Abstract
The effects of various experimental parameters on adsorption of Zn2+ metal ion from its aqueous solution by castor seed hull and also by activated carbon have been investigated using batch adsorption experiments. It has been found that the amount of zinc adsorbed per unit mass of the hull increases with the initial metal ion concentration, contact time, solution pH and with the amount of the adsorbent. Kinetic experiments clearly indicate that adsorption of zinc on both castor hull and activated carbon is a three-step process—a rapid adsorption of the metal ion, a transition phase, and an almost flat plateau. This has also been confirmed by the intraparticle diffusion model. It has also been found that the zinc adsorption process followed pseudo-second order kinetics. The kinetic parameters including rate constants have been determined at different initial metal ion concentration, pH, amount, and type of adsorbent, respectively. The Langmuir and Freundlich adsorption isotherm models have been used to interpret the equilibrium adsorption data. The Langmuir model yields better correlation coefficients. The monolayer adsorption capacities (q m ) of castor hull and activated carbon have been compared with those for others reported in the literature. The value of separation factor (R L ) derived from the Langmuir model gives an indication of favorable adsorption. Finally, from comparative studies, it has been found that castor hull is a potentially attractive adsorbent as compared to commercial activated carbon for the removal of zinc from aqueus effluents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Heavy metals belong to the class of hazardous pollutants due to their toxicity even at low concentrations (Sen and Meimon 2008). Zinc is considered as an essential element for life and act as a micronutrient when present in trace amounts Bhattacharya et al. (2006). The WHO recommended the maximum acceptable concentration of zinc in drinking water as 5.0 mg/L (Mohan and Singh 2002); it is toxic above this limit. The main sources of zinc in waste water are effluents from chemicals, pulp and paper manufacturing processes, steel works with galvanizing lines, zinc, and brass plating, viscous rayon yarn and fiber production etc. (Mohan and Singh 2002). The fate and transport of metal ions including Zn2+ in natural water as well as in industrial effluents are often controlled by their interactions with adsorbents under different environmental conditions (Arias and Sen 2009; Sen and Khilar 2006). Various treatment processes such as precipitation, ion exchange, filtration, oxidation, reduction, dialysis/electrodialysis, solvent extraction, membrane technology and adsorption on activated carbon are the conventional methods for the removal of heavy metal ions from aqueous solutions. But most of these techniques may not be effective or may be extremely expensive when a large volume of waste at relatively low concentrations of the metal is to be treated (Acharya et al. 2009; Zhu et al. 2007; Gurses et al. 2006; Sen et al. 2002). In addition, process limitations including inadequate metal removal, requirements for expensive equipment and monitoring systems, high reagent, and energy requirements exist in practice (Demiral et al. 2008). Among these processes, the adsorption on a suitable cheap material can be an effective technique for the removal of heavy metal ions from waste waters (Arias and Sen 2009; Sen and Meimon 2008; Acharya et al. 2009; Gurses et al. 2006). Basically, adsorption has proven advantages over alternative methods because of simple design with a sludge-free environment and involves low investment in terms of both initial cost and land requirement. Activated carbon has undoubtedly been the most popular and widely used effective adsorbent for the removal of heavy metal ions from concentrated as well as dilute metal-bearing effluents (Sen and Meimon 2008; Zhu et al. 2007). Despite its extensive use in the water and wastewater treatment industries, activated carbon remains an expensive material. Further, regeneration of activated carbon is not easy; it requires the use of a complexing agent such as EDTA for efficient removal of adsorbed metal ions from the spent adsorbent (Babel and Kurniawan 2003). Research efforts for the identification of alternative adsorbents to replace the costly activated carbon have been intensified in recent years as a result.
The potential use of various agricultural products and byproducts for the removal of heavy metals from solution is well-documented (Bhattacharya et al. 2006; Mohan and Singh 2002). Naturally occurring materials such as chitosan, zeolites, and clays and industrial solid wastes such as fly ash, red mud, etc., are categorized as low cost adsorbents (Babel and Kurniawan 2003; Bhattacharyya and Sen Gupta 2008). A variety of agricultural byproducts such as almond shell, olive and peach stones, and waste tea leaves have been explored as effective low cost adsorbents for removal of Zn2+, Cd2+, and Cu2+ (Kandah 2004). Adsorption of heavy metals at solid-liquid interfaces has been extensively studied in the last 10 years or more. In general, adsorption of inorganic and organics at solid/liquid interface depends on many factors such as contact time, initial metal ion concentration, solution pH, ionic strength, amount of adsorbent, and temperature (Sen and Meimon 2008; Acharya et al. 2009; Zhu et al. 2007; Gurses et al. 2006; Naiya et al. 2008).
The primary objective of this research was to explore the potential of castor seed hull (CSH) as a low cost adsorbent for Zn2+ removal from its aqueous solution and to investigate adsorption characteristic of Zn2+ on castor hull as well as activated carbon. Castor seed hull (an agricultural product) is the residue obtained in the process of oil extraction from the seed of the castor plant (Ricinus communis). Since castor seed contains roughly 50% oil and 50% residual meal/hull, a large tonnage of meal/hull is generated in many countries worldwide. In this work, laboratory batch kinetic and equilibrium adsorption studies were conducted to determine the adsorption capacity of castor seed hull, kinetic rate constants, and equilibrium adsorption isotherm parameters under different experimental conditions. The effects of initial solution pH, amount of adsorbent, contact time, and initial concentration of Zn2+ on adsorption by CSH are presented. Finally, the results have been compared with Zn2+ adsorption on activated carbon under identical experimental conditions.
2 Materials and Methods
2.1 The Adsorbents
The castor seed hull used as adsorbent in this study was obtained from a local market in Indonesia. It was repeatedly washed with water to remove adhering dirt and soluble components and color and then oven dried overnight at 50-60°C to a constant weight. The washed and dried material was crushed. It was characterized by Fourier-transformed infrared spectrometry (FTIR, Perkin Elmer), scanning electron microscopy (Oxford Instruments, model. LEO 1430VP) and other measurements. The particle size (0.15-1.18 mm) was determined by Malvern particle size analyzer and Brennaur-Emmet-Taylor (BET) surface area (8.9 m2/g) was measured by a standard BET apparatus (Quantachrome Autosorb) by ususal nitrogen adsorption method. Commercial activated carbon (Merck) was used as such after drying at a temperature of 60°C in a temperature-controlled oven.
2.2 Chemicals Used
All chemicals including activated carbon were of analytical grade and purchased from Merck. Stock standard solution of Zn2+ has been prepared by dissolving the appropriate amount of its nitrate in deionized water. This stock solution was then diluting to specified concentrations.
2.3 Adsorption Experiments
Adsorption rates and equilibrium were measured by separate sets of experiments for a range of values of the relevant system parameters. The former were measured by batch contact of the adsorbent with the solution for varying times (Arias and Sen 2009; Sen and Meimon 2008). In an experiment, 25 ml of zinc nitrate solution of appropriate concentration and the requisite quantity of castor hull (0.05–15 gm) were taken in a 60 ml vial and the pH was adjusted to 3-6 by using NaOH and/or HNO3. The vials were put in a shaker running at 120 rpm for different times up to 350 min. The temperature was maintained at 24 ± 5°C. A small sample of the solution was filtered using Whatman microfilter, suitably diluted then analyzed for Zn2+ concentration using an atomic absorption spectrophotometer with air-acetylene flame. The Zn2+ concentration retained in the adsorbent phase was calculated according to
Where q t is the mass of Zn2+ adsorbed per unit mass of the adsorbent (mg/gm), C 0 (mg/L), and C t (mg/L) are the concentration in the solution at time t = 0 and at time t, V is the volume of solution (L) and m is the amount of adsorbent (g) added.
Equilibrium experiments were conducted following the above procedure except that a solid-liquid contact time of 350 min was allowed. From the above batch experiments, it was found that maximum removal of the metal ion was achieved in about 200 min and an extended time of 350 min was allowed in equilibrium experiments to avoid any uncertainty. Calculation of uptake of metal ion was dome by Eq (1).
3 Results and Discussion
3.1 Effect of Initial Solution pH on Metal Ion Adsorption
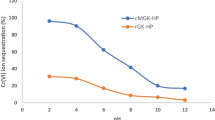
The pH of the adsorbate solutions has been reported as the most important parameter governing adsorption on different adsorbents (Ajmal et al. 2000). It is known that metal species (M(II) = Zn2+) may be present in different forms such as M2+, M(OH)+, M(OH)2(S), etc. At pH ∼5.0, the solubility of the M(OH)2(S) is high and therefore, the M2+ is the main species in the solution (Snoeyink and Jenkins 1980). With an increase in pH of the solution, the solubility of M(OH)2(S) decreases and at pH ∼10.0, the solubility of M(OH)2(s) becomes very small. At this pH, the main species in the solution is M(OH)2(S). Therefore, in the alkaline range, the metal ion precipitation plays the major role in the process of its removal. To avoid precipitation of the metal ion, all the experiments were carried out at a maximum initial solution pH of 6.0. The effect of initial solution pH on adsorption of Zn2+ by castor hull at two different initial concentrations over a period of 240 min is shown in Fig. 1. The extent of removal was found to increase when the solution pH was increased from 3.0 to 6.0 in both cases. The maximum uptake of zinc occurred at a pH of 6.0. This dependence of metal uptake on pH may be related to the functional groups in the constituents of castor hull and/or the solution chemistry (Annadurai et al. 2002). The absorption maxima of the FTIR spectra of CSH show that castor seed hull primarily contain weak acidic and basic functional groups and carboxyl groups (−COOH) as the important groups for metal uptake like other agricultural based biomaterials (Norton et al. 2004). The minimal adsorption at low pH may be due to the higher concentration and high mobility of the H+, which are preferentially adsorbed rather than metal ions (Norton et al. 2004; Ajmal et al. 2000). At a higher pH, a lower H+ concentration, along with more of negatively charged ligands, gives greater zinc adsorption. At a pH higher than 3-4, the carboxylic groups are deprotonated and negatively charged and therefore a strong attraction with positively charged metal ions prevails (Sen and Meimon 2008). Zinc (II) ions are known to precipitate as the hydroxide at pH values above 6. At low pH values such as pH 3, available H+ ion concentrations would neutralize the normally negatively charged surfaces of castor hull prohibiting binding of positively charged Zn(II) ions. Adsorption of the metal ion will be low as a result. Moreover, it is expected that the point of zero charge of castor hull, like similar agro-wastes, would be between 3 and 4. Above the point of zero charge, the surface of an adsorbent becomes negatively charged. The concentration of zinc adsorbed on the solid would increase with increasing pH because of increasing negative charge on the surfaces of CSH. A general increase in adsorption with increasing pH of the solution was observed between pH 3-6 by many other researchers (Jain 2001; Weng and Huang 2004; Bhattacharya et al. 2006; Kargi and Cikla 2007).
The effect of solution pH on Zn2+ adsorption on activated carbon under identical experimental conditions follows a similar pattern. The amount of metal ion adsorbed increases with initial metal ion concentration as well with increase in the solution pH. But the amount of metal ion adsorbed by activated carbon adsorbent at a particular pH is significantly less than that in CSH. The properties of the surface of activated carbon strongly depend on pH (Demiral et al. 2008) and in an acidic medium, the surface is charged positively and electrostatic repulsion forces predominate (Sen and Meimon 2008). The point of zero charge for activated carbon is 3.4 Leyva-Ramos et al. 1997 and at a pH greater than 3.4, a more negatively charged surface results in higher adsorption of the metal. On the other hand, at a pH lower than 3.4, the carbon surface is positively charged and lesser adsorption takes place due to the repulsive forces between the carbon surface and Zn2+ [Sen and Meimon 2008; Sen et al. 2002; Leyva-Ramos et al. 1997].
3.2 Effect of the Amount of Adsorbent
The results of the kinetic experiments with different amounts of the adsorbent (50, 100, and 150 mg in 25-ml solution) are presented in Fig. 2. The amount of metal ion adsorbed increased with increased amount of the adsorbent added to the solution as well as with an increase in solution pH. The effect can be explained by an increase in surface area cum active adsorption sites with the increase in adsorbent mass. Similar observations were reported in the literature (Naiya et al. 2008; Oliveira et al. 2008).
3.3 Kinetics of Adsorption
3.3.1 Effect of Contact Time
Figure 3 presents the time evolution of metal ion adsorption for different initial solution concentrations of 5, 10, 20, and 30 mg/L. The amount of adsorption is found to increase with increasing contact time at all initial metal ion concentrations and equilibrium is attained within about 240 min. It was further observed that the amount of metal ion uptake, q t (mg/g) increases with increasing initial adsorbate concentration. This kinetic experiment clearly indicates that the adsorption phenomenon follows a three-step process similar to that reported by Jain (2001): a rapid adsorption at smaller time (up to about 30 min), a transition phase and an almost flat plateau section at final stage (above 100 min). The first step is attributed to the fast utilization of the most readily available adsorbing sites on the adsorbent surface (bulk diffusion). The next step (up to about 100 min), exhibits additional removal which is attributed to the diffusion of the adsorbate from the surface film into the macro-pores of the adsorbent (pore diffusion or intraparticle diffusion) stimulating further movement of the metal ions onto the adsorbent surface. The last is essentially an equilibrium step. For a solid-liquid adsorption process, the solute transfer is usually characterized by either external mass transfer (boundary layer diffusion) or intraparticle diffusion or both (Vadivelan and Kumar 2005; Arias and Sen 2009). The overall rate of sorption will be controlled by the slowest step, which would be either film or pore diffusion. However, the controlling step might also be distributed between intraparticle and external transport mechanism (Vadivelan and Kumar 2005; Arias and Sen 2009). Therefore, the sorption of zinc onto castor hull particles may be controlled by film diffusion at small time and intraparticle diffusion at a later stage. The most commonly used technique for identifying the mechanism involved in the sorption process is by fitting the experimental data in an intraparticle diffusion plot which is described in the later part of this paper.
Figure 4 shows the time evolution of adsorption on activated carbon for different initial metal ion concentrations of 5, 10, 20, and 50 mg/L. The amount of adsorption increases with increasing contact time and equilibrium is attained within about 240 min which is the same as with Zn-castor hull system. It also consists of three steps. But the adsorption capacity (metal ion uptake in miligram per gram of adsorbent) of castor hull is substantially larger than that of activated carbon (compare Figs. 3 and 4).
In order to investigate the mechanism of adsorption, particularly to identify the rate-controlling step, the transient pattern of adsorption of Zn2+ was analyzed using the pseudo-first order, pseudo-second order, and intraparticle diffusion models are discussed below.
Lagergren pseudo-first order model
The integral form of the model is expressed as (Sen and Meimon 2008; Naiya et al. 2008)
Where q t and q e represent the amount of metal ion adsorbed (mg/g) at any time t and at equilibrium, respectively; K 1 represents the first-order adsorption rate constant (min−1).
A plot of log (q e – q t ) against t should give a straight line (Fig. 5) if the pseudo first-order adsorption kinetics is applicable and should allow computation of the rate constant K 1. The accuracy of fitting represented by the linear regression coefficient (R 2) is also shown in the figure. The corresponding K 1 values are 0.04513, 0.04352, 0.02395, 0.02303, and 0.04513 min−1, respectively. Similar K 1 values such as 0.069 for Zn(II) on clarified sludge, 0.0255 on rice husk ash (Bhattacharya et al. 2006) and 0.07 on alumina (Arias and Sen 2009).
3.3.2 Pseudo-Second-Order Model
The adsorption data were also analyzed in terms of the pseudo-second order model given below (Sen and Meimon 2008; Wu et al. 2002).
Where K 2 is the pseudo-second order rate constant (g/mg min). Integrating and applying the initial conditions t = 0, and q = 0 we get
A plot between t/q t versus t should give a straight line and yield the values of the rate constants K 2 and the equilibrium amount of adsorption, q e .
The constant K 2 may be used to calculate the initial sorption rate h (i.e., the rate at t → 0) as follows
Thus the rate constant (K 2), the initial adsorption rate (h), and the predicted q e can be calculated from the plots of t/q versus time t using Eq. (5). Figures 6, 7, 8 represent the plots for different initial metal ion concentrations, solution pH, and amounts of adsorbents respectively. High values of the R 2 suggest that the adsorption process follows the pseudo-second order kinetic model. The equilibrium adsorption capacity (q e ) and second order constant (K 2) are determined from the slope and intercept the plots (Figs. 6, 7, 8). The estimated correlation coefficients (R 2), the pseudo-second order rate constant (K 2), equilibrium sorption capacity (q e ), and the initial adsorption rate (h) are presented in Table 1.
The higher values of the correlation coefficients suggest that adsorption of zinc on castor hull follows the pseudo second-order kinetics rather than the pseudo-first order model. It also corroborates the assumption behind the pseudo-second order model that the metal ion uptake process is due to chemisorptions (Sen and Meimon 2008; Acharya et al. 2009) and more than one steps are involved in the adsorption process. It further appears in Table 1 that the initial sorption rate (h) and the adsorption capacity (q e ) increase with increasing initial metal ion concentration, initial solution pH as well as the adsorbent dosing. Similar type of model parameters are reported by other researchers for different systems (Kargi and Cikla 2007; Ong et al. 2005).
Figure 9 shows the pseudo-second order kinetic model fitting of Zn2+ adsorption by activated carbon for the initial metal ion concentrations of 5, 10, 20, 30, and 50 mg/L. The corresponding adsorption rate constants (K 2, g/mg.min) are 0.0328, 0.150, 0.0452, 0.0147, and 0.0160, respectively; the equilibrium adsorption capacities (q e , mg/g) are 0.293, 0.369, 0.786, 1.202, and 1.243 which are substantially smaller than the values for castor seed hull within this metal ion concentration range. The initial sorption rates (h) for Zn-activated carbon system are also lower than those for the Zn-castor hull system. Figure 10 shows the pseudo-second order kinetic model fitting of Zn2+ adsorption on activated carbon for different initial solution pH of 3, 4 and 6. The kinetic parameters (K 2, g/mg min) over this pH range are of 0.904, 0.0770, and 0.060, respectively, and the adsorption capacities (q e , mg/g) are 0.409, 0.417, and 0.641, respectively.
3.3.3 Intraparticle Diffusion Model
For most adsorption processes, according to Weber and Morris (1963), the uptake of the metal ion varies almost proportionately with t 1/2 rather than with the contact time
K id is the intra-particle diffusion rate constant (mg g−1 min−1/2). If the plot of q t versus t 1/2 represent multilinearity, it indicates two or more steps involved in the sorption process (Vadivelan and Kumar 2005; Arias and Sen 2009). Values of intercept give information regarding the thickness of boundary layer, i.e., the larger intercept the greater is the boundary layer effect. This parameter can be determined from the slope of the linear plot of q t versus t 1/2 as shown in Fig. 11. The values of the rate constant of intraparticle diffusion are 0.075, 0.139, 0.256, and 0.334 mg/g min1/2 for initial metal ion concentrations of 5, 10, 20, 30, and 50 mg/L, respectively. The correlation coefficients of the intraparticle diffusion model for the different initial metal ion concentrations (see Fig. 11) are quite reasonable, but they are smaller than those for pseudo-second order kinetic model fitting. Moreover, it is evident from Fig. 11 that adsorption of zinc on castor seed hull follows the three-step process discussed before.
3.4 Adsorption Equilibrium Isotherm
It is essential to have knowledge of the adsorption isotherm in order to design the adsorption equipment. The adsorption equilibrium data for castor seed hull as well as for activated carbon were fitted in the Langmuir and the Freundlich isotherms over the metal ion concentration range of 5-50 mg/L. The parameters of the isotherms were evaluated from the plots of the linearized forms of the respective equations (Eq 7 and 8).
The Freundlich adsorption isotherm, which assumes that adsorption takes place on heterogeneous surfaces, can be expressed (Sen and Meimon 2008) as
Where, q e (mg/gm) is the amount of metal ion adsorbed at equilibrium time, C e is equilibrium concentration of zinc metal ion in solution. K f and n are isotherm constants which indicate the capacity and the intensity of the adsorption, respectively (Sen and Meimon 2008; Sen et al. 2002) and can be obtained from the intercept and the slope of the plot of ln q e and ln C e . The greater the value of K f , the greater is the adsorption capacity. The other Freundlich constant n is a measure of the deviation from the linearity of the adsorption and is used to identify the type of adsorption. A value of n (very often the quantity is reported as 1/n) less than unity indicates that adsorption is a chemically driven. Figure 12 shows the Freundlich isotherm fitting for adsorption on CSH. The Freundlich constants calculated from this plot are 1.62 (mg/g)/(L/g)−0.5 and 1/n = 0.5. Figure 12 also shows the Freundlich isotherm plot for zinc adsorption on activated carbon. The Freundlich parameters [1/n = 0.54 and K f = 0.00424 mg/g/(mg/L)−0.54] for this system are considerably smaller than those for the Zn-CSH system.
According to the Langmuir model, adsorption occurs uniformly on the active sites of the adsorbent and once an adsorbate occupies a site, no further adsorption can take place (Demiral et al. 2008). The Langmuir isotherm was tested for both the adsorbents. The linearized form of Langmuir can be written as (Sen and Meimon 2008; Naiya et al. 2008)
The Langmuir constants, q m (maximum adsorption capacity, mg/gm) and K a (L/mg), obtainable from the plot of 1/q e versus 1/C e as shown in Fig. 13 for castor seed hull adsorbent. The maximum adsorption capacity of Zn2+ (q m ), and the Langmuir constant (K a ) that relates to the binding energy for adsorption are calculated and found to be 6.724 mg/g and 0.327, respectively for Zn-castor hull system. Figure 13 also shows the Langmuir plot for Zn-activated carbon system for the same conditions. The Langmuir constant, K a = 0.0895, and the adsorption capacity, q m = 1.433 mg/g, are significantly less than those for the castor hull seed adsorbent. On the whole, the Langmuir model exhibits a higher R 2 compared to the Freundlich isotherm model (compare Figs. 12 and 13) for both the Zn-CSH and Zn-activated carbon systems. This establishes the applicability of the Langmuir adsorption isotherm model for the present adsorption systems.
It will be pertinent at this point to qualitatively assess the practical usefulness of CSH for the removal of zinc(II). An adsorbent should desirably allow separation of a solute and its subsequent recovery rather than separation alone. Recovery of the adsorbed solute and regeneration of the spent adsorbent depend largely upon the shape of the adsorption equilibrium curve. If the equilibrium curve is convex upward, adsorption is favorable (Hall et al. 1966). In an adsorption (or regeneration) column, the breakthrough curve for a favorable system tends to be self-sharpening as it advances through the column. The concept was extended to define the quantity called separation factor (R L ) that gives a measure of effectiveness of recovery of the solute (Hall et al. 1966).
Here, K a is the Langmuir parameter and C o is the concentration of the solute in the bulk fluid. The quantity (R L ) is akin to the relative volatility in vapor-liquid equilibria. It is easy to verify that for a favorable system, RL lies between 0 and 1; it is larger than zero for an unfavorable system. Figure 14 shows the plots of the calculated values of the separation factor, R L , versus initial metal ion concentration for Zn-CSH system and it indicates favorable adsorption (values are between 0 and 1).
The adsorption capacity of CSH towards Zn2+ has been compared with the results with other adsorbents reported in the literature and is presented in Table 2. It is found that the monolayer adsorption capacity of castor seed hull for Zn(II) is comparable to many of the other adsorbents used in previous studies.
4 Conclusion
The results obtained in this study clearly demonstrated that castor hull from castor oil industry can be considered as an attractive low cost adsorbent as compared to activated carbon for the removal of zinc metal ions from aqueous solution. The adsorption characteristics of zinc are strongly affected by initial solution pH and the initial metal ion concentration. In batch adsorption studies, the amount of metal ion adsorption on both castor hull and activated carbon increases with initial metal ion concentration, the contact time and the solution pH. The adsorption capacity of castor hull is significantly larger than that for activated carbon.
Kinetic experiments clearly indicate that adsorption of zinc on both castor hull and activated carbon is a three-step process consists of a rapid adsorption of the metal ion, a transition phase, and an almost flat plateau section. This has also been confirmed by intraparticle diffusion model. The adsorbent rate could be interpreted more satisfactorily by the pseudo-second order model rather than the pseudo-first order model. Langmuir and Freundlich models have been tested to describe the adsorption equilibrium of zinc adsorption on both castor seed hull and activated carbon. Langmuir model shows higher coefficients of determination than the other model for both adsorbents under study. The calculated value of the R L gives an indication of favorable adsorption. The monolayer adsorption capacity of castor hull is larger than that for activated carbon and it is comparable with other reported agricultural waste adsorbents.
References
Acharya, J., Sahu, J. N., Mohanty, C. R., & Meikap, B. C. (2009). Removal of Pb(II) from wastewater by activated carbon developed from tamarind wood activated with zinc chloride. Chemical Enineering Journal, 149, 249–262.
Ajmal, M., Rao, A. K. R., & Ahmad, J. (2000). Adsorption studies on Citrus reticulata: removal and recovery of Ni(II) from electroplating wastewater. Journal of Hazardous Materials, B79(2000), 117–131.
Annadurai, G., Juang, R., & Lee, D.-J. (2002). Use of cellulose-based wastes for adsorption of dyes from aqueous solutions. Journal of Hazardous Materials, 92, 263–274.
Arias, F., & Sen, T. K. (2009). Removal of zinc metal ion (Zn2+) from its aqueous solution by kaolin clay minerals: A kinetic and equilibrium study. Colloids and Surfaces, 348, 100–108.
Babel, S., & Kurniawan, T. A. (2003). Low-cost adsorbents for heavy metals uptake from contaminated water: a review. Journal of Hazardous Materials, 97, 219–225.
Bhattacharyya, K. G., & Sen Gupta, S. (2008). Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: a review. Advances in Colloid and Interface Science, 140, 114–131.
Bhattacharya, A. K., Mandal, S. N., & Das, S. K. (2006). Adsorption of Zn(II) from aqueous solution by using different adsorbents. Chemical Enineering Journal, 123, 43–51.
Choi, J. Y., & Kim, D.-S. (2002). Adsorption behavior of zinc and cadmium on granular activated carbon in singular and binary systems and the influence of nitrilotricetic acid as a complexing agent. Journal of Environmental Science and Health, A37(9), 1701–1719.
Demiral, H., Demiral, L., Tumsek, F., & Karabacakoglu, B. (2008). Adsorption of chromium (VI) from aqueous solution by activated carbon derived from olive bagasse and applicability of different adsorption models. Chemical Enineering Journal., 144, 188–196.
Gharaibeh, S. H., Abu-EI-Shae, W. Y., & Al-Kofahi, M. M. (1998). Removal of selected heavy metals from aqueous solutions using processed solid residue of olive mill products. Water Research, 32, 498–502.
Gurses, A., Dogar, C., Yalcin, Y., Acikyildiz, M., Bayrak, R., & Karaca, S. (2006). The adsorption kinetics of the cationic dye, methylene blue, onto clay. Journal of Hazardous Materials, 131(1–3), 217–228.
Hall, K. R., Eagleton, L. C., Acrivos, A., & Vermeulen, T. (1966). Pore and solid-diffusion kinetics in fixed-bed adsorption under constant-pattern conditions. Industrial and Engineering Chemistry Fundamentals, 5, 212–223.
Jain, C. K. (2001). Adsorption of zinc onto bed sediments of the river Ganga: adsorption models and kinetics. Hydrological Sciences Journal, 46, 419–434.
Kandah, M. I. (2004). Zinc and cadmium adsorption on low-grade phosphate. Separation and Purification Technology., 35, 61–70.
Kargi, F., & Cikla, S. (2007). Kinetics of zinc(II) ion biosorption onto powdered waste sludge (PWS) at different operating conditions. Environmental Engineering Science, 24, 687–695.
Leyva-Ramos, R., Rangel-Mendez, J. R., Mendoza-Barron, J., Fuentes-Rubio, L., & Guerrero-Coronado, R. M. (1997). Adsorption of cadmium(II) from aqueous solution onto activated carbon. Water Science and Technology, 35, 205–211.
Marshall, W. E., & Johns, M. M. (1996). Agricultural by-products as metal adsorbents: sorption properties and resistance to mechanical abrasion. Journal of Chemical Technology and Biotechnology, 66, 192–198.
Meena, A. K., Mishra, G. K., Rai, P. K., Rajagopal, C., & Nager, P. N. (2005). Removal of heavy metal ions from aqueous solutions using carbon aerogel as an adsorbent. Journal of Hazardous Materials, 122, 161–170.
Mohan, D., & Singh, K. P. (2002). Single and multi-component adsorption of cadmium and zinc using activated carbon derived from bagasse—an agricultural waste. Water Research, 36, 2304–2318.
Monser, L., & Adhoum, N. (2002). Modified activated carbon for the removal of copper, zinc, chromium and cyanide from wastewater. Separation and Purfication Technology, 26, 137–146.
Naiya, T. K., Chowdhury, P., Bhattacharya, A. K., & Das, S. K. (2008). Saw dust and neem bark as low-cost natural biosorbent for adsorptive removal of Zn(II) and Cd(II) ions from aqueous solutions. Chemical Enineering Journal. doi:10.1016/J.cej.2008.08.002.
Norton, L., Baskaran, K., & McKenzie, T. (2004). Biosorption of zinc from aqueous solutions using biosolids. Advances in Environmental Research, 8, 629–635.
Oliveira, W. E., Franca, A. S., Oliveira, L. S., & Rocha, D. (2008). Untreated coffee husks as biosorbents for the removal of heavy metals from aqueous solutions. Journal of Hazardous Materials, 15, 1073–1081.
Ong, S. A., Toorisaka, E., Hirata, M., & Hano, T. (2005). The behavior of Ni(II), Cr(III) and Zn(II) in biological wastewater treatment process. Acta Hydrochimica Hydrobiologica, 33, 95–103.
Ricordel, S., Taha, S., Cisse, I., & Dorange, G. (2001). Heavy metals removal by adsorption onto peanut husks carbon: characterization, kinetic study and modeling. Separation and Puriication Technollogy, 24, 389–401.
Sen, T. K., & Khilar, K. C. (2006). Review on subsurface colloids and colloid-associated contaminant transport in saturated porous media. Advances in Colloid and Interface Science., 119, 71–96.
Sen, T. K., & Meimon, V. S. (2008). Removal of cadmium metal ion (Cd2+) from its aqueous solution by aluminium oxide (Al2O3): a kinetic and equilibrium study. Chemical Enineering Journal, 142, 256–262.
Sen, T. K., Mahajan, S. P., & Khilar, K. C. (2002). Adsorption of Cu2+ and Ni2+ on iron oxide and kaolin and its importance on Ni2+ transport in Porous Media. Colloids and Surfaces A, 211, 91–102.
Shukla, S. R., & Pai, R. S. (2005). Adsorption of Cu(II), Ni(II) and Zn(II) on dye loaded groundnut shells and sawdust. Separation and Purification Technology, 43, 1–8.
Snoeyink, V. L., & Jenkins, D. (1980). Water chemistry. New York, USA: Wiley.
Ucer, A., Uyanik, A., & Aygun, S. F. (2006). Adsorption of Cu(II), Cd(II), Zn(II), Mn(II) and Fe(III) ions by tannic acid immobilised activated carbon. Separation and Purification Technology, 47, 113–118.
Vadivelan, V., & Kumar, K. V. (2005). Equilibrium, kinetics, mechanism and process design for the sorption of methylene blue onto rice husk. Journal of Colloid and Interface Science, 286, 90–100.
Weber, W. J., & Morris, J. C. (1963). Kinetics of adsorption on carbon from solution. Journal of Sanitary Engineering Division, American Society of Civil Engineers., 89, 31–60.
Weng, C. H., & Huang, C. P. (2004). Adsorption characteristics of Zn(II) from dilute aqueous solution by fly ash. Colloids and Surfaces A, 247, 137–143.
Wu, F. C., Tseng, R. L., & Juang, R. S. (2002). Adsorption of dyes and humic acid from water using chitosan-encapsulated activated carbon. Journal of Chemical Technology and Biotechnology, 77, 1269–1279.
Zacaria, R., Gerente, C., Andres, Y., & Cloirec, P. L. (2002). Adsorption of several metal ions on to low cost biosorbent: kinetic and equilibrium studies. Environmental Science and Technology., 36, 2067–2073.
Zhu, C., Luan, Z., Wang, Y., & Shau, X. (2007). Removal of cadmium from aqueous solution by adsorption on granular red mud 9GRM. Separation and Purification Technology, 57, 167–175.
Acknowledgement
The authors gratefully acknowledge the Universiti Teknologi PETRONAS (UTP), Malaysia for providing necessary research facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohammod, M., Sen, T.K., Maitra, S. et al. Removal of Zn2+ from Aqueous Solution using Castor Seed Hull. Water Air Soil Pollut 215, 609–620 (2011). https://doi.org/10.1007/s11270-010-0503-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-010-0503-0