatly influence the sorption of phenanthrene in sediment. In a laboratory-based study, Zhang et al. (2010) evaluated how biochar influenced the sorption of phenanthrene in soil. However, the role of biochar on the sorption of phenanthrene onto soil under ambient field conditions is largely unknown and needs further investigations.
The main objective of this study was to investigate the sorption of phenanthrene in biochar-amended soils under different application rates, repeated application, aging, and different soil physiochemical properties. The biochar-amended soils considered in this study were taken from experimental sites in Denmark and therefore were exposed to natural aging in fields under temperate climate and environmental conditions. For comparison, we also used literature data on Danish agricultural soils without biochar, but with natural organic carbon contents broadly comparable to the soils used in this study.
2 Materials and Methods
2.1 Chemicals
Ring-UL-14C-phenanthrene (22.4 × 104 Bq L−1, specific activity = 55 mCi mmol−1; radiochemical purity ≥99 %) from American Radiolabeled Chemicals, Inc. (St. Louis, MO) was used in the experiments.
2.2 Biochar
A commercial biochar (Skogens Kol AB, Sweden) produced from birch wood (pyrolyzed at 500 °C) was used in this study. Its properties are fully characterized in the EU Interreg Biochar program, where the selected biochar is used as one of the model biochars in soil fertility experiments. The biochar had 81 % C, 0.24 % N, pH = 9.6, specific surface area (SSA) = 322 m2 g−1, and CEC = 9.26 cmol kg−1. Additional characteristics can be found in Kumari et al. (2014).
2.3 Study Sites, Soils, and Analysis
A total of 20 soil samples were collected from two biochar-amended agricultural sites located at Risoe (55°41′N, 12°05′E) and Kalundborg (55°42'N, 11°18'E) in Denmark. The soils from the two sites are different with respect to physicochemical properties, biochar application rates, and aging. The soil at Risoe site is a sandy loam (Table 1) and a total of eight plots were established at the site (Fig. 1a). The field experiment was block-designed and biochar was applied to selected plots at two different rates (0 and 20 t ha−1). A distinct natural pH gradient was observed across the field. A detailed characterization of soil and biochar at the Risoe experimental site, together with a description on the sampling, can be found in Kumari et al. (2014).
The soil at Kalundborg is loamy sand (Table 1) and 12 plots, each measuring 6 m × 6 m, were established at the site (Fig. 1b). Biochar was incorporated by harrowing into the 0–10-cm layer at different rates, ranging from 0 to 50 t ha−1. Plots K1–K4 received biochar in 2011 while plots K5–K8 received biochar in 2012. Plots K9 to K12, on the other hand, received biochar in both 2011 and 2012, in each year at a rate ranging between 0 to 50 t ha−1, thereby making the total application rate vary between 0 and 100 t ha−1. Animal manure was also applied in 2011 at 21 t ha−1 (plots K1 to K8) and 42 t ha−1 (plots K9 to K12). Bulk soil samples were collected from 0 to 20 cm depth from six sampling points (see Fig. 1b) and pooled to one composite sample per plot. The procedure for the analysis of basic soil properties is explained in detail in Kumari et al. (2014). The physicochemical properties of soils are given in Table 1.
2.4 Sorption Experiment
The sorption coefficient (K d) of phenanthrene was obtained in a batch equilibration experiment on three replicates. Soil aliquots (0.5 g) were hydrated with 0.5 mL of 2 mM CaCl2 for 24 h in glass centrifuge tubes closed with Teflon caps. Nine milliliters of aqueous solutions of phenanthrene (0.12 mg L−1) was added and the samples rotated end-over-end (30 rpm) for another 24 h at 20 ± 2 °C followed by centrifugation at 5,000 rpm for 1 h. Three-milliliter from the supernatant were taken and mixed with 17 mL of scintillation cocktail (Packard Ultima Gold). The concentrations were quantified using a liquid scintillation analyzer (Packard 2250 CA, Downers Grove, IL). The amount of compound sorbed was calculated by the difference between the initial and final solution concentrations. Tubes without soil were used to give the initial amount of 14C activity. No significant adsorption of the test substances occurred on the tubes.
2.5 Calculation of Sorption Parameters
The sorption coefficient of phenanthrene, K d [L kg−1], was calculated by
where C s is the amount of compound sorbed by the soil [g kg−1] and C e is the compound concentration of the soil solution at equilibrium [g L−1].
The amount of phenanthrene sorption per unit soil organic carbon (SOC), K oc, is given by
where f oc is organic carbon content [kg kg−1].
The phenanthrene K oc can be estimated from the octanol/water distribution coefficient, K ow, based on the following empirical relation:
where A and B are model empirical coefficients. Karickhoff (1981) suggested A = 0.989 and B = 0.346 based on measurements on soils and sediments with SOC content between 0.001 and 0.024 kg kg−1 while Abdul et al. (1987) proposed A = 1.04 and B = 0.88 for sediments with SOC ranging between 0.004 and 0.020 kg kg−1.
In order to analyze the effects of non-complexed organic carbon (NCOC) on phenanthrene sorption, NCOC in soil was calculated based on the approach taken by Dexter et al. (2008) given in Eq. [4], assuming a value of n = 10 is valid for the identification of NCOC in both reference and biochar-amended soils.
2.6 Statistical Analyses
Statistical testing of K d for Risoe soils was done using a mixed-effects model including soil pH, biochar, and their interaction as fixed effects and block as a random effect (Montgomery 2013). Data analysis was done with R version 3.0.2 (R Core Team 2013) and the R package lme4 (function lmer with REML=FALSE). P values for effects of soil pH, biochar, and their interaction were obtained by Likelihood ratio tests (function ANOVA) of models with and without the tested effects (Winter 2013).
The absence of true replicates at the Kalundborg experimental site did not permit us a full statistical analysis of the effects of biochar on phenanthrene sorption. Alternatively, as an approximate approach, significant effects of biochar were tested for individual years of biochar application (i.e., 2011, 2012 and 2011 + 2012) as suggested by Sun et al. (2014). A representative soil sample for each treatment was obtained by combining six individual soil samples collected from six different sampling points in each plot. We envisage that the subsamples of this composite soil sample will adequately reflect the field-scale variation in soil properties. Analyses of variance were performed using the Holm–Sidak test (α = 0.05) in SigmaPlot 11.0 (Systat Software, San Jose, CA).
Linear relationships between measured variables were evaluated by the Pearson test. All procedures were performed at P < 0.05.
3 Results and Discussion
3.1 Physicochemical Properties of Soils
The clay content of the soils, irrespective of their location, varied from 0.08 to 0.17 kg kg−1 (Table 1), with the Risoe soils having a higher clay content than the Kalundborg soils. With respect to soil pH, Risoe soils were alkaline with a pH gradient (7.4–8.3) while Kalundborg soils were slightly acidic to neutral (6.3–7.0). When considering organic carbon content, Risoe soils had a lower organic carbon content than Kalundborg soils and there were significant differences in organic carbon content between reference and biochar-amended plots. The organic carbon content increased with the biochar application rate. For example, in 7-month-aged soils from Kalundborg, the organic carbon content increased by 18, 52, and 136 % at biochar application rates (t ha−1) of 10, 20, and 50, respectively, compared to reference soils. A similar increasing trend (14, 38, and 70 %) was observed for 19-month-aged soils as well. However, it was evident that in longer aged soils, the increase in organic carbon content due to biochar addition declined, and the decline was more pronounced at higher biochar application rates. This implies that in biochar-amended soils, the organic carbon content declines with time and the degree of decline depends on the initial biochar application rate. The exact reason for the decline in organic carbon content with time in biochar-amended soils is not clear and needs further research to clarify it.
3.2 Phenanthrene Sorption Onto Fresh Biochar and Soils
The phenanthrene sorption parameters (K d and K oc) onto fresh biochar are presented in Table 2. Note the markedly higher phenanthrene K d (=7,254 L kg−1) on Skogens kol biochar compared to that of soil, implying favorable conditions for phenanthrene sorption onto biochar. This is not surprising since polycyclic aromatic hydrocarbons are strongly sorbed to the biochar surface through specific π-π interactions between the aromatic rings of PAHs and those of the biochar (Smernik 2009). Entrapment into small and narrow pores in biochar can also be considered to be another possible mechanism for increased sorption. Sun et al. (2013) studied phenanthrene sorption on various types of biochar produced from different feedstocks and temperatures, and observed phenanthrene K d varying from 3.1 to 38,019 L kg−1, suggesting that phenanthrene sorption is highly dependent on the characteristics of the biochar.
As expected with the high phenanthrene K d observed with pure biochar, an enhanced sorption coefficient of phenanthrene onto biochar-amended soil was observed, implying a direct positive effect of biochar amendment on phenanthrene sorption. This is in line with the results from Zhang et al. (2010) who related the sorption mechanism of phenanthrene to partitioning into soil organic matter and biochar. Entrapment of phenanthrene molecules in meso and microporous structures within soil aggregates, organic matter, and biochar is another possible mechanism (Carroll et al. 1994).
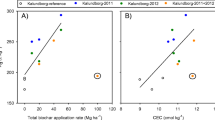
3.3 Effects of Biochar Application Rate on Sorption of Phenanthrene
Phenanthrene sorption increased continuously with an increasing biochar application rate (Fig. 2). This enhancement became more pronounced at higher biochar application rates. For example, in Kalundborg soils after seven months of biochar amendment, phenanthrene K d increased by 13 %, 26 % and 93 % at biochar application rates (t ha−1) of 10, 20, and 50, respectively. Similar results were also observed by Zhang et al. (2010) but provided no clear explanation of enhanced sorption at higher application rates. Presumably, this is due to the pronounced negative effect of mineral–biochar interactions on sorption at lower application rates. It is known that biochar in soils potentially interacts with soil minerals (Laird et al. 2008; Liang et al. 2008), obscuring sorption sites on biochar. The number of vacant sites for sorption becomes higher at higher application rates compared to the lower rate, resulting in enhanced sorption. In a recent study, Sun et al. (2013) reported that the interactions between biochar and soil minerals affect sorption and hence supported the present hypothesis. This is supported also by many previous studies that found extensive portions of biochar in the organo-mineral fraction of soils (Brodowski et al. 2006; Laird et al. 2008; Liang et al. 2008), strongly suggesting biochar–mineral interactions. However, further studies required to arrive at a clear conclusion
Variation of phenanthrene K d at different biochar application rates (0–50 t ha−1) in Risoe and Kalundborg experimental sites. Note that for Kalundborg (2011 + 2012), the soils received double doses; therefore, the biochar amount corresponds to 0–100 t ha−1. Data for K d are shown as mean ± standard error. Significant effects of biochar were tested for individual years of biochar applications (i.e., 2011, 2012, and 2011 + 2012) on Kalundborg soils by a Holm–Sidak test. Different letters indicate the significant difference. For Risoe soils, significant effects of biochar were tested using a mixed-effects model (P < 0.001)
3.4 Effects of Biochar Aging on Sorption of Phenanthrene in Soils
When comparing the biochar effects on phenanthrene K d between 7-month-aged soils and 19-month-aged soils, 19-month-aged soils had significantly lower phenanthrene K d than the 7-month-aged soils. It is also evident that in longer aged soils, the biochar-enhancing sorption effect has declined, and the decline is more pronounced with higher biochar application rates. For example, in Kalundborg soils after 19 months of biochar amendment, the phenanthrene K d increased by just 12, 22, and 41 % at biochar application rates (t ha−1) of 10, 20, and 50, respectively. This may be due to the negative effect of increased interactions of biochar with the soil mineral fraction over time, corroborating the argument above. Zhang et al. (2010) made similar observations in a study with a 28-day incubation period and reported a decrease in the overall sorption of phenanthrene in biochar-amended soil, which they attributed to the increased association of dissolved organic carbon (DOC) with biochar surfaces over time. We presume that the combination of both mechanisms (i.e., increased association of DOC on biochar surfaces and interaction with the soil mineral fraction) has caused the decrease in the sorption capacity in biochar-amended soils over time. However, further research warrants for a clear conclusion.
3.5 Effects of Reapplication of Biochar on Phenanthrene Sorption
When comparing the enhancement effect of biochar on phenanthrene sorption, the repeated application of biochar reduced the enhancement effect of biochar on phenanthrene K d. For example, in soils that received biochar in 2012 or 2011 only, K d increased by 13 or 12 % at a biochar application rate of 10 t ha−1. On the other hand, for soils that received biochar by repeated application (10 + 10 t ha−1), phenanthrene K d increased by just 17 %. Note also that this decrease in expected K d (13 + 12 %) is without regard to the observed increasing effect of manure in reference soils, which should expectedly increase K d in biochar-amended soils as well. Therefore, it is clear that a mechanism other than increased biochar–mineral interactions and DOC association plays a role in the decrease in sorption over time. We ascribe this decline in enhancement effect to the increased DOC association with sorption sites as a result of reapplication. In support of this, Quilliam et al. (2012) documented that the repeated application of biochar produced significantly higher DOC compared to single application. However, further research is needed to clarify our hypotheses.
3.6 Effects of Biochar on Sorption of Phenanthrene in Soils with Different Physicochemical Properties
As with Kalundborg soils, biochar-enhancing phenanthrene sorption in Risoe soils also was observed to be statistically significant (P < 0.001). However, the enhancement effect was less pronounced in Risoe soils than in Kalundborg soils. For example, at the same application rate and same aging time, phenanthrene K d increased by 16 % in Risoe soils, while it increased by 26 % in Kalundborg soils. Zhang et al. (2010) also observed the variation in biochar effects on phenanthrene K d among different soil types, which they ascribed to the difference in organic carbon content in those soils. They speculated that the coating of biochar particles with the dissolved organic matter from soil may have reduced overall accessibility to the sorption sites. However, it is unlikely that this is the case in the present study as Risoe soils had lower organic carbon content than Kalundborg soils. This is again attributable to the pronounced mineral–biochar interaction in Risoe soils owing to their higher clay content. However, further investigations required to meet a clear conclusion.
3.7 Effects of Soil Physicochemical Properties on Phenanthrene Sorption
Pearson correlation coefficient analyses were performed between the relevant physicochemical soil properties and sorption parameters (Table 3).
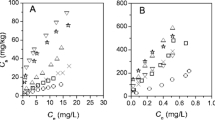
As shown in Table 3 and Fig. 3, a strong linear positive correlation (r = 0.92, P < 0.01) exists between phenanthrene K d and organic carbon content, as also reported in several previous studies (Celis et al. 2006; Pan et al. 2006). This is to be expected since soil organic carbon has been widely believed to be the main sorbent for non-ionic species (Kile et al. 1995; Fall et al. 2003; Ahangar et al. 2008). For comparison, two previous sorption models proposed by Karickhoff (1981) and Abdul et al. (1987) are shown (Fig. 3) which provide upper and lower boundaries respectively for the considered data. However, it must be taken into account that the two models were based on the values of an organic carbon content ranging from 0.001 to 0.033 kg kg−1 only. In this figure, as discussed above, the less enhancing effect of biochar in Risoe soils is clear.
de Jonge et al. (2008) concluded that phenanthrene sorption could not be accurately predicted from SOC content alone. In line with this, Dexter et al. (2008) pointed out that it is not the total amount of organic carbon, but the amount of complexed and non-complexed organic carbon (COC and NCOC), that controls the soil physical behavior. Based on a range of differently textured and managed Danish soil data, de Jonge et al. (2009) discussed the applicability of the Dexter concept on “clay saturation” to distinguish NCOC and its potential role on soil carbon sequestration. Recently, Soares et al. (2013), working with Danish cultivated top soils, concluded that NCOC had a higher sorption affinity for phenanthrene than COC. Figure 4 shows the variation of phenanthrene K d against NCOC calculated based on the Dexter et al. (2008) clay capacity factor (n) (Eq. [4]). Phenanthrene K d showed a strong linear increase (r = 0.98, P < 0.001) with increasing NCOC. Notably, the scatter around the regression lines diminished when phenanthrene K d was plotted against NCOC instead of total organic carbon, and hence agrees more closely with the previous discussion about the blockage effect of sorption sites by mineral interactions. Note also the strong linear correlation achieved by extending the Dexter concept, even for differently aged biochar-amended soils, confirming the validity of the approach taken by Dexter et al. (2008) across differently aged biochar-amended soils as well.
3.8 The Variation in Organic Matter Normalized Sorption Coefficient (K oc)
The estimated K d values were normalized over the soil organic carbon content to derive K oc values (Table 2). Despite the strong tendency to consider K oc as a universal constant independent of soil type (Wauchope et al. 2002), greatly differing phenanthrene K oc (9.54 × 103 to 1.53 × 104 L kg−1) was observed in reference soils, as found in other studies as well (Wauchope et al. 2002; Ahangar et al. 2008). Research over the last decade has revealed that organic matter heterogeneity, solution chemistry, and the contribution of inorganic constituents to the sorption process are among the most important causes of the variability of K oc. Risoe soils with a higher clay content had lower phenanthrene K oc than Kalundborg soils with a lower clay content. Further confirming the discussion above regarding the blockage effect of clay minerals, phenanthrene K oc strongly and negatively correlated (Table 3) with clay content (r = −0.82). Similar to these results, Celis et al. (2006), who worked with agricultural topsoils, also observed that the sorption of phenanthrene was negatively related to clay content, which they attributed to the combination of soil organic matter domain blockage and associated pH shifts. Similarly, several previous studies (e.g., Pusino et al. 1992; Bonin and Simpson 2007) reported that soil minerals may indirectly reduce K oc by blocking organic matter sorption sites or by causing conformational changes in its structure. Even within Risoe soils with the same clay content, we observed a significant variation in K oc, implying the marked effect of soil pH in controlling K oc. Laor et al. (1998) also observed the similar effect of pH on phenanthrene sorption. The decrease in phenanthrene sorption can be explained as the increased polarity of humic materials with increasing pH, which creates a lower affinity for hydrophobic compounds (Schlautman and Morgan 1993).
Even though K d for fresh biochar was higher than that of soils with natural soil organic carbon, it is clear that the K oc value for fresh biochar (8.96 x 103 L kg−1) was lower than that of soils (9.54 to 15.3 × 103 L kg−1), suggesting that the biochar exhibited lower sorptivity to phenanthrene than the natural soil organic carbon. Furthermore, the phenanthrene K oc values of amended soils were lower than reference soils. This is in contrast with the findings of Zhang et al. (2010) who reported that the apparent K oc values of biochar-amended soils were generally higher than those not amended with biochar. However, we should not ignore the fact that the results of Zhang et al. (2010) were based on freshly spiked biochar and soils in which biochar–mineral interactions are not possible. Furthermore, Smernik (2009) concluded that the sorption affinity of soils that contain large amounts of biochar was not as high as would be expected based on the sorption properties of fresh biochar. As shown in Fig. 5, K oc did not change significantly at the lowest application rate (10 t ha−1). However, at the application rate of 20 t ha−1, K oc declined significantly. Interestingly, beyond this point, K oc remain unchanged except in longer aged soils. A pronounced decline of K oc was further observed in Risoe soils compared to Kalundborg soils.
Variation of phenanthrene K oc at different biochar application rates (0–50 t ha−1) in Risoe and Kalundborg experimental sites. Note that for Kalundborg (2011 + 2012), the soils received double doses therefore the biochar amount corresponds to 0–100 t ha−1. Data for K oc are shown as mean ± standard error.
Figure 6 shows the variation of phenanthrene K oc with soil organic carbon content. For comparison, we further considered K oc data from literature (data from Soares et al. 2014) which were obtained using the same method on Danish agricultural field soils (without biochar) representing a similar range of organic carbon content as in the present study. A decreasing trend, in general, could be observed with increasing organic carbon in all types of soils. At a lower organic carbon content (below 0.02 kg kg−1), K oc generally decreased sharply with OC content, implying the pronounced effect of inorganic fraction and solution chemistry (pH). In literature, data where the organic carbon in soils is above 0.02 kg kg−1, K oc decreased linearly with OC content. Similarly, decreasing K oc was observed with increasing organic carbon content in biochar-amended soils as well. However, at higher values of organic carbon content (above 0.03 kg kg−1), K oc tended to be constant.
Variation of K oc (L kg-1) for phenanthrene against organic carbon (OC) (kg kg−1) in soils from Risoe and Kalundborg experimental sites. Estrup and Fardrup soil data are from Soares et al. (2014)
3.9 Added Effect of Biochar on Sorption Coefficient (K d) in Biochar-Amended Soil
We finally examined the added effect of biochar on soil sorption coefficient (K d) of phenenthrene in biochar-amended soils by considering the variation of ΔK d (=K d of biochar amended soils − K d of reference soils) with total biochar application rate (Fig. 7). The results reveal an increase in K d of ∼3 L kg−1 for each ton per hectare biochar added for Kalundborg soils, as compared to the corresponding value of ∼1 L kg−1 for Riso soils. The higher ΔK d values in Kalundborg soils can possibly be explained linking to the higher NCOC contents in Kalundborg soils compared to Riso soils, taking also into account the extra effects of organic manure added to Kalundborg soils.
4 Conclusions
This study investigated the effect of birch wood biochar on the sorption coefficient (K d) and organic carbon normalized sorption coefficient (K oc) of phenanthrene in agricultural soils under differing biochar application rates, biochar aging, and soil types. Compared to soils, fresh biochar showed higher phenanthrene K d. Biochar application enhanced sorption of phenanthrene in agricultural soils; however, the degree of enhancement is controlled by the application rate, aging, repeated application, and physicochemical properties of the soils. The enhancement effect on phenanthrene sorption was increased with an increasing biochar application rate, while aging decreased phenanthrene sorption. Repeated application was found to suppress the biochar-enhanced effect on the sorption of phenanthrene. At the same biochar application rate and aging time, the effects of biochar on phenanthrene K d were less pronounced in the soil with higher clay content. Phenanthrene K d was strongly correlated with both total and non-complexed organic carbon. K oc of phenanthrene in biochar-amended soils was lower than that in reference soils. The overall results show strong evidence of the effect of biochar–mineral interactions in soils on phenanthrene sorption. The results further highlight that chemical sorption in a complex soil–biochar–chemical system is controlled by the physicochemical characteristics of each component.
References
Abdul, A. S., Gibson, T. L., & Rai, D. N. (1987). Statistical correlations for predicting the partition-coefficient for nonpolar organic contaminants between aquifer organic-carbon and water. Hazardous Waste & Hazardous Materials, 4, 211–222.
Ahangar, A. G. (2010). Sorption of PAHs in the soil environment with emphasis on the role of soil organic matter: a review. World Applied Sciences Journal, 11(7), 759–765.
Ahangar, A. G., Smernik, R. J., Kookana, R. S., & Chittleborough, D. J. (2008). Separating the effects of organic matter–mineral interactions and organic matter chemistry on the sorption of diuron and phenanthrene. Chemosphere, 72, 886–890.
Bonin, J. L., & Simpson, M. J. (2006). Variation in phenanthrene sorption coefficients with soil organic matter fractionation: the result of structure or conformation? Environmental Science & Technology, 41(1), 153–159.
Brodowski, S., John, B., Flessa, H., & Amelung, W. (2006). Aggregate occluded black carbon in soil. European Journal of Soil Science, 57(4), 539–546.
Carroll, K. M., Harkness, M. R., Bracco, A. A., & Balcarcel, R. R. (1994). Application of a permeantrpolymer diffusional model to the desorption of polychlorinated biphenyls from Hudson River sediments. Environmental Science & Technology, 28, 253–258.
Celis, R., de Jonge, H., de Jonge, L. W., Real, M., Hermosin, M. C., & Cornejo, J. (2006). The role of mineral and organic components in phenanthrene and dibenzofuran sorption by soil. European Journal of Soil Science, 57, 308–319.
Cornelissen, G., & Gustafsson, Ö. (2005). Importance of unburned coal carbon, black carbon, and amorphous organic carbon to phenanthrene sorption in sediments. Environmental Science & Technology, 39, 764–769.
de Jonge, L. W., Møldrup, P., de Jonge, H., & Celis, R. (2008). Sorption and leaching of short-term-aged PAHs in eight European soils: link to physicochemical properties and leaching of dissolved organic carbon. Soil Science, 173, 13–24.
de Jonge, L. W., Møldrup, P., & Schjønning, P. (2009). Soil Infrastructure, Interfaces & Translocation Processes in Inner Space ("Soil-it-is"): towards a road map for the constraints and crossroads of soil architecture and biophysical processes. Hydrology and Earth System Sciences, 13, 1485–1502.
Dexter, A. R., Richard, G., Arrouays, D., Czyz, E. A., Jolivet, C., & Duval, O. (2008). Complexed organic matter controls soil physical properties. Geoderma, 144, 620–627.
Fall, C., Chaouki, J., Chavarie, C., & Elena-Ortega, R. (2003). Multivariate study on phenanthrene sorption in soils. Journal of Environmental Engineering, 129, 1030–1040.
Graber, E. R., Tsechansky, L., Khanukov, J., & Oka, Y. (2011). Sorption, volatilization and efficacy of the fumigant 1,3-dichloropropene in a biochar-amended soil. Soil Science Society of America Journal, 75, 1365–1373.
Karickhoff, S. W. (1981). Semi-empirical estimation of sorption of hydrophobic pollutants on natural sediments and soils. Chemosphere, 10, 833–846.
Kile, D. E., Chiou, C. T., Zhou, H., Li, H., & Xu, O. (1995). Partition of nonpolar organic pollutants from water to soil and sediment organic matter. Environmental Science & Technology, 29, 1401–1409.
Kleineidam, S., Rügner, H., Ligouis, B., & Grathwohl, P. (1999). Organic matter facies and equilibrium sorption of phenanthrene. Environmental Science & Technology, 35, 1637–1644.
Kookana, R.S., Sarmah, A.K., Van Zwieten, L., Krull, E. & Singh, B. (2011). Chapter three—Biochar application to soil: agronomic and environmental benefits and unintended consequences. In L.S. Donald (Ed.), Advances in agronomy (pp.103-143). Academic, New York.
Kumari, K. G. I. D., Moldrup, P., Paradelo, M., Elsgaard, L., Nielsen, H. H., & de Jonge, L. W. (2014). Effects of biochar on air and water permeability and colloid and phosphorus leaching in soils from a natural calcium carbonate gradient. Journal of Environmental Quality, 43(2), 647–657.
Laird, D. A., Chappell, M. A., Martens, D. A., Wershaw, R. L., & Thompson, M. (2008). Distinguishing black carbon from biogenic humic substances in soil clay fractions. Geoderma, 143(1–2), 115–122.
Laor, Y., Farmer, W. J., Aochi, Y., & Strom, P. (1998). Phenanthrene binding and sorption to dissolved and to mineral-associated humic acid. Water Research, 32, 1923–1931.
Liang, B., Lehmann, J., Solomon, D., Sohi, S., Thies, J. E., Skjemstad, J. O., Luizão, F. J., Engelhard, M. H., Neves, E. G., & Wirick, S. (2008). Stability of biomass-derived black carbon in soils. Geochimica et Cosmochimica Acta, 72(24), 6069–6078.
Lin, C. H., Lerch, R. N., Goyne, K. W., & Garrett, H. E. (2011). Reducing herbicides and veterinary antibiotics losses from agroecosystems using vegetative buffers. Journal of Environmental Quality, 40, 791–799.
Martin, S. M., Kookana, R. S., Van Zwieten, L., & Krull, E. (2012). Marked changes in herbicide sorption–desorption upon ageing of biochars in soil. Journal of Hazard Materials, 70(8), 231–232.Montgomery, D. C. (2013). Design and analysis of experiments. 8th edn. John Wiley and Sons, Singapore.
Montgomery, D. C. (2013). Design and analysis of experiments. 8th edn. John Wiley and Sons, Singapore.
Pan, B., Xing, B. S., Liu, W. X., Tao, S., Lin, X. M., Zhang, X. M., Zhang, Y. X., Xiao, Y., Dai, H. C., & Yuan, H. S. (2006). Distribution of sorbed phenanthrene and pyrene in different humic fractions of soils and importance of humin. Environmental Pollution, 143, 24–33.
Pignatello, J. J., Kwon, S., & Lu, Y. F. (2006). Effect of natural organic substances on the surface and adsorptive properties of environmental black carbon (char): attenuation of surface activity by humic and fulvic acids. Environmental Science Technology, 40(24), 7757–7763.
Pusino, A., Liu, W., & Gessa, C. (1992). Influence of organic matter and its clay complexes on metolachlor adsorption on soil. Pesticide Science, 36, 283–286.
Quilliam, R. S., Marsden, K., Gertler, G., Rousk, J., DeLuca, T. H., & Jones, D. L. (2012). Nutrient dynamics, microbial growth and weed emergence in biochar amended soil are influenced by time since application and reapplication rate. Agriculture, Ecosystems and Environment, 158, 192–199
R Core Team (2013). R: a language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria. http://www.R-project.org/. Accessed 15 Apr 2014
Schlautman, M. A., & Morgan, J. (1993). Effects of aqueous chemistry on the binding of polycyclic aromatic-hydrocarbons by dissolved humic materials. Environmental Science and Technology, 27, 961–969.
Sheng, G., Yang, Y., Huang, M., & Yang, K. (2005). Influence of pH on pesticide sorption by soil containing wheat residue-derived char. Environmental Pollution, 134, 457–463.
Shin, K. H., Kim, K. W., Kim, J. Y., Lee, K. E., & Han, S. S. (2008). Rhamnolipid morphology and phenanthrene solubility at different pH values. Journal of Environmental Quality, 37, 509–514.
Smernik, R. J. (2009). Biochar and sorption of organic compounds. In J. Lehmann & S. Joseph (Eds.), Biochar for environmental management science and technology (pp. 289–296). London: Earthscan.
Soares, A. A., Moldrup, P., Minh, L. N., Vendelboe, A. L., Schjonning, P., & de Jonge, L. W. (2013). Sorption of phenanthrene on agricultural soils. Water, Air, and Soil Pollution, 224, 1519–1531.
Soares, A. A., Paradelo, M., Moldrup, P., Tuller, M., Matos, C. & de Jonge, L. W. (2014). Spatial variation of phenanthrene sorption in soil across two loamy fields. Water, Air, & Soil Pollution. (Submitted).
Spokas, K. A., Koskinen, W. C., Baker, J. M., & Reicosky, D. C. (2009). Impact of woodchip biochar additions on greenhouse gas production and sorption/degradation of two herbicides in a Minnesota soil. Chemosphere, 77, 574–581.
Sun, K., Kang, M., Zhang, Z., Jin, J., Wang, Z., Pan, Z., Xu, D., Wu, F., & Xing, B. (2013). Impact of de-ashing treatment on biochar structural properties and potential sorption mechanisms of phenanthrene. Environmental Science & Technology, 47, 11473–11481.
Sun, Z., Bruun, E.W., Arthur, E., de Jonge, L.W., Moldrup, P., Hauggaard-Nielsen, H., & Elsgaard, L. (2014). Effects of biochar on aerobic processes, enzyme activity, and crop yields in two sandy loam soils. Biology & Fertility of Soils. doi:10.1007/s00374-014- 0928-5.
Wauchope, R. D., Yeh, S., Linders, J. B. H. J., Kloskowski, R., Tanaka, K., Rubin, B., Katayama, A., Kordel, W., Gerstl, Z., Lane, M., & Unsworth, J. B. (2002). Pesticide soil sorption parameters: theory, measurement, uses, limitations and reliability. Pest Management Science, 58, 419–445.
Winter, B. (2013). Linearmodels and linearmixed effects models in R with linguistic applications. arXiv:1308.5499. http://arxiv.org/pdf/1308.5499.pdf. Accessed 15 Apr 2014
Zhang, H., Lin, K., Wang, H., & Gan, J. (2010). Effect of Pinus radiata derived biochars on soil sorption and desorption of phenanthrene. Environmental Pollution, 158, 2821–2825.
Zhang, P., Sheng, G., Feng, Y., & Miller, D. M. (2006). Predominance of char sorption over substrate concentration and soil pH in influencing biodegradation of benzonitrile. Biodegradation, 17(1), 1–8.
Acknowledgments
The authors would like to thank P. Jørgensen, J.M. Nielsen, and S.T. Rasmussen for their technical assistance in sampling and taking laboratory measurements. Esben W. Bruun is also thanked for establishing the field trial. The study was funded by the international project Soil Infrastructure, Interfaces and Translocation Processes in Inner Space (Soil-it-is), which is funded by the Danish Research Council for Technology and Production Sciences (http://www.agrsci.dk/soil-it-is/). The field trial was funded by the North Sea Region Program IVB through the “Biochar: climate saving soils” project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumari, K.G.I.D., Moldrup, P., Paradelo, M. et al. Phenanthrene Sorption on Biochar-Amended Soils: Application Rate, Aging, and Physicochemical Properties of Soil. Water Air Soil Pollut 225, 2105 (2014). https://doi.org/10.1007/s11270-014-2105-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-014-2105-8