Abstract
The presence of emerging compounds was investigated in surface water samples from Iguassu River watershed in the metropolitan region of Curitiba (Brazil). Emerging compounds are substances present in domestic and industrial sewage. Generally, the emerging compounds are present in the environment due to the indiscriminate release of untreated domestic and industrial sewage. Even treated sewage may contain emerging compounds due to the difficulty in removing them. In this work, the presence of caffeine, musk xylene (a fragrance), and bisphenol-A was investigated in surface water samples. Also, traditional parameters used in water quality monitoring were determined, such as biochemical oxygen demand (BOD), nitrate, and fecal coliforms. The emerging compounds were extracted in solid phase and analyzed by high performance liquid chromatography. Caffeine is eliminated in the urine (approximately 0.5% to 10% of the consumption), and the only source is domestic sewage. Bisphenol is widely used in food packs, while musk xylene is found in personal care products, easily eliminated in sewage. The caffeine concentration was between 1.74 ± 0.54 and 123.45 ± 0.81 µg/L. Musk xylene and bisphenol-A had their concentrations between 0.04 ± 0.07 and 0.56 ± 0.12 and between 0.62 ± 0.15 and 12.61 ± 0.21 µg/L, respectively. Positive correlations were found between caffeine and traditional monitoring parameters (BOD and fecal coliforms). Higher values of emerging compounds were determined at points considered extremely polluted. The positive correlations confirm the origin of emerging compounds and show that chemical markers are good parameters for monitoring pollution when the use of traditional parameters makes the diagnosis doubtful.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Concern about the preservation of water resources has increased in recent years (Froehner et al. 2009a). The degradation of water resources can occur from a variety of point and non-point sources caused by both anthropogenic and natural factors (Aufdenkampe et al. 2006). The most common sources of contamination are domestic sewage (treated and poorly treated), livestock manure, agricultural runoff (road and urban), industrial effluent, mining activities, atmospheric deposition, and even wildlife. Such sources have many chemical compounds with much toxicological power. These compounds, due to their physical–chemical characteristics, have a highly negative effect on the ecosystem and also make the water unfit for drinking. Thus, in order for policy makers and managers to maintain water resource quality, targeted efforts to reduce or eliminate primary contamination sources require the accurate identification and quantification of all contaminant sources that contribute to the degradation of water quality (Aufdenkampe et al. 2006).
In general, the water quality is assessed, or contamination is found using molecular tracers or micropollutants (Barber et al. 1995). The use of molecular tracers to identify sources of contaminants is a technique that qualitatively links chemical fingerprints unique to these sources with contaminants of concern (Leeming and Nichols 1996; Standley et al. 2000; Kolpin et al. 2002; Yunker et al. 2002; Buerge et al. 2003; Aufdenkampe et al. 2006). Chemical markers or molecular tracers need not necessarily be toxic but should be specific sources and therefore act as proxies for contaminants originating from those same sources. For instance, a recent and increasingly used proxy to detect potential sewage contamination is the fecal steroid coprostanol (Froehner et al. 2009b). While not considered toxic to humans or to aquatic life at any measured environmental concentration, coprostanol is much more abundant in human feces than in that of any other animals (Froehner et al. 2009b; Leeming and Nichols 1996). Therefore, high aquatic concentrations of coprostanol are a strong indicator of human sewage and or septic contamination (Leeming and Nichols 1996, Aufdenkampe et al. 2006).
Due to the growing chemical industry, other compounds found in the environment can also be associated with specific contaminations. Many of these compounds, however, are not degraded in sewage treatment plants and are released into water bodies, representing a major problem for the ecosystem. Among the compounds that are currently found in water resources are the fragrances. Fragrances are a group of structurally diverse chemicals that are used at low concentrations in consumer products, to deliver consumer preferred odors (Simonich et al. 2000). Approximately 4,000 mT of fragrances are produced every year (van de Plassche and Balk 1997). Fragrances are semi-volatile compounds with vapor pressure ranging from 10 to 105 Pa and are highly soluble in water (10−3–10−1 mg/L). The primary route of these materials into the environment is through down the drain disposal of consumer products and untreated sewage. Treated sewage can also contribute (Barber et al. 1995). In recent years, there have been reports on the detection of some fragrances and their metabolites in surface water and aquatic organisms (Simonich et al. 2000). Fragrances are partially removed in a wastewater treatment plant (WWTP); usually 40% to 60% are removed (Yang and Metcalf 2006).
Bisphenol compounds are another example of tracer contaminant. Moreover, bisphenol can be a problem due its endocrine disruption properties. Bisphenol is a monomer used in the resins and plastic industry to produce varnishes that is the inner coating of food cans and thermal paper (Danzl et al. 2009).
It is known that health risks concerns are increasing. Such concerns led the European Union to ban the manufacture of bisphenol as a food packaging material. Fromme et al. (2002) stated that diphenyl and diphenyl methane derivates containing two hydroxyl groups in the para positions, among them bisphenol-A and bisphenol-F, showed estrogenic activity. The two compounds mentioned tested 100% positive for estrus response. Most of these estrogen-like chemicals are widely used persistent organic compounds, which are ubiquitous in the environment and in biological samples (Fromme et al. 2002). Abundant data on estrogenic activity, androgen activity, carcinogenicity, and toxicity have been published since then, mostly on bisphenol, and recently, low-dose effects of bisphenol-A have been discussed (Vom Saal and Hughes 2005). Estrogenic activity of bisphenol-A was quite similar to other bisphenols (Chen et al. 2002). Although the toxicity of these compounds is known, especially those that act as environmental estrogens, there are few studies in the literature that show their presence in water resources in Brazil. Another significant emerging compound, caffeine, is present in many products consumed daily, rapidly metabolized in liver, and about 0.5% to 10% is excreted through urine (Arnaud 1993).
The main objective of this work was to investigate the presence of emerging compounds currently used as contamination tracers and linked with the origin of pollution. The area investigated has been extensively studied and characterized as polluted, but there is a lack of information about organic compounds as emerging compounds that can be present in water associated with domestic and industrial sewage inputs.
2 Materials and Methods
2.1 Study Area
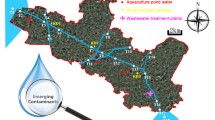
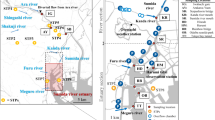
The investigation of emerging compounds was carried out in the Upper Iguassu watershed (Fig. 1), specifically in the metropolitan region of Curitiba. The Upper Iguassu watershed has its sources along the Serra do Mar, whose main river is about 90-km long to the boundary of the metropolitan region of Curitiba, with a drainage area of about 2,800 km2. The population of this area is approximately three million in 14 municipalities. It is worthwhile noting the low rate of domestic sewage collection and treatment. The area covered is heavily urbanized and has been undergoing a process of irregular occupation of flood plains and watershed areas, which has led to the clear degradation of rivers in the region. This work presents a collection campaign in urban rivers considered in the basin. Water samples were collected in rivers Irai, Barigüi, and Iguaçu, although the Upper Iguassu watershed has 26 major tributaries.
2.2 Sample Collection
Samples were collected at points already established as control points and sampling for the purpose of monitoring of water quality according to Brazilian standards for classification and use of water (IBAMA 2000); moreover, the analysis were restricted to physical–chemical and biological parameters, and the presence of toxic or persistent organic compounds that may pose risks to the ecosystem is unknown. Surface water samples were collected from six stations. The collection campaign took place in May and October 2009 during a low rainfall period. The location of stations is shown in Fig. 1. Water samples were collected in 1-L pre-cleaned amber glass bottles 10–15 cm below the water surface to avoid collecting the surface microlayer. The samples were stored in a styrene box with ice (approximately 4°C) until arrival in the laboratory for further analysis biochemical oxygen demand (BOD), nitrate, and emerging compounds).
The most probable number (MPN) of fecal coliform bacteria per unit volume of a sample was also determined using an IDEXX Quanti-Tray/2000. The samples were collected in bottles previously sterilized and containing sodium thiosulfate as a preservative.
For control’s purpose, the removing of studied compounds (caffeine, bisphenol-A, and galaxolide) was also investigated in two wastewater treatment plants located near the sampling sites (P3 and P5) where treated effluent is discharged into the basin.
2.3 Analytical Methods
Standard Methods for Water and Wastewater Examination (APHA 1998) were used to analyze samples for parameters such as nitrate, BOD, and fecal coliforms.
2.4 Caffeine, Bisphenol-A Extraction, and Analysis
The water samples were filtered through a combusted 0.45-μm Whatman before analysis (Papadopoulou-Mourkidou et al. 2001; Chen et al. 2009), and caffeine and bisphenol-A were extracted together. For extraction, 1 L of filtered water was extracted in solid phase microextraction (SPME C18).
The solid phase extraction used a rate of 1 L of filtered sample, which was previously acidified with concentrated HCl to pH 3. A C18 cartridge previously conditioned with 6 mL of methanol, 6 mL of deionized water, and 6 mL of HCl solution with pH 3 was used. The elution of compounds was done by the addition of four servings of 3 mL of methanol to each cartridge. The extracts were nitrogen dried and redissolved in 0.5 mL of methanol, giving a concentration factor of 2,000 times the analytes in the extract (Thomas and Hilton 2003). Quantitative analytical determination of caffeine and bisphenol-A was carried out by high performance liquid chromatography (HPLC). All samples were analyzed on a Shimadzu HPLC LC20A model, equipped with an absorbance detector (274 nm). Fifty microliters of sample were injected. Chromatographic separation was accomplished using column ODS Hipersil 250 × 4 mm, 5-μm particles. The mobile phase was an isocratic mixture of methanol/acetonitrile at a proportion of 1:1, and the flux rate was 0.65 mL/min. The retention time for caffeine was between 7 and 8 min.
2.5 Musk Xylene (Fragrance) Extraction and Analysis
The extraction and analysis of musk xylene was conducted according to Fu et al. (2005). Due to the widespread use of fragrances in many household products, great care must be taken to avoid contamination during sample treatment and analyses. In order to minimize contamination, soaps and hand cream were not used, whereas disposable plastic gloves were used. All the glass apparatus were washed with K2CrO4–H2SO4 lotion, then washed with redistilled water, and then dried at 450°C. Water samples (approximately 1 L) were extracted with dichloromethane 50 mL in an ultrasonic bath for 20 min. Possible sulfur content was removed from the extracts by adding activated copper (Fu et al. 2005). The organic phases were separated in a separatory funnel and concentrated using a rotary evaporator to approximately 1 mL. The concentrated extracts were loaded in a combined column of silica gel and alumina. Three fractions (n-hexane, mixture of n-hexane and dichloromethane) were used for separation. The collected fractions were concentrated to 0.5 mL, exchanged into n-hexane, concentrated again to 0.5 mL, and adjusted to 0.2–0.5 mL under a gentle stream of nitrogen. The concentrated extracts were ready to inject in gas chromatography–flame ionization detector (GC-FID). Recovery was higher than 54%. The detection limit was 0.05 ng/L.
The GC analyses were carried out in a HP7890 GC chromatograph equipped with an HP-5 fused silica capillary column (30 m × 0.32 mm ID × 0.25-µm film, Agilent Technology). The oven temperature was programmed as follows: 80°C held for 1 min, raised at 5°C/min to 150°C, then 1°C/min to 180°C, and 10°C/min to 300°C (held for 15 min); the carrier gas was N2 with a flux of 37 cm/s; the injection was set on a split/splitless mode at 270°C, splitless time 60 s, the injection volume 1 µL. Detection was conducted by FID at 300°C. Data acquisition and processing were controlled by an HP EZChromStation data system.
3 Results and Discussions
The selected area for the identification of emerging compounds was already monitored previously in some parts of the watershed (Froehner et al. 2009a, b); however, that was the first time the monitoring assessment was conducted using emerging compounds. Previous monitoring programs concluded that most areas of the watershed were polluted with domestic and industrial sewage. In fact, the discharge of domestic sewage has been the main factor responsible for the contamination of water resources. Table 1 shows the parameter values obtained for the sites investigated.
Typically, water quality monitoring in rivers is accomplished through parameters such as nitrates and BOD, which are generally associated with contamination by domestic sewage, in addition to fecal coliforms; however, these parameters did not reveal the presence of industrial sewage. Nitrate, which can be from other sources and not only from domestic sewage (Sankararamakrishnan and Guo 2005), ranged from 0.35 ± 0.05 to 2.23 ± 0.08 mg/L, while parameter BOD ranged from 0.23 ± 0.06 to 7.21 ± 0.09 mg/L. These values were expected because the points named P3, P4, P5, and P6 are located in areas considered contaminated and near industrial and urbanized areas. A previous report showed that there are several points of discharge of untreated sewage close to these sampling sites (Froehner et al. 2009a). The presence of domestic sewage is confirmed by the high values of fecal coliforms, which ranged from 79.1 to more than 2,400 for site P6 (severed polluted). By the results, it can be concluded that the area is contaminated in many of the points investigated, but it is suspected that the major source of contamination is due to the presence of untreated and improperly treated sewage. Using only the physico-chemical and biological parameters leaves suspicions about the source of pollution. However, the presence of domestic or industrial sewage can be much more severe to the ecosystem. Micropollutants as markers of pollution instead of biological parameters could be more trustful since the molecules of micropollutants are resistant to degradation. The biological parameters can be sensible to the presence of inorganic and organic contaminants, intervening in the diagnostic. Emerging compounds have often been used as indicators to assess contamination by domestic and industrial sewage, due to their toxic effects on ecosystems and concern about the removal capacity of WWTP. Such compounds can be used as pollution tracers due to their physico-chemical characteristics and mainly due to the emission source because there is no natural source for such compounds (Buerge et al. 2003).
In this work, three compounds associated with sewage were investigated, caffeine, bisphenol-A, and musk xylene. Caffeine was found at all sites investigated. The concentration was between 1.74 ± 0.054 and 123.45 ± 0.81 µg/L. Caffeine is found in urine at concentrations of only 3 mg/L; it seems to be unique to the molecular tracer of sewage. Caffeine has been used as a tracer of pollution by several authors. For instance, Aufdenkampe et al. (2006) found high concentrations of caffeine in streams (5 to 16 µg/L). Also, Sankararamakrishnan and Guo (2005) used caffeine as a molecular tracer for assessing the water quality of a local river in India. The highest concentration was 1,912 µg/L. The most complete report on caffeine occurrence in surface waters (Standley et al. 2000) reported a range of concentrations between almost zero and 0.115 µg/L. Ternes et al. (1999) reported concentrations of caffeine in rivers and wastewaters in Germany ranging from 0.010 to 147 µg/L. Although caffeine is quite soluble (2.2 mg/mL), the degradation is slow (Buerge et al. 2003). The concentrations of caffeine at some sampling sites confirm contamination by domestic sewage. The values found are close to the values found in other works whose scenario had the same characteristics. For instance, the caffeine concentrations are in the same range of the values found by Buerge et al. (2003). According to Buerge et al. (2003), caffeine removal in WWTP is higher than 99%. Only for comparisons, the caffeine concentration in untreated sewage was 68.20 ± 0.62 µg/L; meanwhile, the caffeine content in treated effluent was only 0.02 ± 0.05 µg/L, close to detection limit. Therefore, removal was higher than 99%. Although there is WWTP, it does not appear sufficient for all residences, justifying the high values of caffeine found, although there are plenty of illegal sewage discharges. Other sources of caffeine are unknown, in addition to domestic sewage. Thus, it is expected that positive correlations are found between caffeine and the traditional parameters attributed to the presence of domestic sewage. Positive correlations were found between caffeine and nitrate (0.83), BOD (0.97), and fecal coliforms (0.92; Fig. 2). Here, positive correlations were found, indicating that the caffeine in surface water samples is from sewage.
The anthropogenic burden to a water body by domestic wastewater increases with the population in the catchment; however in rivers, spatiotemporal variations can be expected. For example, P2 and P5 showed concentrations of 1.74 ± 0.054 and 123.45 ± 0.81 ng/L at the same and opposite bank with respect to the WWTP, respectively, indicating somewhat incomplete lateral mixing. Also, flushing contribution can increase the amount of caffeine in water bodies. Caffeine is source specific for domestic wastewater, and the compound will surely be consumed in the future at comparable amounts. Furthermore, the concentrations in surface waters are sufficiently high for reliable analytical quantification. The use of caffeine as anthropogenic marker is, of course, limited to areas without significant natural or industrial sources.
As the caffeine was found in high concentrations, the other physico-chemical and biological parameters are high, confirming the presence of domestic sewage. The investigated area has already been characterized as polluted, but the diagnosis has always been done using physical and chemical parameters and not with chemical markers or micropollutants. Besides caffeine, the presence of musk xylene, a fragrance commonly used in personal care products, was also investigated. Musk xylene is a synthetic substance, whose only sources are domestic and industrial sewage. Whereas the sampling sites were in areas contaminated by domestic sewage, the concentration of musk xylene ranged from 0.040 ± 0.070 to 0.56 ± 0.12 µg/L. Musk xylene, as well as other fragrances, has been investigated in rivers and lakes. For instance, in less polluted areas, Bester et al. 2008a found fragrances ranging from 0.029 to 0.039 µg/L, while in polluted areas the concentration was between 0.14 and 0.81 µg/L. The annual per capita consumption of fragrances is approximately 0.8 g. Musk xylene enters rivers from sewage and mostly from untreated sewage; however, treated sewage can also contain significant amounts of fragrances, whereas removal by WWTP is approximately 65% (Bester et al. 2008b). Two local sewage treatment plants showed that removal of the fragrance was approximately 42% when treatment was physical and 68% for treatment with activated sludge. Therefore, most of the musk xylene still remains even if the sewage has already been treated.
The presence of chemical compounds such as fragrances could be very worrying considering that the degradation and/or removal of such compounds have proved inefficient. There is a lack of information about the toxic effects on humans and wild animals; however, such compounds have been found in water for drinking purpose (Bester et al. 2008a). The main source of musk xylene, as other fragrances, is sewage (treated and untreated); thus, a positive correlation can be found with biological parameters (BOD and coliforms). Here, a correlation of 0.83 for BOD and 0.91 for coliforms and musk xylene was found. Removal of fragrances by atmospheric photodegradation has been reported (Aschmann et al. 2001), but no register on biodegradation or aqueous photo degradation available. It was assumed that volatilization and sedimentation were the only removal processes from surface water (Bester et al. 2008a). Here, considering the low light penetration in some samples sites (P3, P4, P5, and P6), photo degradation was neglected, supported by the eutrophic situation of the river (Froehner et al. 2009b).
The number of chemical compounds found in the environment is rising, following industry developments and humans needs. Hence, there is increased consumption of products based on epoxy resins and polycarbonates that contain bisphenol-A (Staples et al. 1998). The presence of bisphenol compounds became a worldwide concern due to their endocrine disruption effects (Chen et al. 2002). Bisphenol-A was present at all sites sampled. The concentration ranged from 0.62 ± 0.15 to 12.61 ± 0.21 µg/L. The highest concentrations were found at sites P3 and P5. Historically, these two points are located in areas close to industry and densely populated, and in addition, there is a history of pollution by both domestic and industrial sewage identified in previous campaigns (Froehner et al. 2009b). The presence of bisphenol-A has been common in water bodies. In this study, the values found are comparable to those measured in other countries. For example, Chen et al. (2009) found bisphenol-A in surface waters of rivers with a concentration from 37 to 4,230 µg/L. In Germany, the values ranged from 0.50 to 410 µg/L (Fromme et al. 2002). Also, Suzuki et al. (2004) found concentrations in surface water between 20 and 230 µg/L.
Bisphenol-A is highly water soluble; also, it is biodegraded rapidly in surface waters that received a continued discharge of bisphenol-A. According to Klecka et al. (2001), the half-lives for bisphenol-A biodegradation in surface waters ranged from 1.2 to 3.4 days. However, even with such characteristics, high concentration of bisphenol-A in water samples was observed. Following Kang and Kondo (2002), the biodegradation of bisphenol-A is much slower in anaerobic conditions than in aerobic conditions. The scenario studied showed anaerobic conditions in most of the sampled points, accounting for the high concentration of bisphenol-A; even the physico-chemical characteristics were taken to rapid removal by microbiological reactions of bisphenol-A.
The presence of bisphenol-A in the environment is mainly due to industrial waste and domestic sewage. Bisphenol-A is highly soluble in water; thus, its presence in non-polluted water has become common. However, this is extremely worrying given its toxic characteristics. As the source of bisphenol-A may also be from industrial waste and domestic sewage and other sources, the correlations between biological parameters and bisphenol-A showed positive values but low compared with the correlations between BOD and musk xylene for instance, probably due to the source of this contaminant, industrial and domestic.
4 Conclusions
Emerging compounds were used for the first time as tracers of sewage pollution in the metropolitan region of Curitiba. The results show that the sites labeled as very polluted areas had the highest concentration of biomarkers. Positive correlations were found between the emerging compounds and fecal coliforms, but there is no quantitative correlation yet. The presence of emerging compounds in water resources has been constant in recent years. There is concern due to the low removal rate of some compounds by WWTP, and in this study, we observed less than 70% for musk xylene. Because of the chemical and physico-chemical properties of these compounds and the fact that they come from a single source only found in domestic and industrial waste, they are excellent markers of pollution. The relevance of these findings is that if emerging compounds are present in water, numerous pathogens are certainly present as well. Finally, the scenario of pollution was expected; however, biomarkers can reveal the main source of pollution because some compounds are exclusively from domestic sources while others come from industrial sources.
Moreover, the presence of emerging compounds can indicate that there is pollution. So far, no quantitative correlation has been established between concentration of emerging compounds and amount of pollution.
References
APHA. (1998). Standard methods for the examination of water and wastewater (20th ed.). Washington DC: American Public Health Association American Water Works Association/Water Environment Federation.
Arnaud, M. J. (1993). Metabolism of caffeine and other components of coffee. In S. Guaratini (Ed.), Caffeine, coffee and health (pp. 43–95). New York: Raven.
Aschmann, S. M., Arey, J., Atkinson, R., & Simonich, S. L. (2001). Atmospheric life times and fates of selected fragrance materials and volatile model compounds. Environmental Science & Technology, 35, 3595–3600.
Aufdenkampe, A. K., Arscott, D. B., Dow, C. L., & Standley, L. J. (2006). Molecular tracers of soot and sewage contamination in streams supplying New York City drinking water. Journal of the North American Benthological Society, 25(4), 928–953.
Barber, L. B., Leeheer, J. A., Pereira, W. E., Noyes, T. L., Brown, G. K., & Tabor, C. F. (1995). Organic compounds in sewage-derived contaminants. In R. H. Meade (Ed.), Contaminants in the Mississippi River, 1997–1992 (pp. 115–135). Reston: USGS Circular 1133.
Bester, K., Hüffmeyer, H., Schaub, E., & Klasmeier, J. (2008a). Surface water concentrations of the fragrance compound OTNE in Germany—a comparison between data from measurements and models. Chemosphere, 73(8), 1366–1372.
Bester, K., Klasmeier, J., & Kupper, T. (2008b). Emissions of OTNE (Iso-E-super) mass flows in sewage treatment plants. Chemosphere, 71(11), 2003–2010.
Buerge, I. J., Poiger, T., Müller, M. D., & Buser, H. R. (2003). Caffeine, an anthropogenic marker for wastewater contamination of surface waters. Environmental Science & Technology, 37(4), 691–700.
Chen, M. Y., Ike, M., & Fujita, M. (2002). Acute toxicity, mutagenicity, and estrogenicity of bisphenol-A and other bisphenols. Environmental Toxicology, 17(1), 80–86.
Chen, T. C., Shue, M. F., Yeh, Y. L., & Kao, T. J. (2009). Bisphenol A occurred in Kao-Pin River and its tributaries in Taiwan. Environmental Monitoring Assessment. doi:10.1007/s10661-008-0733-4.
Danzl, E., Sei, K., Soda, S., Ike, M., & Fujita, M. (2009). Biodegradation of Bisphenol A, Bisphenol F and Bisphenol S in seawater. International Journal of Environmental Research and Public Health, 6, 1472–1484.
Froehner, S., Machado, K. S., & Souza, D. B. (2009a). Tracking anthropogenic inputs in Barigui River-Brazil using biomarkers. Environmental Monitoring Assessment. doi:10.1007/s11270-009-0220-8.
Froehner, S., Martins, R. F., & Errera, M. R. (2009b). Assessment of fecal sterols in Barigui River sediments in Curitiba, Brazil. Environmental Assessment Monitoring, 157(1–4), 591–600.
Fromme, H., Küchler, T., Otto, T., Pilz, K., Müller, J., & Wenzel, A. (2002). Occurrence of phthalates and bisphenol A and F in the environment. Water Research, 36(6), 1429–1438.
Fu, J., Xiong, Y., Sheng, G., & Zeng, X. (2005). Determination of polycyclic musks in sewage sludge from Guangdong, China using GC–EI-MS. Chemosphere, 60(6), 817–823.
IBAMA (2000) Directive 357/2000. Water classification for uses. Brazilian Institute of Environment. www.ibama.gov.br. Accessed November 2009.
Kang, J. H., & Kondo, F. (2002). Bisphenol A degradation by bacteria isolated from river water. Archives of Environmental Contamination and Toxicology, 43, 265–269.
Klecka, G. M., Gonsior, S. J., West, R. J., Goodwin, P. A., & Markham, D. A. (2001). Biodegradation of bisphenol-A in aquatic environments: River die-away. Environmental Toxicology and Chemistry, 20, 2725–2735.
Kolpin, D. W., Furlong, E. T., Meyer, M. T., Thurman, M., Zaugg, S. D., Barber, L. B., et al. (2002). Pharmaceutical, hormones, and other organic wastewater contaminants in U.S. streams, 1999–2000: A national reconnaissance. Environmental Science & Technology, 36(6), 1202–1211.
Leeming, R., & Nichols, P. (1996). Concentrations of coprostanol that correspond to existing bacterial indicator guideline limits. Water Research, 30(12), 2997–3006.
Papadopoulou-Mourkidou, E., Patsias, J., Papadakis, E., & Koukourikou, A. (2001). Use of an automated on-line SPE HPLC method to monitor caffeine and selected aniline and phenol compounds in aquatic systems of Macedonia-Thrace. Greece. Fresenius' Journal of Analytical Chemistry, 371(4), 491–496.
Sankararamakrishnan, N., & Guo, Q. (2005). Chemical tracers as indicator of human fecal coliforms at storm water outfalls. Environment International, 31(8), 1133–1140.
Simonich, S. L., Begley, W. M., Debaere, G., & Eckhoff, W. S. (2000). Trace analysis of fragrance materials in wastewater and treated wastewater. Environmental Science & Technology, 34(6), 959–965.
Standley, L. J., Kaplan, L. A., & Smith, D. (2000). Molecular tracers of organic matter sources to surface water resources. Environmental Science Technology, 34(15), 3124–3130.
Staples, C. A., Dorn, P. B., Klecka, G. M., O’Block, S. T., & Harris, L. R. (1998). A review of the environmental fate, effects, and exposures of bisphenol A. Chemosphere, 36(10), 2149–2173.
Suzuki, T., Nakagawa, Y., Takano, I., Yaguchi, K., Yasuda, K. (2004) Environmental Fate of Bisphenol-A and its Biological metabolites in River Water and their Xeno-estrogenic Activity. Environmental Science Technology 38, 2389–2396.
Ternes, T. A., Stumpf, M., Mueller, J., Haberer, K., Wilken, R. D., & Servos, M. (1999). Behavior and occurrence of estrogens in municipal sewage treatment plants. Investigations in Germany, Canada and Brazil. The Science of the Total Environment, 225(1), 81–90.
Thomas, K. V., & Hilton, M. J. (2003). Determination of selected human pharmaceuticals compounds in effluent and surface water samples by HPLC-ESI-MS. Journal of Chromatography A, 1015(1–2), 129–141.
van de Plassche, E. J., & Balk, F. (1997). Environmental risk assessment of the polycyclic musks AHTN and HHCB according to the EU-TGD. Bilthoven, The Netherlands: National Institute of Public Health and the Environment (RIVM).
Vom Saal, F. S., & Hughes, C. (2005). An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environmental Health Perspectives, 113(8), 926–933.
Yang, J. J., & Metcalf, C. D. (2006). Fate of synthetic musks in a domestic wastewater treatment plant and in an agricultural field amended with biosolids. The Science of the Total Environment, 363(1–3), 149–165.
Yunker, M. B., Macdonald, R. W., Vingarzan, R., Mitchell, R. H., Goyette, D., & Sylvestre, S. (2002). PAHs in the Fraser River basin: A critical appraisal of PAH ratios as indicators of PAH source and composition. Organic Geochemistry, 33(4), 489–515.
Acknowledgments
The study was made possible by financial support from the CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico; Project 577060/2008-2 and 473238/2008-0). We are very grateful to Prof. Cristovão Scapulatempo Fernandes for the helpful discussions. We also would like to thank Mr. Luis Carlos Barbosa for the help in field works.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Froehner, S., Machado, K.S., Falcão, F. et al. Inputs of Domestic and Industrial Sewage in Upper Iguassu, Brazil Identified by Emerging Compounds. Water Air Soil Pollut 215, 251–259 (2011). https://doi.org/10.1007/s11270-010-0475-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-010-0475-0