Abstract
The phosphorus (P) and cadmium (Cd) speciation was analyzed in a contaminated soil having a Cd concentration of 1,028 mg kg−1 in order to assess the value of bone char (BC) as a Cd immobilizing agent. The soil was incubated with BC and triple superphosphate (TSP, as control) in the dark at 60 to 70 % water holding capacity for time periods between 1 and 145 days. Samples from the various incubation periods were sequentially extracted and investigated by X-ray absorption near edge structure (XANES) spectroscopy. The sequential P extraction revealed that BC increased the H2SO4 extractable P fraction, mainly consisting of Ca- and Mg-phosphates, by 14 %, whereas TSP increased the water extractable P fraction by 7 % of total P at day 1. Subsequently, the proportions of these two P fractions decreased during incubation. The increase in these two P fractions is explained by the solubility of the main components of BC (hydroxylapatite (HAP)) and TSP (Ca(H2PO4)2). Furthermore, this finding was confirmed for BC by the P K-edge XANES spectra using partial least square regression that provided independent evidence for increased proportion of HAP after BC application. As a result of BC dissolution, the soil pH increased resulting in Cd immobilization as indicated by significantly reduced concentrations of mobile Cd in the sequential extraction up to 23 mg Cd kg−1. This observed immobilization was explained by the Cd L 3 -edge XANES spectra revealing an immediate increase in the proportion of insoluble Cd3(PO4)2. By contrast, none of the speciation methods indicated a Cd immobilizing capability of TSP. Thus, by using this multi-methodological approach, we could show that besides its potential as renewable and clean P fertilizer, BC is a superior immobilization agent for Cd compared to TSP.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
High cadmium (Cd) concentrations are a serious environmental problem in various agro-ecosystems and urban areas due to industrial pollution and the progressive use of Cd-contaminated phosphorus (P) fertilizer (Wuana and Okieimen 2011). Therefore, cost-effective remediation techniques for in situ immobilization of Cd in soil are urgently required to prevent mobile Cd from entering the food chain by plant uptake (Adriano 2001). Several studies have shown that Cd can be immobilized by P application, reducing the amount of Cd taken up by plants (Naidu et al. 1994; Bolan et al. 1999, 2003; Chaiyarat et al. 2011). However, P is getting a worldwide limited resource because estimates indicated that the world’s reserves will only last for about 50 to 125 years (Gilbert 2009). Thus, benefits of alternative P sources have to be investigated in more detail not only for their potential to immobilize Cd effectively, but also for their fertilizer value to provide sufficient plant-available P for crop production.
Bone char (BC) is one of these promising alternative P-rich renewable resources, containing 150 g P kg−1, 280 g calcium (Ca) kg−1, and 6.5 g magnesium (Mg) kg−1 but virtually no Cd and uranium (U) (Siebers and Leinweber 2013). Initial incubation experiments have proven that BC increased the concentration of labile P immediately after application and immobilized up to 75 % of the mobile Cd in various contaminated soils (Siebers and Leinweber 2013). The immobilization of Cd after BC application was mainly attributed to a soil pH increase resulting in more negatively charged functional groups at reactive soil surfaces that absorb mobile Cd (Naidu et al. 1994; Siebers and Leinweber 2013). This liming effect did not last over the complete incubation period, since 34 days after BC application the pH started to slowly decrease (Siebers and Leinweber 2013). However, there was no concomitant re-mobilization of Cd observed, yet Cd binding increased up to an incubation of 145 days for most soils, suggesting pH-independent Cd immobilization mechanisms. So far, these mechanisms and the explicit role of P are unknown. Thus, it is of crucial importance to clarify involved changes in Cd speciation and the P composition in soil in order to understand the mechanisms of Cd immobilization following BC application.
Several wet-chemical sequential extractions have been developed to characterize and track changes in P (e.g., Hedley et al. 1982) and Cd (e.g., Zeien and Brümmer 1989) fractions in soils. These fractionations are widely applied because of simplicity and low costs although they yield operationally defined fractions based on chemical solubility leaving the chemical nature of species incompletely understood. An attempt to compensate these method-dependent limitations is the application of a complementary speciation technique such as Raman spectroscopy to identify metal phosphates in soil (Lanfranco et al. 2003). However, problematic are the background fluorescence masking the underlying Raman spectrum or the high concentrations of metal phosphates necessary to clearly distinguish various metal phosphates like Cd- or Pb-phosphates from each other (Lanfranco et al. 2003). Alternatively, the use of X-ray adsorption near edge structure (XANES) spectroscopy to the same sample set is promising, as was already done for P in environmental samples (e.g., Toor et al. 2005; Shober et al. 2006; Kruse et al. 2010). XANES spectroscopy enables element-specific in situ speciation and requires only minimal sample preparation (e.g., Beauchemin et al. 2003; Lombi and Susini 2009). However, so far, only Cd L-edge XANES was applied to various biological samples (e.g., Pickering et al. 1999; Isaure et al. 2006; Jalilehvand et al. 2009) and Cd K-edge XANES was not yet applied to Cd speciation in soils. Until now, there was only one recent successful proof-of-concept study using Cd L 3 -edge XANES for soil Cd speciation (Siebers et al. 2012b). In summary, to the best of our knowledge, sequential Cd fractionation and Cd XANES (neither K- nor L-edge) have not been applied in conjunction to speciate Cd in soil so far or combined with analogous measurements for P speciation.
Therefore, the objectives of the present study were (1) to track the changes in P and Cd speciation following P application and incubation, (2) to reveal Cd immobilization mechanisms, and (3) to compare the ability of BC as an immobilization agent with triple superphosphate (TSP). We hypothesize that complementing the sequential extractions by P and Cd XANES spectroscopy enables disclosing Cd phases newly formed after BC application.
2 Materials and Methods
2.1 Soil Sample Analyses
A topsoil (0 to 30 cm) sample was taken from a site contaminated with a mixture of sludge waste from electronic industries in southern Thuringia, Germany. All samples for soil characterization were analyzed in duplicate. Soil texture is determined using the pipette method (Rowell 1994). The pH of the soil is measured in 0.1 M CaCl2 solution at a soil/solution ratio of 1:2.5 (Rowell 1994) and cation exchange capacity (CEC) is determined as described in Blume et al. (2011). Total C concentration is determined by using a CNS-Analyzer (CNS elemental analyzer; Vario EL Fa. Foss Heraeus, Hanau, Germany). The concentration of inorganic C is determined by digestion of carbonates with 10 % HCl using the Scheibler apparatus (Austrian Standards Institute 1999) and organic C estimated by subtraction from the total C content. Total Cd (Cdt) and P (Pt) concentrations of the soil samples are determined after microwave-assisted digestion with HNO3 and HCl (USEPA 1997) using inductively coupled plasma–optical emission spectroscopy (ICP-OES) (JY 238, Jobin Yvon, France) at wavelengths of 214.914 nm (P) and 214.438 nm (Cd). Two blanks were included in each run in order to determine the method error. The soil had a particle size distribution of 27 % clay, 55 % silt and 18 % sand, a pH of 6.2, a CEC of 25.2 cmol kg−1, 70 g kg−1 organic C, and a Pt and Cdt concentration of 3642 mg kg−1 and 1028 mg kg−1, respectively.
2.2 Fertilizer Treatments and Soil Incubation Experiment
Samples of BC, produced from degreased animal bone chips by pyrolysis at 700 to 800 °C, and TSP fertilizer were crushed using a mortar and pestle to pass a 90 μm mesh sieve to minimize particle size effects among fertilizers. For detailed chemical and physical properties of both fertilizers see Siebers and Leinweber (2013). In short, BC had the following concentrations: total P 152.0 g kg−1, water extractable P 6.6 g kg−1, total Ca 280.0 g kg−1, total Mg 6.5 g kg−1, total C 130.1 g kg−1, total N 18.7 g kg−1, total S 1.7 g kg−1, and total Cd 0.3 mg kg−1, whereas the concentrations of TSP were: total P 178.2 g kg−1, water extractable P 9.2 g kg−1, total Ca 129.1 g kg−1, total Mg 8.9 g kg−1, and total Cd 27.4 mg kg−1. Bone char and TSP were added to ten grams air dry soil (<2 mm) to increase the initial P concentration of the soil by 0 (control) and 1000 mg P kg−1. The addition of P fertilizer increased the total P to 4739 mg kg−1 (BC) and 4641 mg kg−1 (TSP). Samples were placed in 30 mL plastic containers which were sealed with a perforated lid to allow ventilation during incubation at 20 °C in the dark. The soil moisture content was maintained constant between 60 to 70 % of the water holding capacity by daily addition of deionized water. Triplicate samples were removed from the incubation experiment after 1, 3, 5, 13, 34, 70, and 145 days, air dried, and ground to <2 mm for further analyses. For XANES data analysis replicates of each variant were pooled to reduce the number of samples.
2.3 Sequential P and Cd Fractionation
The sequential P fractionation proposed by Hedley et al. (1982) was a slightly modified. Briefly, 0.5 g air dried and ground soil sample was placed into a centrifuge tube and 30 mL DI-water was added. The solution was shaken for 18 h on a reciprocal shaker with a rotary speed of 20 rpm. Afterwards the sample was centrifuged (2700 × g for 20 min) and decanted. The next subsequent extractions were done using 0.5 M NaHCO3 (pH 8.5), 0.1 M NaOH, and 1 M H2SO4 to access the following P fractions: (1) H2O-P (easily exchangeable and soil solution P), (2) NaHCO3-P (labile inorganic and organic P as well as some microbial P), (3) NaOH-P (inorganic and organic P sorbed and/or fixed by Al and Fe oxide minerals and P in humic and fulvic acids), (4) H2SO4-P (P associated with Ca and Mg minerals). Total P concentrations in all extracts were measured by ICP-OES (JY 238, Jobin Yvon, France). The concentrations of inorganic P (Pi) were measured colorimetrically using the malachite-green method. The absorption at 630 nm was determined using a UV–Vis spectrophotometer (Spectonic Genesys 5, Milton Roy Company, Rochester, NY 14625-2783, USA). The concentrations of organic P (Po) were calculated as the difference between Pt and Pi.
The Cd was sequentially fractionated according to Zeien and Brümmer (1989). Briefly, 1 g of air-dried and sieved soil was shaken on a reciprocal shaker with a rotary speed of 20 rpm together with 25 mL of extracting agent. Afterwards the sample was centrifuged (3,000 × g for 10 min) and decanted. The extractions steps 2 to 6 were repeated once using 25 mL of the extracting agent of the step before (shaken 10 min) and both filtrates for each fraction were combined. Fractions 1 to 3 were stabilized with 0.5 mL 65 % HNO3 before ICP measurement. The following seven Cd fractions were differentiated: (1) 1 M NH4NO3 (mobile Cd), (2) 1 M NH4-acetate (easily mobilizable Cd), (3) 0.1 M [NH3(OH)]Cl + 1 M NH4-acetate (Cd bound to Mn-(hydr)oxides), (4) 0.025 M NH4-EDTA (Cd bound to soil organic matter), (5) 0.2 M NH4-oxalatebuffer-Cd (Cd bound by low crystalline Fe-(hydr)oxides), (6) 0.2 M NH4-oxalatebuffer + ascorbic acid-Cd (Cd bound by crystalline Fe-(hydr)oxides), and (7) residual Cd being the total Cd concentration minus the sum of fraction 1 to 6.
2.4 XANES Data Collection
All XANES experiments were conducted at the Canadian Light Source in Saskatoon, Saskatchewan, Canada, on the Soft X-Ray Microcharacterization beamline (ΔE/E:~10−4), covering an energy range of 1.7 to 10 keV. Air-dried soil samples were ground in an agate stone mortar and spread as a thin film onto a P-free carbon tape that was mounted onto a cupper sample holder. The sample holder was then placed into a vacuum chamber. All spectra were recorded in fluorescence yield mode at photon energies between 2,130 and 2,200 eV for P K-edge and 3,500 to 3,600 eV for Cd L 3-edge. The step size for P and Cd measurement was 1 eV in the pre-edge region (P, 2,130 to 2,140 eV; Cd, 3,500 to 3,530 eV), 0.25 eV at the edge step (P, 2,140 to 2,180 eV; Cd, 3,530 to 3,580 eV), and 0.5 in the post-edge region (P, 2,180 to 2,200 eV; Cd, 3,580 to 3,650 eV) for P and 1 for Cd. A dwell time of 4 s for soil samples was used and at least four scans were averaged. After each scan, the sample holder position was changed so that the beam always hit a “fresh” sample spot.
2.5 XANES Data Analyses
Background and baseline correction of the XANES spectra was done using ATHENA (Ravel and Newville 2005). For partial least square (PLS) analysis, the PLS package (Wehrens and Mevik 2007) of R 2.13.0 (R Development Core Team 2011) was used. Qualitative data evaluation of Cd was based on the previously published PLS regression model, which was developed in a previous publication (Siebers et al. 2012). This model incorporated XANES spectra measured at the Cd L 3-edge XANES of the following compounds: Cd(NO3)2·4H2O, CdCl2, CdSO4, Cd3(PO4)2/Cd-acetate, CdCO3, and Cd(OH)2. Pretest showed that PLS regression was not able to differentiate Cd3(PO4)2 and Cd-acetate (representing Cd bound to organics) (Siebers et al. 2012) so that only Cd3(PO4)2 was used for further analysis representing both compounds (Cd3(PO4)2/Cd-acetate). For the P K-edge PLS regression model, the following P reference compounds were used: Cd3(PO4)2, FePO4·4H2O, Ca(H2PO4)2, KH2PO4, phytic acid Na salt, Mg3(PO4)2 5H2O, AlPO4·H2O, and hydroxylapatite (HAP). All reference compounds were purchased from Sigma Aldrich in reagent grade and ground to a fine powder using an agate stone mortar and pestle. Mathematical binary to quaternary mixtures (Siebers et al. 2012) were computed by integrating normalized spectra of the respective reference compounds in varying proportions and normalization of the resulting spectra as described below. The PLS model was built using 190 independent variables (data points) in the energy range between 2,145.25 and 2,200 eV.
2.6 Statistical Analyses
Data were statistically analyzed with IBM SPSS 20 using general analysis of variance (ANOVA) (Tukey test, *P < 0.05; **P < 0.01; ***P < 0.001) for the effect of BC and TSP.
3 Results and Discussion
3.1 Soil pH
The initial soil pH was 6.2, and incubation of soils with BC significantly increased the soil pH relative to the control, whereas soil pH was significantly lowered when TSP was added (Fig. 1).
For BC, the maximum rise in soil pH was observed after 34 days of incubation with an increase of 0.12 units. By contrast, TSP reduced the soil pH most strongly right after application by about 0.36 units. A similar effect of BC and TSP was observed in comparable incubation experiments with various acidic soils (Siebers and Leinweber 2013) and explained by (1) consumption of protons during the dissolution of hydroxylapatite (main component of BC), (2) ligand exchange reactions (Bolan et al. 2003), and (3) the release of bases during BC dissolution and their corresponding neutralization by protons. The increase in soil pH after TSP addition is due to the release of phosphoric acid during TSP dissolution (Kolay 2007) because the water soluble Ca(H2PO4)2 is the main component of TSP. This also explains the immediate drop in pH after TSP application, whereas BC had a delaying effect on soil pH because it consists of less soluble hydroxylapatites.
3.2 Sequentially Extracted P and Cd Fractions
The total recovery of P for all treatments was relatively constant between 82 and 99 % during incubation (Table 1). In the untreated (control) soil, NaOH-P (47 % of Pt) made up the highest proportion amongst all fractions with, followed by H2SO4-P (40 % of Pt). The labile fractions (H2O-P, NaHCO3-Pi, NaHCO3-Po) had much lower P concentrations and proportions of Pt (Table 1). This distribution pattern of the P fractions was similar to those reported in many other studies as compiled by Negassa and Leinweber (2009). However, it changed significantly after fertilizer application (day 1) and differed between both treatments. In general, TSP application resulted in a significantly (P < 0.01) higher proportion of labile P (H2O-P, NaHCO3-Pi, and NaHCO3-Po) compared to BC during the whole incubation, whereas BC application significantly (P < 0.01) increased the proportion of insoluble P (H2SO4-P). With ongoing incubation, the soluble P (H2O-P) gradually decreased by 5 % of Pt between days 1 and 145. A similar but less pronounced trend was observed for insoluble P (H2SO4-P) which decreased by 6 % of Pt in the same time period. TSP significantly increased the NaHCO3-Po pool at all incubation times, unlike the NaOH-Po fraction which ranged relatively constant from 23 to 26 % of Pt (Table 1).
In the TSP treatment, the increase in labile P was anticipated as TSP mainly consists of the highly water soluble Ca(H2PO4)2, whereas the addition of BC did not change the proportion of labile P due to its lower solubility (Warren et al. 2009; Siebers and Leinweber 2013). The successive reduction of labile P in the TSP treatment with incubation time might be a result of microbial incorporation and fixation, which has been indicated by the significant increase in NaOH-Po that contains some microbial P (Tiessen et al. 1983). Additionally, H2O-P is likely to be redistributed to less labile P with time. The NaHCO3-Pi fraction in the TSP treatment remained constant at a high level during the incubation period, which could be a result of completely covered adsorption sites. Phosphorus possibly redistributed from the NaHCO3-P fraction to more immobile forms is then replaced by P of the H2O-P fraction as anticipated from the ongoing decrease of H2O-P with incubation. The increase in NaHCO3-Po after TSP application is in accordance with literature, as Po has been found to build up in soils as a result of Pi fertilization (Dalal 1977; Stewart and Tiessen 1987). A similar increase in NaHCO3-Po was also observed by Zheng et al. (2004) after addition of a mineral P fertilizer, likely from microbial growth with increased availability of labile P. Linquist et al. (1997) found that the NaHCO3-Po pool was positively correlated with labile Pi, also confirming the observed increase in NaHCO3-Po after TSP application. The significant increase in NaOH-Po after TSP application for day 1 might be a result of the same reason as for the NaHCO3-Po pool. However, for the subsequent incubation, the increase was insignificant. In the BC treatment, the immediate significant increase in the H2SO4-P fraction indicates a direct effect of Ca-P from BC that contains about 80 % of H2SO4-P. The subsequent decrease in this fraction is due to BC dissolution.
The total recovery of Cd for all treatments was between 76 and 95 % (Table 2). In the untreated soil, the Cd concentrations in the different fractions followed the order 2 > 1 = 3 > 7 > 4 > 5 > 6, and 65 to 85 % of the total Cd were found in the first three fractions (Table 2). The overall order was not altered with fertilizer addition, but the relative proportions of sequentially extracted fractions differed among treatments. After BC addition the first fraction was significantly (P < 0.01) reduced by 1 to 3 % compared to the control, whereas in the TSP treatment the second fraction was significantly (P < 0.01) lowered up to 10 % for most of the samples. Additionally, the fourth fraction was significantly (P < 0.05 and P < 0.01) increased after BC addition for most samples. The other Cd fractions did not change through fertilizer application. Additionally, the Cd fractions remained constant over incubation time in all treatments (Table 2).
The estimated Cd composition of the soil confirms various studies that showed the majority of total Cd bound in the first three labile fractions (e.g., Ahnstrom and Parker 1999; Zimmer et al. 2011). The redistribution of labile Cd to more immobile fractions after BC application is explained by the increase in pH, as it promotes the adsorption of Cd onto the solid phase (Seuntjens et al. 2004; Amini et al. 2005). With increase in soil pH, the negative charging of soil surfaces is promoted, resulting in an increased adsorption of Cd (Naidu et al. 1994). However, besides the pH effect, Cd immobilization might also be a result of the formation and precipitation of other insoluble Cd, i.e., Cd as metal phosphates, or the adsorption of Cd onto the surface of not yet dissolved BC particles (Valsami-Jones et al. 1998; Hodson et al. 2000; Bolan and Hedley 1990). The reduced Cd concentration in fraction 2 with TSP application resulted from the change in soil pH, since this fraction incorporates Cd bound to carbonates, which dissolve with decreasing pH and release bound Cd (Madrid and Diaz-Barrientos 1992). However, this Cd was not recovered in fraction 1 as was anticipated, yet the residual Cd was significantly increased. This indicates the saturation of the soil solution with Cd and a complete coverage of all potential Cd adsorption sites, which can be explained by the extraordinary high Cd concentrations. The slight increase in Cd bound to organic matter (fraction 4) after BC application is a result of the introduction of organic matter with BC (C = 130 g kg−1), most likely of highly aromatic structure. This increase in fraction 4 render the Cd present in more labile forms immobile (Antoniadis and Alloway 2000), as was confirmed by a decrease of Cd found in fraction 1. A first application of the multiple equation (Eq. 1) established in a previous publication (Siebers and Leinweber 2013) to estimate the Cd immobilization at day 34 after BC addition revealed an amount of 21.4 mg Cd kg−1 to be immobilized. For calculation, the initial soil pH and the difference between NaHCO3-P of the control and the BC treatment for 34 days of incubation was taken (Table 1).
The actual amount of immobilized Cd is 18 mg kg−1 as determined by the difference between NH4NO3 extractable Cd of the control and the BC treatment for day 34 (Table 2). The error between the calculated and measured Cd immobilization is 15.9 %. Thus, by using this equation, a good approximation for immobilized Cd is obtained, confirming the value of this multiple equation for the first time. However, to the best of our knowledge, the mechanisms of the Cd immobilization were not yet directly proven for BC, calling for a direct in situ solid-state P and Cd speciation.
3.3 P K- and Cd L 3 -Edge XANES
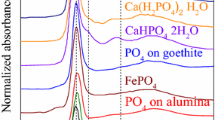
P K-edge and Cd L 3-edge XANES spectra were recorded to directly elucidate the involved changes in P and Cd speciation in the soil due to BC and TSP application. In general, the P K-edge XANES spectra were all similar in shape and visual comparison revealed no pronounced trend in spectral features neither due to fertilizer addition nor incubation time (Fig. 2).
However, since XANES data evaluation by visual comparison is very limited in its validity, PLS regression analysis was applied to deconvolute the P K-edge XANES spectra and estimate individual proportions of P species in the samples. In general, P composition in the samples followed the order: HAP ≥ Cd3(PO4)2 > AlPO4·H2O > Mg3(PO4)2·5H2O (Table 3), whereas FePO4·4H2O, Ca(H2PO4)2, KH2PO4, and phytic acid were not determined. This order did not change with fertilizer addition or with incubation. However, individual proportions differed among the treatments. For instance, the application of BC slightly increased the proportion of HAP compared to the control treatment for days 1, 13, 34, and 145, whereas TSP had no effect. The proportion of Cd3(PO4)2 was also increased after BC addition for days 1, 34, and 145. The TSP application generally decreased the Cd3(PO4)2 proportion. The proportion of AlPO4·H2O increased with fertilizer application at day 1 (only for TSP), 13, 34, and 70. Furthermore, there was a trend towards higher proportions with ongoing incubation in all samples, whereas no systematic effect was visible for Mg3(PO4)2·5H2O (Table 3).
The increased proportions of HAP after BC application are not surprising, as this is the main P compound in BC (Warren et al. 2009). This finding also agrees with the sequential P fractionation showing an increase of H2SO4-P (P associated with Ca) after BC application, which was not observed for the TSP treatment (Table 1). The detection of Cd3(PO4)2 at relatively high proportions in the control sample is explained by the high Cd and P concentrations facilitating the formation of Cd-phosphates (Soon 1981; Xiong 1995). The increase of Cd3(PO4)2 in the BC treatment can be attributed to the additional increase in P concentration and pH in the soil (Fig. 1), as both factors promote the formation of insoluble Cd-phosphates. It is very likely that Cd-phosphates are particularly formed in the direct surrounding or at the surface of BC particles in soil, since the lowest Cd to P ratio can be expected in this region. Then, the pH likely increases stronger in the vicinity of a dissolving BC particle than in the bulk soil. If the same is true for pH deceases close to dissolving TSP particles, this explains why the application of TSP did not lead to the formation of Cd3(PO4)2. Furthermore, the fact that no Ca(H2PO4)2 was detected in the TSP treatments indicated that TSP was already dissolved in the early incubation period. This confirms the P dissolution kinetics of TSP in various acidic soils by Siebers and Leinweber (2013). Organic P (Phytic acid) was not assigned by the fitting procedure (Table 3), which disagrees with sequential P fractionation that found 22 to 33 % of Pt as Po (Table 1). However, it is known that P K-edge XANES fails to identify and quantify Po species (Beauchemin et al. 2003). Therefore, the proportions of P species in Table 3 are considered as relative rather than absolute proportions of Pt (Hesterberg et al. 1999). However, the overall P speciation estimated by P XANES confirmed the results from the sequential P fractionation by showing the majority of P in the moderately labile to stable forms.
The Cd L 3-edge XANES spectra were all characterized by three main features: a sharp peak at around 3,540.2 eV, a shoulder around 3,551.0 eV, and a broad peak around 3,565.5 eV (Fig. 3), which differed only marginally in their intensity and energy position.
A PLS regression analysis model was applied to deconvolute the Cd L 3-edge to estimate individual proportions of Cd species in the soil samples (Table 4). In general, the Cd composition in the samples followed the order Cd-P/Cd-org > CdCl2 ≥ CdCO3 > Cd(OH)2 > CdNO3, whereas no CdSO4 could be assigned. This order did not change with fertilizer application and incubation time. However, compared to the control treatment, an immediate increase in the proportions of Cd-P/Cd-org with BC and TSP addition were observed, whereas the proportions of CdCO3 declined until day 70 of incubation, being more pronounced for TSP (Table 4).
The substantial contribution of CdCO3 (about 20 %) to the soil Cd composition (Table 4) agrees with the view that Cd can precipitate as CdCO3 (fraction 2) at high Cd concentrations in soil (McBride 1980; Prieto et al. 2003). Additionally, the high proportions (about 40 %) of Cd-P/Cd-org (representing fraction 3 and 4) as derived from L 3-edge XANES spectra further support Cd fractionation results found in literature (Ahnstrom and Parker 1999; Zimmer et al. 2011). In general, the overall Cd composition revealed by Cd L 3-edge XANES was slightly different compared to results of the Cd fractionation, estimating Cd-P/Cd-org (representing fraction 3 and 4) as the main component instead of the easily mobilizable Cd fraction 2. However, Cd XANES data confirmed, to some extent, trends of the sequential Cd extraction. The observed decline in the proportion of CdCO3 after TSP addition, which is a result of the decreased soil pH, agrees with observed significant (P < 0.01) reduction of fraction 2 (Table 2), which is commonly assigned to carbonate-bound Cd (Zeien and Brümmer 1989).
4 Conclusions
In conclusion, the present study demonstrated for the first time that the combined application of two separate sequential P and Cd extractions and synchrotron-based P K-edge and Cd L 3-edge XANES spectroscopy enables tracking changes in P and Cd speciation in soils. Use of a direct solid-state P and Cd speciation uncovered the formation of insoluble Cd-phosphates as one reaction resulting in Cd immobilization after BC application. Furthermore, the results indicated that besides its potential as renewable and clean P fertilizer (Siebers and Leinweber 2013), BC is a better immobilization agent for Cd compared than TSP. In a forthcoming study, we will assess the applicability of BC as a clean and renewable P fertilizer with Cd immobilizing capability in the cultivation of various crops.
References
Adriano, D. C. (2001). Trace elements in the terrestrial environments—biochemistry, bioavailability and risk of metals (pp. 450–800). New York: Springer.
Ahnstrom, Z. S., & Parker, D. R. (1999). Development and assessment of a sequential extraction procedure for the fractionation of soil cadmium. Soil Science Society of America Journal, 63, 1650–1658.
Amini, M., Khademi, H., Afyuni, M., & Abbaspour, K. C. (2005). Variability of available cadmium in relation to soil properties and landuse in an arid region in central Iran. Water, Air, and Soil Pollution, 162, 205–218.
Antoniadis, V., & Alloway, B. J. (2000). Availability of Cd, Ni and Zn to ryegrass in sewage sludge-treated soils at different temperatures. Water, Air, and Soil Pollution, 132, 201–214.
Austrian Standards Institute. (1999). ÖNORM L 1084. Chemical analyses of soils: Determination of carbonate. Vienna: Österreichisches Normungsinstitut.
Beauchemin, S., Hesterberg, D., Chou, J., Beauchemin, M., Simard, R. R., & Sayers, E. (2003). Speciation of phosphorus in phosphorus-enriched agricultural soils using X-ray adsorption near-edge structure spectroscopy and chemical fractionation. Journal of Environmental Quality, 32, 1809–1819.
Blume, H. P., Stahr, K., & Leinweber, P. (2011). Bodenkundliches praktikum, vol 3. Heidelberg: Spektrum Akademischer Verlag.
Bolan, N. S., & Hedley, M. J. (1990). Dissolution of phosphate rocks in soils. 2. Effect of pH on the dissolution and plant availability of phosphate rock in soil with pH dependent charge. Fertilizer Research, 24, 125–134.
Bolan, N. S., Naidu, R., Khan, M. A. R., Tillman, R. W., & Syers, J. K. (1999). The effects of anions sorption on sorption and leaching of cadmium. Australian Journal of Soil Research, 3, 445–460.
Bolan, N. S., Adriano, D. C., Duraisamy, P., Mani, A., & Arulmozhiselvan, K. (2003). Immobilization and phytoavailability of cadmium in variable charge soils. I. Effect of phosphate addition. Plant and Soil, 250, 83–94.
Chaiyarat, R., Suebsima, R., Putwattana, N., Kruatrachue, M., & Pokethitiyook, M. (2011). Effects of soil amendments on growth and metal uptake by Ocimum gratissimum grown in Cd/Zn-contaminated soil. Water, Air, and Soil Pollution, 214, 383–392.
Dalal, R. C. (1977). Soil organic phosphorus. Advances in Agronomy, 29, 83–113.
Gilbert, N. (2009). The disappearing nutrient. Nature, 461, 716–718.
Hedley, M. J., Stewart, J. W. B., & Chauhan, B. S. (1982). Changes in organic and organic soil phosphorus fractions induced by cultivation practices and laboratory incubations. Soil Science Society of America Journal, 46, 970–976.
Hesterberg, D., Zhou, W. Q., Hutchison, K. J., Beauchemin, S., & Sayers, D. E. (1999). XAFS study of adsorbed and mineral forms of phosphate. Journal of Synchrotron Radiation, 6, 636–638.
Hodson, M. E., Valsami-Jones, E., & Cotter-Howells, J. D. (2000). Bone meal additions as a remediation treatment for metal contaminated soil. Environmental Science and Technology, 34, 3501–3507.
Isaure, M. P., Fayard, B., Sarret, G., Pairis, S., & Bourguignon, J. (2006). Localization and chemical forms of cadmium in plant samples by combining analytical electron microscopy and X-ray spectromicroscopy. Spectrochimica Acta, 12, 1242–1252.
Jalilehvand, F., Leung, B. O., & Mah, V. (2009). Cadmium(II) complex frmation with cysteine and penicillamine. Inorganic Chemistry, 48, 5758–5771.
Kolay, A. K. (2007). Manures and fertilizer. New Delhi: Atlantic Publisher and Distributors.
Kruse, J., Negassa, W., Appathurai, N., Zuin, L., & Leinweber, P. (2010). Phosphorus speciation in sequentially extracted agro-industrial by-products: evidence from X-ray absorption near edge structure spectroscopy. Journal of Environmental Quality, 39, 2179–2184.
Lanfranco, A. M., Schofield, P. F., Murphy, P. J., Hodson, M. E., Mosselmans, J. F. W., & Valsami-Jones, E. (2003). Characterization and identification of mixed-metal phosphates in soils: the application, of Raman spectroscopy. Mineralogical Magazine, 67, 1299–1316.
Linquist, B. A., Singleton, P. W., & Cassman, K. G. (1997). Inorganic and organic phosphorus dynamics during a build-up and decline of available phosphorus in an ultisol. Soil Science, 162, 254–264.
Lombi, E., & Susini, J. (2009). Synchrotron-based techniques for plant and soil science: opportunities, challenges and future perspectives. Plant and Soil, 320, 1–35.
Madrid, L., & Diaz-Barrientos, E. (1992). Influence of carbonate on the reaction of heavy metals in soils. Journal of Soil Science, 43, 709–721.
McBride, M. B. (1980). Chemisorption of Cd2+ on calcite surfaces. Soil Science Society of America Journal, 44, 26–28.
Naidu, R., Bolan, N. S., Kookana, R. S., & Tiller, K. G. (1994). Ionic strength and pH effects on the adsorption of cadmium and the surface charge of soils. European Journal of Soil Science, 45, 19–429.
Negassa, W., & Leinweber, P. (2009). How does the Hedley sequential phosphorus fractionation reflect impacts of land use and management on soil phosphorus: a review. Journal of Plant Nutrition and Soil Science, 172, 305–325.
Pickering, I. J., Prince, R. C., George, G. N., Rauser, W. E., Wickramasinghe, W. A., Watson, A. A., et al. (1999). X-ray absorption spectroscopy of cadmium phytochelatin and model systems. Biochimica et Biophysica Acta, 1429, 351–364.
Prieto, M., Cubillas, P., & Fernández-González, A. (2003). Uptake of dissolved cadmium by biogenic and abiogenic aragonite: a comparison with sorption onto calcite. Geochimica et Cosmochimica Acta, 67, 3859–3869.
R Development Core Team (2011). Vienna, Austria. ISBN 3-900051_07_0, URL: http://www.R-project.org/.
Ravel, B., & Newville, M. (2005). Athena, Artemis, Hephaestus: data analysis for X-ray absorption spectroscopy using IFEFFIT. Journal of Synchrotron Radiation, 12, 537–541.
Rowell, D. L. (1994). Soil science: Methods and applications. Harlow: Longman.
Seuntjens, P., Nowack, B., & Schulin, R. (2004). Root-zone modeling of heavy metal uptake and leaching in the presence of organic ligands. Plant and Soil, 265, 61–73.
Shober, A. L., Hesterberg, D. L., Thomas Sims, J., & Gardner, S. (2006). Characterization of phosphorus species in biosolids and manures using XANES spectroscopy. Journal of Environmental Quality, 35, 1983–1993.
Siebers, N., & Leinweber, P. (2013). Bone char—a clean and renewable fertilizer with cadmium immobilizing capability. Journal of Environmental Quality. doi:10.2134/jeq2012.0363.
Siebers, N., Kruse, J., Eckhardt, K.-U., Hu, Y., & Leinweber, P. (2012). Solid-phase cadmium speciation in soil using L3-edge XANES spectroscopy with partial least-square regression. Journal of Synchrotron Radiation, 19, 579–585.
Soon, Y. K. (1981). Solubility and sorption of cadmium in soils amended with sewage sludge. Journal of Soil Science, 32, 85–95.
Stewart, J. W. B., & Tiessen, H. (1987). Dynamics of soil organic phosphorus. Biogeochemica, 4, 41–60.
Tiessen, H., Stewart, J. W. B., & Moir, J. O. (1983). Changes in organic and inorganic phosphorus composition of two grassland soils and their particle size fractions during 60–90 years of cultivation. Journal of Soil Science, 34, 815–823.
Toor, G. S., Peak, J. D., & Sims, J. T. (2005). Phosphorus speciation in broiler litter and turkey manure produced from modified diets. Journal of Environmental Quality, 34, 687–697.
U.S. Environmental Protection Agency. (1997). Method 305la: Microwave assisted acid dissolution of sediments, sludges, soils, and oils (2nd ed.). Washington: U.S. Gov. Print. Office.
Valsami-Jones, E., Ragnarsdottir, K. V., Putnis, A., Bosbach, D., Kemp, A. J., & Cressey, G. (1998). The dissolution of apatite in the presence of aqueous metal cations at pH 2–7. Chemical Geology, 151, 215–233.
Warren, G. P., Robinson, J. S., & Someus, E. (2009). Dissolution of phosphorus from animal bone char in 12 soils. Nutrient Cycling in Agroecosystems, 84, 167–178.
Wehrens, R., & Mevik, B. H. (2007). R package version 2.1-0-http://mevik.net/work/software/pls.html.
Wuana, R. A., & Okieimen, F. E. (2011). Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. International Scholarly Research Network Ecology. doi:10.5402/2011/402647.
Xiong, L. M. (1995). Influence of phosphate on cadmium adsorption by soils. Fertilizer Research, 40, 31–40.
Zeien, H., & Brümmer, G. W. (1989). Chemische Extraktionen zur Bestimmung von Schwermetallbindungsformen in Böden. Mitteilungen der Deutschen Bodenkundlichen Gesellschaft, 59, 505–510.
Zheng, Z., MacLeod, J. A., Sanderson, J. B., & Lafond, J. (2004). Phosphorus dynamics after ten annual applications of mineral fertilizers and liquid dairy manure: fractionation and path analyses. Soil Science, 169, 449–456.
Zimmer, D., Kiersch, K., Baum, C., Meissner, R., Müller, R., Jandl, G., et al. (2011). Scale-dependent variability of As and heavy metals in a River Elbe floodplain. Clean - Soil, Air, Water, 39, 328–337.
Acknowledgments
Research described in this paper was performed in the scope of the Leibniz WissenschaftsCampus “Phosphor-Forschung-Rostock” and was done at the Canadian Light Source, which is supported by the Natural Sciences and Engineering Research Council of Canada, the National Research Council Canada, the Canadian Institutes of Health Research, the Province of Saskatchewan, Western Economic Diversification Canada, and the University of Saskatchewan. We thank Dr. Volkmar König, Thuringian State Research Centre for Agriculture (TLL), for providing the soil used in this study. N. Siebers also acknowledges a Ph.D. grant from the Federal State of Mecklenburg, Western Pommeria in Germany.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Siebers, N., Kruse, J. & Leinweber, P. Speciation of Phosphorus and Cadmium in a Contaminated Soil Amended with Bone Char: Sequential Fractionations and XANES Spectroscopy. Water Air Soil Pollut 224, 1564 (2013). https://doi.org/10.1007/s11270-013-1564-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-013-1564-7