Abstract
In this work, the sorption of pentachlorophenol (PCP) by non-viable biomass of Rhizopus oryzae ENHE was evaluated. The kinetics and isotherm studies were performed at pH 5.0, 6.0, and 8.0. The point of zero charge of the biomass was determined; this value allowed us to explain the changes of pH during sorption studies. The analyzed experimental kinetic data revealed that Ho’s model adjusted better to the experimental data than Lagergren’s model. PCP sorption was fast; an equilibrium sorption time was reached within 30 min, regardless of pH. PCP sorption at pH 5.0 and 6.0 was better described by the Freundlich isotherm than by the Langmuir isotherm. In contrast, at pH 8.0, the Langmuir isotherm describes better the PCP sorption. Sorption data showed that at pH 5.0 and 6.0, the sorption capacity of PCP was higher than at pH 8.0. Sorption of PCP by the fungal biomass occurred spontaneously; it was endothermic and due to physical sorption. Finally, FT-IR analysis of the dried biomass indicated that amino and hydroxyl groups were involved in the sorption of PCP. This work is one of the few reporting the effect of pH and temperature on the sorption of PCP by microbial biomass from a filamentous fungus belonging to the genus Rhizopus.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Pentachlorophenol (PCP) was once used as biocide and extensively as wood preservative. Currently, PCP is still used under a restricted-use criterion to preserve wood for utility poles (EPA 2010). The widespread use of PCP in the past and its recalcitrant nature has resulted in the global contamination of soils and groundwater. PCP is very toxic because it uncouples oxidative phosphorylation and forms adducts with DNA (Vaidyanathan et al. 2007). Due to its toxicity, PCP has been classified as a “priority toxic pollutant” by the United States Environmental Protection Agency (US-EPA). PCP removal from a natural system by photolysis, volatilization, or degradation is exceedingly slow; an alternative is bioremediation. For this reason, PCP bioremoval processes, as biosorption and biodegradation, from the environment have been studied; degradation by fungus is one of the assessed processes (Fahr et al. 1999; Montiel et al. 2004; Szewczyk and Dlugonski 2009; Tomasini et al. 2001). The biosorption approach is another alternative technique to uptake PCP (Estevinho et al. 2007). Sorption processes are useful tools to remove chemicals at low concentrations and the mechanism could be absortion or adsorption.
The amount of solute sorbed can be calculated by using the following equation:
where q x is the sorption capacity of PCP (at time t or at equilibrium) sorbed per unit mass (mg g−1), C 0 is the initial PCP concentration (mg L−1) and C x is the PCP concentration at time t or at equilibrium (mg L−1), V is the volume of the PCP solution (L), and m is the weight of the sorbent (g).
Sorption kinetics describes the solute removal rate that controls the residence time of the sorbate in the solid/liquid interface (Febrianto et al. 2009). The pseudo-first order kinetics equation was developed by Lagergren, it was the first equation that described sorption on a liquid/solid system, and it is based on the solid’s capacity to sorb molecules (Lagergren 1898; Chowdhury and Saha 2011). The pseudo-second order kinetics model was developed by Ho and describes that the limiting step of the sorption rate may be chemical sorption involving valence forces through sharing or exchanging electrons between sorbent and sorbate (Ho and McKay 1999).
A sorption isotherm describes the equilibrium relationship between the amount of solute sorbed by unit mass of sorbent and the amount of solute remaining in the liquid phase. The most common isotherm models used to determine the sorption capacity are the Langmuir (1916), Freundlich (1906), and Redlich and Peterson (1959) models.
The Langmuir isotherm describes that molecules can form a monolayer on the sorbent surface; hence, sorption can be accomplished only in a finite number of binding sites and there is no interaction among molecules sorbed. The sorption explained by the Langmuir isotherm refers to homogeneous sorption, in which each molecule possesses constant enthalpies and sorption activation energy. In other words, all binding sites possess equal affinity for the sorbate. This isotherm is thermodynamically consistent with Henry’s law at low concentration (Foo and Hameed 2010).
The Freundlich isotherm is an exponential equation that describes a reversible and non-ideal sorption onto heterogeneous surfaces, where the formation of monolayer is not restricted. Therefore, interaction among molecules can occur. In this context, the amount of molecules sorbed is the summation of sorption at all sites (each having bond energy), where the stronger binding sites are occupied first, until the sorption energy is exponentially decreased upon completion of the sorption process. The Freundlich model does not follow Henry’s law (Freundlich 1906; Foo and Hameed 2010; Allen et al. 2004).
The Redlich-Peterson isotherm is a non-linear model that combines features of both the Langmuir and Freundlich isotherms. This empirical model reduces to a linear isotherm at low solute concentration, whereas at high concentration of solutes, its behavior is closer to the Freundlich isotherm and is similar to the Langmuir isotherm when g = 1. Therefore, the g value will always vary from 0 to 1 (Redlich and Peterson 1959; Garcia-Calzon and Diaz-Garcia 2007).
The pH plays an important role in biosorption processes because solubility of PCP depends largely on the pH of the aqueous phase (Arcand et al. 1995). The pH also alters the functional groups that carry out the sorption process onto the sorbent, producing changes in the kinetic and equilibrium behavior.
Earlier sorption technologies used all kind of inorganic sorbents; nowadays, new sorption technologies, biosorption, are focused on finding and developing organic sorbents, such as agro-industrial wastes that are cheap and biodegradable. Many sorbents have been studied for PCP removal from water, such as sediments, ash, black carbon, and biological sorbents such as biomass (Estevinho et al. 2007; Luo et al. 2011; Pizzigallo et al. 2011; Yang et al. 2009; Wang et al. 2000). In general, chemical conditioned biomass has been used as sorbent of toxic compounds; in this study, biomass was not treated chemically, therefore it is a low cost process and friendly with the environment. Besides, few works have studied and characterized PCP sorption by the fungus Rhizopus oryzae that is widely used in the food industry.
The aim of the present work was to study the effect of pH and temperature on sorption of PCP by non-viable R. oryzae ENHE biomass, a zygomycete isolated from a PCP-contaminated soil.
2 Material and Methods
2.1 Biomass Preparation
The strain of R. oryzae ENHE was used and spore suspension was obtained according to Leon-Santiesteban et al. (2011). R. oryzae ENHE biomass was produced using a bubble column reactor of 40 cm in height and 6 cm in diameter with nominal volume of 1.1 L. The working volume of the bioreactor was 600 mL, inoculated with 1 × 106 spores mL−1 of enriched Merlin-Norkran (EMN) medium (Montiel et al. 2004), 10 g L−1 of saccharose was used instead of glucose, because more biomass is produced. Bioreactor was maintained at 30 °C, fed with an air flow rate of 740 mL min−1 during 24 h. After growing, the biomass of R. oryzae ENHE was recovered by filtration. The recovered biomass was washed 10 times with deionized water. Next, the biomass was dried during 5 days at 30 °C and at 60 °C for 2 days. Dried biomass, that means non-viable biomass, because the fungus degrades PCP during its growth, was ground and sieved through a No. 50 mesh (0.297 mm); straightaway, it was dried once more at 60 °C during 2 days. Finally, the dried-powdered biomass (non-viable biomass) was taken to a constant temperature and used for the biosorption studies.
2.2 Batch Biosorption Experiments
Biosorption studies were carried out in deionized water. The pH of the water was adjusted with 0.1 M NaOH and 0.1 N HCl, when necessary. An industrial-grade PCP (95 % purity) was used to prepare a stock solution of 500 mg L−1 in methanol. Working solutions of PCP were prepared from the stock solution by appropriate dilutions. Kinetic and equilibrium experiments were conducted at three different values of pH, which were 5.0, 6.0, and 8.0. All experiments were assessed in triplicate, and the average values were used in the regression analysis. Controls without biomass were run to check the abiotic loss.
2.3 Determination of Point of Zero Charge (pzc) of R. oryzae ENHE Biomass
The point of zero charge (pzc) of R. oryzae ENHE was determined by the potentiometric mass titration (PMT) method. This technique was carried out under N2 atmosphere. A control solution, without biomass, and suspensions of three different amounts of biomass, 5.4, 8.2, and 10.2 mg, were placed into contact with 100 mL of a 0.03 M KNO3 solution. This solution was agitated for 24 h to obtain a constant pH. The pH of the solution was adjusted to 11.0 with 0.1–10 M KOH. Titrations were achieved by adding HNO3 (0.1 M) under continuous agitation until the pH was 3.0 (Bourikas et al. 2003; Fiol and Villaescusa 2009).
2.4 Kinetic Experiments
Kinetic experiments were carried out in 250-mL Erlenmeyer flasks with 100 mL of solution at 10 mg PCP L−1 and 100 mg of non-viable biomass. Biosorption kinetics studies were maintained at 30 ± 1 °C at 150 rpm for 48 h. The samples were taken from the aqueous phase at the following time intervals: 1, 5, 10, 30, 60, 120, 180, 240, and 300 min to determine the concentration of non-sorbed PCP. The quantified PCP was used to calculate the sorption capacities at time t (q t ).
The non-linear pseudo-first order model (Lagergren 1898) and pseudo-second order model (Ho and McKay 1999) were used to obtain the kinetic parameters (Lagergren 1898; Ho and McKay 1999):
2.5 Isotherm Experiments
Isotherm experiments were carried out by contacting 30 mL of the PCP solution at 10 mg PCP L−1 in 150-mL Erlenmeyer flasks with different masses of non-viable biomass, which varied from 5 to 120 mg. The flasks were shaken at 150 rpm at 30 ± 1 °C for 8 h. Once the sorption equilibrium was reached, samples were taken from the supernatant to determine the PCP concentration in the aqueous phase. The quantified PCP was considered the concentration of PCP at equilibrium (C e ), which was used to calculate the sorption capacities at equilibrium (q e ). The non-linear Langmuir, Freundlich, and Redich-Peterson models were used to describe the PCP sorption by the fungus (Langmuir 1916; Freundlich 1906; Redlich and Peterson 1959):
2.6 Temperature Effect on Biosorption of PCP
Temperature studies were carried out at pH 5.0 in 150-mL Erlenmeyer flasks with 30 mL of PCP solution at 10 mg PCP L−1. Assayed temperatures were 20, 25, 30, 35 and 40 ± 1 °C; supernatant samples were taken after 8 h, for their analysis of PCP content.
2.7 Error Analysis
To weigh the best fit of experimental data to kinetics and isotherm models, the non-linear coefficients of determination (R 2) and chi-squared (χ 2) test (Ho et al. 2005) were applied.
2.8 PCP Quantification
The samples were centrifuged at 12,000 × g during 30 min and filtered through 0.22-μm nitro-cellulose membranes. PCP was quantified by HPLC, performed in a Water System 600 solvent delivery system equipped with a 996 Photodiode Array Detector at 303 nm (Leon-Santiesteban et al. 2011).
2.9 Fourier Transformation Infrared Spectroscopy
Fourier transformation infrared spectroscopy (FT-IR) spectra of solid samples of non-viable R. oryzae ENHE biomass were obtained by using a spectrophotometer FT-IR GX System (Perkin-Elmer, Shelton, CT, USA) coupled to an ATR DuranSample II accessory. The spectra were an average of 16 scans from 4,000 to 650 cm−1 at a resolution of 2 cm−1.
3 Results and Discussion
3.1 pH Studies
In the present work, we observed the effect of pH on PCP sorption, three different values of pH: 5.0, 6.0, and 8.0 were assayed. After adjusting the pH of the solutions, PCP was added at a concentration of 10 mg L−1. The pH of the solutions changed when PCP was added, after few minutes of stirring. Besides, when the biomass was added to the solutions, the pH of each solution changed again. After 8 h of contact, the measured pHs at equilibrium were 6.2, 6.0, and 6.5 for experiments with an initial pH of 5.0, 6.0, and 8.0, respectively. Koumanova et al. (2002) used R. oryzae biomass to sorb chlorophenols at pH 6.0. They found that, after adding the biomass, the pH of the solutions was maintained without changes, but at the end of the sorption process, the pH was 7.0. Deng et al. (2009) worked with aminated Penicillium chrysogenum biomass to adsorb PCP and 2,4-dichlorophenoxyacetic acid (2,4-D), and they found that the aminated biomass in acidic solutions increased the pH at equilibrium, because of the protonation of amine groups on the aminated biomass.
The pH pzc value indicates that the pH is in equilibrium where the biomass presents a neutral charge due to the negative and positive charges in the biomass. At pH < pH pzc , the biomass charge is positive and at pH > pH pzc , the biomass charge is negative. Results show that the value of pH pzc of R. oryzae ENHE biomass was 6.43 ± 0.3 (Fig. 1). Figure 1 shows a zone between pH 8.0 and pH 5.0 where the pH decreased slowly. The large amount of functional groups that the R. oryzae ENHE biomass presents produces a buffering-like zone where the pH of solution tends to achieve the pH at equilibrium, i.e., the pH pzc . The titration curves obtained in this work by the PMT method explain the pH change to 6.2–6.5, when biomass was added to the PCP solutions, regardless of the initial pH adjusted in this work.
Experimental PMT curves from non-viable biomass of R. oryzae ENHE. White squares (□) indicate control; black-filled circles (●) indicate 0.054; black-filled diamonds (▲) indicate 0.082; and black-filled stars (★) indicate 0.102 mg biomass mL−1. pH pzc value is the pH at which the four titration curves intersect. The value of each curve is the means of three replicates
3.2 Kinetic Studies
The kinetic and statistical parameters obtained from the pseudo-first and pseudo-second order models described by Lagergren (1898) and Ho and McKay (1999) are listed in Table 1. Both kinetics models adjusted very well to the experimental data (R 2 > 0.98). However, the pseudo-second order model (Ho and McKay 1999) described better the kinetic behaviors because this model presents the biggest determination coefficients (R 2) and the least chi-squared values (χ 2) for each assessed pH.
Abiotic experiments (control without biomass) showed that the PCP concentration was maintained constant throughout the 48 h, at the three assessed pH.
The sorption rate of PCP by the non-viable R. oryzae ENHE biomass was strongly affected by the pH (Fig. 2). The sorption capacities of PCP, at equilibrium, obtained from the pseudo-second kinetics model were 3.65, 2.84, and 1.69 mg g−1 at pH 5.0, 6.0, and 8.0, respectively (Table 1). The sorption capacity obtained in this work at pH 5.0 was 3.7-fold higher than the sorption capacity of PCP stated by Mathialagan and Viraraghavan (2009) using chemically conditioned biomass of Aspergillus niger.
On the other hand, the initial sorption rate of PCP at pH 6.0 was higher (1.14 mg min−1 g−1) than the initial sorption rates at pH 5.0 and 8.0 (0.62 and 0.33 mg min−1 g−1, respectively). Apparently, at pH 6.0, non-viable R. oryzae ENHE biomass can initially sorb PCP faster than at pH 5.0 or 8.0, but more amount of PCP can be sorbed by non-viable R. oryzae ENHE biomass at pH 5.0.
Figure 2 shows the sorption kinetics of PCP by non-viable R. oryzae ENHE biomass; results indicate that PCP sorption is very fast, the sorption equilibrium was reached at 30 min. In this moment, the fungal biomass sorbed 34.8, 29.8, and 18.1 % of the initial PCP at pH 5.0, 6.0, and 8.0, respectively. Other types of biosorbents of microbial origin have been used to take up PCP from aqueous solutions, but they have not sorbed PCP as fast as the non-viable R. oryzae ENHE biomass. Wang et al. (2000) found that the equilibrium sorption time was reached in less than 2 h using activated sludge biomass. Deng et al. (2009) used an aminated P. chrysogenum biomass to sorb PCP and found an equilibrium sorption time at 6 h, whereas Mathialagan and Viraraghavan (2009) computed an equilibrium sorption time of 2 h for the sorption process of PCP using A. niger biomass treated with cetyltrimethylammonium bromide as biosorbent.
3.3 Sorption Isotherms
Table 2 shows the parameters obtained from isotherm models and the statistical parameters from non-linear regression. Using the Langmuir model, it was found that the maximum sorption capacities (q m ) were 16.99, 7.64, and 2.04 mg PCP g−1 biomass at pH 5.0, 6.0, and 8.0, respectively. The q m obtained at pH 8.0 was 8.4-fold less than the q m obtained at pH 5.0. That means, at acidic pH, the sorption of PCP by the dried biomass was higher than at alkaline pH. This sorption behavior agrees with other results reported previously (Brandt et al. 1997; Deng et al. 2009; Mathialagan and Viraraghavan 2009).
The Freundlich isotherm showed that the sorption capacities of the sorbent (K F ) were 0.94, 0.68, and 1.45 (mg PCP g−1 biomass) (L mg−1 PCP)1/n at pH 5.0, 6.0, and 8.0, respectively. Results show that the sorption experiments carried out at pH 8.0 apparently reached the saturation level at 2.03 mg PCP L−1 at equilibrium, probably because the biomass at this pH could not sorb more PCP molecules, as compared to the experiments carried out at pH 5.0 and 6.0, where the saturation level was not achieved. By comparing the Langmuir and Freundlich results with the parameters obtained from the Redlich-Peterson isotherm (Fig. 3), it was seen that the g parameter from isotherms at pH 5.0 and 6.0 were 0.41 and 0.58, respectively, indicating that the sorption of PCP is better described by the Freundlich than by the Langmuir model. Whereas the g value calculated at pH 8.0 was 0.94, meaning that the sorption of PCP is closer to Langmuir’s than to Freundlich’s behavior (Table 2).
As stated by Arcand et al. (1995) and Brandt et al. (1997), the solubility of PCP is highly dependent on the pH of the solution. PCP presents a pKa of 4.75, that means that, at a pH equal to pKa, 50 % of PCP is found in a neutral form (undissociated PCP) and the other 50 % is found in an anionic form (ionized PCP). At pH 5.0, approximately 36 % of PCP is found in an undissociated form, whereas, at pH 8.0, 99.9 % of PCP is found in anionic form.
The lowest amount of PCP sorbed by the non-viable R. oryzae ENHE biomass was found at pH 8.0. At this pH, PCP is principally in anionic form, and the non-viable R. oryzae ENHE biomass presents negative charge. Thus, electrostatic repulsion between anionic PCP and biomass can be carried out, causing a decrease in the amount of PCP sorbed by the fungal biomass (Mathialagan and Viraraghavan 2009; Sathishkumar et al. 2009). On the other hand, at pH 5.0, PCP is either neutral or positively charged and less negative charges are on the biomass surface. Since at pH 5.0, 36 % of PCP is in a neutral form, a lesser amount of electrostatic repulsion forces are presented, as a consequence, an enhanced PCP sorption by the non-viable R. oryzae ENHE biomass is obtained. When neutral species of PCP are sorbed by the non-viable R. oryzae ENHE biomass, the anionic molecules of PCP in the bulk are quickly equilibrated to form new neutral molecules of PCP that will probably be sorbed by the fungal biomass.
As described previously, the initial pH of the isotherm experiments changed after adding PCP and biomass. In the case of experiments performed at pH 5.0, a pH of 6.2 was reached at equilibrium. Under these experimental conditions, the surface of the non-viable R. oryzae ENHE biomass was protonated, which would enhance the interaction of PCP with the binding sites of the biomass by greater attractive forces improving the sorption capacity, as stated by Aksu and Yener (2001) and Mathialagan and Viraraghavan (2009).
3.4 Effect of Temperature
The ΔG 0 values were calculated using Gibbs’s equation:
where R is the universal gas constant, T is the absolute temperature (Kelvin), and K is an equilibrium constant of the sorption process. Assuming the solvent activity (water) to be the unit, the equilibrium constant can be associated with K a constant obtained from a Langmuir isotherm (Chattoraj and Birdi 1984; Hong et al. 2009). Considering that temperature changes can affect directly the K a value, the equation of van’t Hoff can be used to determine the enthalpy changes (ΔH 0) and the entropy changes (ΔS 0) from the sorption process:
In this work, the effect of temperature on PCP sorption by the non-viable R. oryzae ENHE biomass was assayed at pH 5.0 and five temperatures were used to obtain the thermodynamic parameters, 20, 25, 30, 35, and 40 °C. The results show that the K a values increased from 12,123 to 17,929 L mol−1 PCP with an increase in temperature, while the ΔG 0 values decreased from −22.9 to −25.5 KJ mol−1. The negative values of ΔG 0 obtained confirm that the sorption of PCP has a spontaneous nature (Mathialagan and Viraraghavan 2009). The decreasing ΔG 0 values with an increase in the temperature reveals that PCP sorption becomes more favorable at higher temperatures, conversely to that found in the sorption of PCP by cetyltrimethylammonium bromide-treated A. niger biomass where the sorption of PCP becomes more favorable at lower temperatures (Mathialagan and Viraraghavan 2009). The sorption of PCP by the non-viable R. oryzae ENHE biomass presented a positive value of ΔH 0 (17.69 KJ mol−1), suggesting that the sorption of PCP is of endothermic nature and indicating that the sorption of PCP by the dried biomass is due to physical interactions, such as hydrogen bonding (Biggar and Cheung 1973). The positive value of ΔS 0 obtained (0.138 KJ mol−1 K−1) suggests the increased randomness at the solid/solution interface during sorption of PCP on non-viable biomass (Han et al. 2009).
3.5 Functional Groups of non-viable R. oryzae ENHE Biomass
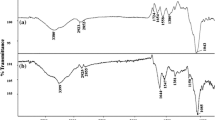
The IR spectrums were determined to identify the functional groups present in the non-viable R. oryzae ENHE biomass. The assigned bands in the IR spectrum marked with capital letters with an apostrophe (A’, B’, and so on) correspond to fungal biomass that was in contact with PCP. While the IR spectra marked with capital letters without an apostrophe (A, B, and so on) correspond to fungal biomass without PCP (control). Figure 4 shows a broad adsorption band, A, at 3,284.6 cm−1; this could be the result from the acetamide asymmetric stretching vibrations of the chitin fraction. Although, it has also been reported that the O–H stretching vibration can be found in the range of 3,500–3,200 cm−1 (Deng and Ting 2005). The bands, B and C, observed at 2,924.5 and 2,855.1 cm−1 are characteristic of alkyl chains (Yee et al. 2004). The band, F, observed at 1,641.3 cm−1 is a product of the C═O stretching vibration of the primary amides. The band, G, found at 1,541.2 cm−1 may be due to the bending of both chitin and chitosan on the fungus cell wall, and C–N stretching vibrations are found also to be overlapping with the asymmetric stretching vibration of COO– groups from carboxylates. The band, I, observed at 1,401.2 cm−1 may be due to the tertiary amides band. However, C–H deformations have also been reported at the same wave number. The band, J, found at 1,248.2 cm−1 is characteristic of C–O stretching vibrations of the carboxylic acids. The band, K, observed at 1,148.3 cm−1 may be due to the P═O group or the –SO2 group. The band, L, observed at 1,070 cm−1 may be due to a phosphate group (–PO4 3−) of the fungal biomass. The band, M, found at 1,035 cm-1 could be due to the P–OH groups. The band, N, observed at 873.3 cm − 1 is representative of N–H (Khambhaty et al. 2009; Majumdar et al. 2008; Mathialagan and Viraraghavan 2009; Sanghi et al. 2009).
The IR spectrum of the fungal biomass with PCP sorbed, in general, was similar to the IR spectrum of the control biomass. However, the biomass with PCP sorbed presented some functional groups that could not be identified in the control biomass, D’, E’ and H’, may be due to the resolution of the equipment used. Besides, some displacements in the absorption patterns were observed.
The band A’, corresponding to the R. oryzae ENHE biomass with PCP sorbed, was shifted from the band A, corresponding to the R. oryzae ENHE biomass without PCP; hydroxyl, or amino groups probably were involved in PCP sorption as reported by Deng et al. (2009). A similar displacement was found from the band F’, at 1,628.1 cm−1 and F at 1,641.3 cm−1, suggesting that C═O from the primary amide is also linked to the sorption of PCP. Finally, a displacement between the bands I’ at 1,379.7 cm−1 and I at 1,401.2 cm−1, suggests that tertiary amides or sulfamide groups may contribute to the sorption of PCP by R. oryzae ENHE biomass.
4 Conclusions
This work is one of the few reports that have used biomass of Rhizopus, without chemical treatment, to study the sorption of PCP. Tsezos and Bell (1989) reported PCP sorption by dead and live biomass of Rhizopus arrhizus, 0.8 and 4.8 mg PCP g−1 biomass, at equilibrium time, 24 h. But they did not report the effect of pH and temperature. In this work, it was shown that R. oryzae ENHE sorbed 3.65 mg PCP g−1 biomass at equilibrium time, 30 min, which means 48 times faster than dead R. arrhizus biomass.
We showed the importance of the pH on the sorption of PCP by non-viable R. oryzae ENHE biomass. The pH affected the solubility of PCP, as well as the electrical charge of the biomass. The pH pzc of R. oryzae ENHE biomass is 6.4, showing a buffer role around this pH. The sorption capacity of PCP by the non-viable R. oryzae ENHE biomass is favored at low pH, where a high proportion of molecules of PCP are in a neutral form and less electrostatic repulsions are exerted between the PCP molecules and the fungal biomass. From the kinetics standpoint, the pseudo-second order model developed by Ho is the best to describe the experimental data based on the highest non-linear coefficients of determination (R 2) and the lowest chi-squared values (χ 2). In 30 min of contact, PCP reached sorption equilibrium with the fungal biomass, regardless of the pH; nevertheless, sorption capacity at equilibrium is lower at alkaline pH than at acidic pH. The equilibrium time observed in this work is one of the lowest reported in the literature for the sorption of PCP using biosorbents.
The highest q m (maximum sorption capacity) of PCP for the non-viable R. oryzae ENHE biomass obtained in this study from the Langmuir isotherm was found at pH 5.0. Based on the non-linear regression analysis, we have a 95 % confidence that the q m obtained at pH 5.0 lies between 9.31 and 17.45 mg PCP g−1 biomass. On the other hand, the sorption isotherm studies showed that the Freundlich isotherm may explain better the sorption of PCP on the biomass at pH 5.0 and 6.0. Whereas the Langmuir isotherm probably predicts better the sorption of PCP at pH 8.0 than the two other models.
In the sorption studies varying the temperature, it was observed that the sorption of PCP on the non-viable R. oryzae ENHE biomass is achieved spontaneously; it is endothermic and the interaction between PCP and biomass is due to a physical sorption. FT-IR analysis of non-viable biomass of R. oryzae ENHE showed that hydroxyl and amino groups were probably related to the sorption of PCP.
References
Aksu, Z., & Yener, J. (2001). A comparative adsorption/biosorption study of mono-chlorinated phenols onto various sorbents. Waste Management, 21(8), 695–702. doi:10.1016/s0956-053x(01)00006-x.
Allen, S. J., McKay, G., & Porter, J. F. (2004). Adsorption isotherm models for basic dye adsorption by peat in single and binary component systems. Journal of Colloid and Interface Science, 280(2), 322–333. doi:10.1016/j.jcis.2004.08.078.
Arcand, Y., Hawari, J., & Guiot, S. R. (1995). Solubility of pentachlorophenol in aqueous solutions: the pH effect. Water Research, 29(1), 131–136. doi:10.1016/0043-1354(94)e0104-e.
Biggar, J. W., & Cheung, M. W. (1973). Adsorption of picloram (4-amino-3,5,6-trichloropicolinic acid) on Panoche, Ephrata, and Palouse soils: a thermodynamic approach to the adsorption mechanism. Soil Science Society of America Journal, 37(6), 863–868.
Bourikas, K., Vakros, J., Kordulis, C., & Lycourghiotis, A. (2003). Potentiometric mass titrations: experimental and theoretical establishment of a new technique for determining the point of zero charge (PZC) of metal (hydr)oxides. Journal of Physical Chemistry B, 107(35), 9441–9451. doi:10.1021/jp035123v.
Brandt, S., Zeng, A. P., & Deckwer, W. D. (1997). Adsorption and desorption of pentachlorophenol on cells of Mycobacterium chlorophenolicum PCP-1. Biotechnology and Bioengineering, 55(3), 480–489. doi:10.1002/(sici)1097-0290(19970805)55:3<480::aid-bit3>3.0.co;2-8.
Chattoraj, D. K., & Birdi, K. S. (1984). Adsorption and the Gibbs surface excess. New York: Plenum Press.
Chowdhury, S., & Saha, P. (2011). Adsorption Kinetic modeling of safranin onto rice husk biomatrix using pseudo-first- and pseudo-second-order kinetic models: comparison of linear and non-linear methods. Clean: Soil, Air, Water, 39(3), 274–282. doi:10.1002/clen.201000170.
Deng, S. B., & Ting, Y. P. (2005). Characterization of PEI-modified biomass and biosorption of Cu(II), Pb(II) and Ni,(II). Water Research, 39(10), 2167–2177. doi:10.1016/j.watres.2005.03.033.
Deng, S. B., Ma, R., Yu, Q., Huang, J., & Yu, G. (2009). Enhanced removal of pentachlorophenol and 2,4-D from aqueous solution by an aminated biosorbent. Journal of Hazardous Materials, 165(1–3), 408–414. doi:10.1016/j.jhazmat.2008.10.029.
EPA, U. S. (2010). IRIS toxicological review of pentachlorophenol. U.S. Environmental Protection Agency, Washington, DC, EPA/635/R-09/004F.
Estevinho, B. N., Martins, I., Ratola, N., Alves, A., & Santos, L. (2007). Removal of 2,4-dichlorophenol and pentachlorophenol from waters by sorption using coal fly ash from a Portuguese thermal power plant. Journal of Hazardous Materials, 143(1–2), 535–540. doi:10.1016/j.jhazmat.2006.09.072.
Fahr, K., Wetzstein, H. G., Grey, R., & Schlosser, D. (1999). Degradation of 2,4-dichlorophenol and pentachlorophenol by two brown rot fungi. FEMS Microbiology Letters, 175(1), 127–132. doi:10.1016/s0378-1097(99)00184-6.
Febrianto, J., Kosasih, A. N., Sunarso, J., Ju, Y. H., Indraswati, N., & Ismadji, S. (2009). Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: a summary of recent studies. Journal of Hazardous Materials, 162(2–3), 616–645. doi:10.1016/j.jhazmat.2008.06.042.
Fiol, N., & Villaescusa, I. (2009). Determination of sorbent point zero charge: usefulness in sorption studies. Environmental Chemistry Letters, 7(1), 79–84. doi:10.1007/s10311-008-0139-0.
Foo, K. Y., & Hameed, B. H. (2010). Insights into the modeling of adsorption isotherm systems. Chemical Engineering Journal, 156(1), 2–10. doi:10.1016/j.cej.2009.09.013.
Freundlich, H. (1906). Concerning adsorption in solutions. Zeitschrift Für Physikalische Chemie–Stochiometrie Und Verwandtschaftslehre, 57(4), 385–470.
Garcia-Calzon, J. A., & Diaz-Garcia, M. E. (2007). Characterization of binding sites in molecularly imprinted polymers. Sensors and Actuators B: Chemical, 123(2), 1180–1194. doi:10.1016/j.snb.2006.10.068.
Han, R., Zhang, J., Han, P., Wang, Y., Zhao, Z., & Tang, M. (2009). Study of equilibrium, kinetic and thermodynamic parameters about methylene blue adsorption onto natural zeolite. Chemical Engineering Journal, 145(3), 496–504. doi:10.1016/j.cej.2008.05.003.
Ho, Y. S., & McKay, G. (1999). Pseudo-second order model for sorption processes. Process Biochemistry, 34(5), 451–465. doi:10.1016/s0032-9592(98)00112-5.
Ho, Y. S., Chiu, W. T., & Wang, C. C. (2005). Regression analysis for the sorption isotherms of basic dyes on sugarcane dust. Bioresource Technology, 96(11), 1285–1291. doi:10.1016/j.biortech.2004.10.021.
Hong, S., Wen, C., He, J., Gan, F., & Ho, Y. S. (2009). Adsorption thermodynamics of methylene blue onto bentonite. Journal of Hazardous Materials, 167(1–3), 630–633. doi:10.1016/j.jhazmat.2009.01.014.
Khambhaty, Y., Mody, K., Basha, S., & Jha, B. (2009). Biosorption of Cr(VI) onto marine Aspergillus niger: experimental studies and pseudo-second order kinetics. World Journal of Microbiology and Biotechnology, 25(8), 1413–1421. doi:10.1007/s11274-009-0028-0.
Koumanova, B., Peeva, P., Allen, S. J., Gallagher, K. A., & Healy, M. G. (2002). Biosorption from aqueous solutions by eggshell membranes and Rhizopus oryzae: equilibrium and kinetic studies. Journal of Chemical Technology and Biotechnology, 77(5), 539–545. doi:10.1002/jctb.601.
Lagergren, S. (1898). Zur theorie der sogenannten adsorption gelöster stoffe. Kungliga Svenska Vetenskapsakademiens, Handlingar, 24(4), 1–39.
Langmuir, I. (1916). The constitution and fundamental properties of solids and liquids. Part I. Solids. Journal of the American Chemical Society, 38, 2221–2295. doi:10.1021/ja02268a002.
Leon-Santiesteban, H., Meraz, M., Wrobel, K., & Tomasini, A. (2011). Pentachlorophenol sorption in nylon fiber and removal by immobilized Rhizopus oryzae ENHE. Journal of Hazardous Materials, 190(1–3), 707–712. doi:10.1016/j.jhazmat.2011.03.101.
Luo, L., Lou, L. P., Cui, X. Y., Wu, B. B., Hou, J. A., Xun, B., et al. (2011). Sorption and desorption of pentachlorophenol to black carbon of three different origins. Journal of Hazardous Materials, 185(2–3), 639–646. doi:10.1016/j.jhazmat.2010.09.066.
Majumdar, S. S., Das, S. K., Saha, T., Panda, G. C., Bandyopadhyoy, T., & Guha, A. K. (2008). Adsorption behavior of copper ions on Mucor rouxii biomass through microscopic and FTIR analysis. Colloids and Surfaces B: Biointerfaces, 63(1), 138–145. doi:10.1016/j.colsurfb.2007.11.022.
Mathialagan, T., & Viraraghavan, T. (2009). Biosorption of pentachlorophenol from aqueous solutions by a fungal biomass. Bioresource Technology, 100(2), 549–558. doi:10.1016/j.biortech.2008.06.054.
Montiel, A. M., Fernandez, F. J., Marcial, J., Soriano, J., Barrios-Gonzalez, J., & Tomasini, A. (2004). A fungal phenoloxidase (tyrosinase) involved in pentachlorophenol degradation. Biotechnology Letters, 26(17), 1353–1357. doi:10.1023/b:bile.0000045632.36401.86.
Pizzigallo, M. D. R., Di Leo, P., Ancona, V., Spagnuolo, M., & Schingaro, E. (2011). Effect of aging on catalytic properties in mechanochemical degradation of pentachlorophenol by birnessite. Chemosphere, 82(4), 627–634. doi:10.1016/j.chemosphere.2010.09.076.
Redlich, O., & Peterson, D. L. (1959). A useful adsorption isotherm. Journal of Physical Chemistry, 63(6), 1024–1026. doi:10.1021/j150576a611.
Sanghi, R., Sankararamakrishnan, N., & Dave, B. C. (2009). Fungal bioremediation of chromates: conformational changes of biomass during sequestration, binding, and reduction of hexavalent chromium ions. Journal of Hazardous Materials, 169(1–3), 1074–1080. doi:10.1016/j.jhazmat.2009.04.056.
Sathishkumar, M., Binupriya, A. R., Kavitha, D., Selvakumar, R., Jayabalan, R., Choi, J. G., et al. (2009). Adsorption potential of maize cob carbon for 2,4-dichlorophenol removal from aqueous solutions: equilibrium, kinetics and thermodynamics modeling. Chemical Engineering Journal, 147(2–3), 265–271. doi:10.1016/j.cej.2008.07.020.
Szewczyk, R., & Dlugonski, J. (2009). Pentachlorophenol and spent engine oil degradation by Mucor ramosissimus. International Biodeterioration & Biodegradation, 63(2), 123–129. doi:10.1016/j.ibiod.2008.08.001.
Tomasini, A., Flores, V., Cortes, D., & Barrios-Gonzalez, J. (2001). An isolate of Rhizopus nigricans capable of tolerating and removing pentachlorophenol. World Journal of Microbiology and Biotechnology, 17(2), 201–205. doi:10.1023/a:1016694720608.
Tsezos, M., & Bell, J. P. (1989). Comparison of the biosorption and desorption of hazardous organic pollutants by live and dead biomass. Water Research, 23(5), 561–568. doi:10.1016/0043-1354(89)90022-5.
Vaidyanathan, V. G., Villalta, P. W., & Sturla, S. J. (2007). Nucleobase-dependent reactivity of a quinone metabolite of pentachlorophenol. Chemical Research in Toxicology, 20(6), 913–919. doi:10.1021/tx600359d.
Wang, J. L., Qian, Y., Horan, N., & Stentiford, E. (2000). Bioadsorption of pentachlorophenol (PCP) from aqueous solution by activated sludge biomass. Bioresource Technology, 75(2), 157–161.
Yang, C., Yu, Z. Q., Xiao, B. H., Huang, W. L., Fu, J. M., & Dang, Z. (2009). Impact of Kerogen heterogeneity on sorption of organic pollutants. 2. Sorption equilibria. Environmental Toxicology and Chemistry, 28(8), 1592–1598.
Yee, N., Benning, L. G., Phoenix, V. R., & Ferris, F. G. (2004). Characterization of metal-cyanobacteria sorption reactions: a combined macroscopic and infrared spectroscopic investigation. Environmental Science & Technology, 38(3), 775–782. doi:10.1021/es0346680.
Acknowledgments
H. H. León-Santiesteban acknowledges the financial support from Consejo Nacional de Ciencia y Tecnología, Mexico, through fellowship number 211618.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
León-Santiesteban, H.H., Wrobel, K., García, L.A. et al. Pentachlorophenol Sorption by Rhizopus oryzae ENHE: pH and Temperature Effects. Water Air Soil Pollut 225, 1947 (2014). https://doi.org/10.1007/s11270-014-1947-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-014-1947-4