Abstract
Under the present investigation phytoremediation of mercury and arsenic from a tropical open cast coalmine effluent was performed. Three aquatic macrophytes Eichhornia crassipes, Lemna minor and Spirodela polyrrhiza removed appreciable amount of mercury and arsenic during 21 days experiment. Removal capacities of these macrophytes were found in the order of E. crassipes > L. minor > S. polyrrhiza. Translocation factor (shot to root ratio of heavy metals) revealed low transportation of metals from root to leaves leading higher accumulation of metals in root as compared to leaves of the plant. It was evident from plant tissue analysis that mercury and arsenic up take by macrophytes had deteriorated the N, P, K, chlorophyll and protein content in these macrophytes. Correlations between removal of arsenic and mercury from mining effluent and its increase in plant parts were highly significant. Results favoured selected species to use as promising accumulator of metals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Mercury (Hg) and arsenic (As) has been considered as highly toxic substances due to their persistent nature and magnification through the food chain in the ecosystem. Mercury is a liquid metal at ambient temperature and pressure. It forms salts in two ionic states Hg (I) and Hg (II) (Brooks and Robinson 1998). Mercury contamination to the aquatic ecosystem is associated with coalmines. Coal rich in pyrite experiences greater mercury reduction during washing (Indiana Geological Survey 2004). Hg can cause brain damage, heart, and lung diseases in human beings (US Environmental Protection Agency 2006).
Arsenic (As) is widely distributed into the nature in form of either metalloids or chemical compounds, which causes a variety of pathogenic conditions including cutaneous and visceral malignancies (Matsui et al. 1999). Arsenic shows toxicity even at low exposures (Dikshit et al. 2000) and causes black foot disease (Lin et al. 1998). Arsenic contamination is also associated with coalmines (Manahan 1997). It exists in two forms i.e. As (V) and As (III), former is the predominant form in the environment since As (III) is oxidized by atmospheric oxygen (Bissen and Frimmel 2000).
Heavy metals including arsenic and mercury cannot be degraded easily and their cleanup usually requires their removal (Lasat 2002). Traditional treatment methodologies do not remove the metals up to satisfactory level (Spearot and Peck 1984). Therefore, treated effluent may contain high concentration of metals which require further treatment for their complete removal. Hyper accumulative capacities of aquatic plants offer a method of heavy metal treatment from waste water. Aquatic plants are known to accumulate heavy metals from the environment (Kamal et al. 2004). Higher tolerance of many aquatic plants for heavy metals has attracted considerable interest in recent years (Antunes et al. 2001; Cohen-Shoel et al. 2002). Accumulation of metals in the roots and leaves of macrophytes has identified Eichhornia crassipes as a bioindicator of metal pollution (Zaranyika and Ndapwadza 1995). Lemna minor and Spirodela polyrrhiza also possess excellent heavy metal removal capabilities (Noraho and Gaur 1996; Rahmani and Sternberg 1999). Most members of the Lemna genus are used as model plants for phytoremediation, nutrient and metal uptake studies, and bioassays (Ensley et al. 1996.
The possibility of using hyper accumulator plants to extract arsenic from aquatic environments was discussed by Brooks and Robinson (1998). Arsenic up take by plants is associated with the phosphate uptake mechanism, where presumably arsenate is taken up as a phosphate analogue (Pickering et al. 2000). Removal of Hg by these plants was also studied from some workers. Brown and Rattigen (1979) reported damage to Elodea canadensis and Lemna minor after 14 and 28 day exposure to varying mercuric chloride. Kamal et al. (2004), Skinner et al. (2007) also had shown the removal of Hg by aquatic macrophytes. Most of these studies were performed the removal of these metals from metal solution prepared in laboratory conditions.
Under present investigation we have assessed the Hg and As removal capacities of aquatic macrophytes from coal mining effluent. Three aquatic macrophytes, i.e. Eichhornia crassipes, Lemna minor, and Spirodela polyrrhiza were used as experimental plants. Mercury and arsenic contamination may lead to disastrous level in near future if no preventing steps are taken. Therefore, present work was conducted with the aim to develop an eco friendly treatment option for mercury and arsenic from the coal mining effluent with the help of aquatic macrophytes and also to demonstrate the translocation and accumulation of metals in the plant tissues.
2 Methodology
2.1 Study Area
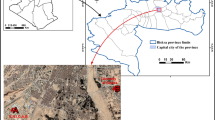
Present work has been done in relation to the coal mines of Northern Coalfields Limited (NCL), Singrauli, (India). This coalfield is located between 24°37′ to 24°12′ N and 81°48′ to 82°52′ E. Singrauli coalfield is one of the largest coal-power complexes in the world. Huge quantities of effluents are released from these opencast coalmine projects in Govind Ballabh Pant (GBP) Sagar, which is in close vicinity of the coal mines and is one of the Asia’s largest man-made water reservoirs. This reservoir is facing severe contamination due to discharges from these coal mines. Therefore this area was chosen for the study (Fig. 1).
2.2 Experimental Design
Aquaculture experiments were conducted using pollution tolerant plant species, i.e. Eichhornia crassipes, Lemna minor and Spirodela polyrrhiza (Kelly et al. 1999; Axtell et al. 2003; Mishra et al. 2008a, b). These aquatic macrophytes were collected from the agrofarm pond of the Banaras Hindu University, Varanasi, India. Plants were cultured in glass aquariums of 150 l capacities (100 × 50 × 30 cm) containing 95 l of mining effluent collected from Bina open cast coal mine of the NCL, Singrauli. Roots of the plant were washed thoroughly with distilled water before they were placed in separate glass aquaria. Monocultures were prepared with 100% coverage of the total surface area of the aquarium used for aquaculture. Control experimental sets contained only mining effluent without any macrophytes. Three replicates of each experimental set were prepared. Effluent used for aquaculture experiments were analyzed at initial level, 10th, 15th, 20th and 25th day. Plant tissues (root and leaves) were also analyzed on similar intervals. A constant water level was maintained in the aquariums with the addition of distilled water.
2.3 Chemical and Biochemical Analysis
Sampling and analysis of coal mine effluent and biological samples were done using Standard Methods for Examination of Water and Wastewater 1995. Plant tissues were oven dried on 80°C. The dried tissues were weighed and ground to powder for analysis of N, P and K. Plants were analysed for total nitrogen-N by microKjeldahl method (Peach and Tracey 1956) and total phosphorus-P by wet oxidation method (Jackson 1962). Potassium was analysed by flame photometer from Elico. For chlorophyll analysis, chlorophyll was extracted in 80% chilled acetone by using Arnon’s method (Arnon 1949). The protein content of leaf material was estimated following Lowry et al. (1951). Bovine serum albumin was used as a standard. Cold vapor technique was used for mercury analysis employing Perkin-Elmer MSH-10 connected to a Perkin-Elmer 2380 spectrophotometer. The procedure described in European Standard EN 1483 (European standard EN 1483 1997) was followed using a solution of 3% NaBH4 in 1% NaOH as a reducing agent. For arsenic, hydride generation technique was used employing Perkin-Elmer MSH-10 connected to a Perkin-Elmer 2380 spectrophotometer. The procedure described in ISO standard 11969 (International Standards Organization ISO 11969 1996) was used for preparing and analyzing the samples. The certified reference materials were analyzed according to ISO 11969 for arsenic and EN 1483 for mercury.
2.4 Statistical Analysis
Statistical comparisons of means were examined with one-way ANOVA. Correlations were also used for statistical significance. SPSS 10 statistical package was used for statistical analysis.
3 Results and Discussion
3.1 Hg and As Concentrations in Mining Effluent and its Removal
Results shown in Figs. 2 and 3 revealed concentration of mercury as 0.007 ± 0.0001 and arsenic 0.05 ± 0.001 mg l−1, respectively in effluent at initial stage in all the experimental sets, which was found in decreasing order up to 20th day of analysis. This indicates continuous absorption of metals by the plants. Highest removal of Hg and As from the effluent by E. crassipes (71% and 80% Hg and As, respectively) may be due to its fast growth (Muramoto and Oki 1983; Kelly et al. 1999), greater biomass accumulation, and higher affinity towards up take. Variations in the metal up take may be associated with the difference in the rate of plant growth and efficiency towards metal absorption. Whereas, a little increase in the mercury and arsenic concentration at the 25th day analysis reveals decaying of plant tissues from which metals are again released to the effluent. This happens when up take mechanisms break down, due to overload of the regulatory mechanism. When this phenomenon occurs the plants show toxicity symptoms and biomass production is reduced (Wenzl et al. 2001). Higher reduction in Hg and As content in the effluent containing E. crassipes as compared to the experimental sets containing L. minor and S.polyrrhiza indicates higher affinity of E. crassipes towards the mercury and arsenic up take. Analysis of variance (Dunnett t test) revealed significant variations (p < 0.001) in the removal of metals in different experimental set.
3.2 Accumulation of Hg and As by Macrophytes and its Translocation
The ratio of leaves to root metals indicates internal metal transportation. Higher concentration of metal was recorded in roots as compared to leaves (Table 1) for both the metals. Highest accumulation was noted on 20th day analysis. Analysis on 25th day revealed decrease in concentration of metal in plant tissues. This may be associated with decaying of plants and release of metals into the effluent. E. crassipes accumulated 0.45 ± 0.02 and 0.34 ± 0.012 μg g−1mercury and arsenic in root which was highest amongst the selected plant species. Leaves of E. crassipes accumulated 0.29 ± 0.02 and 0.25 ± 0.01 μg g−1 mercury and arsenic respectively. Plant species growing on metal contaminated water have restricted translocation of metals to the aerial parts (Zaranyika and Ndapwadza 1995). All the species under present investigation were found to be root accumulator. Lower accumulation of metals in leaves than root can be associated with protection of photosynthesis from toxic levels of trace elements (Landberg and Greger 1996). L. minor and S. polyrrhiza accumulated 0.38 ± 0.03 and 0.35 ± 0.01 μg g−1 mercury, 0.29 ± 0.01 and 0.26 ± 0.01 μg g−1 arsenic, respectively in their roots (Table 1).
The translocation factor, i.e. the ratio of leaves to root metals, indicated the internal metal transportation. Translocation of metals from roots to leaves of plant demonstrated their transportation from roots to shoot. This transportation was higher for arsenic, as compared to mercury. Higher translocation factor for arsenic in comparison to mercury can be related with lower concentration of arsenic than mercury in mining effluent used for study (Wang and Lewis 1977). Translocation was highest on 20th day analysis in all the selected species. Translocation factor of E. crassipes, L. minor and S. polyrrhiza was recorded as 0.73, 0.77, and 0.61 for arsenic as well as 0.64, 0.65, and 0.65 for mercury, respectively on 20th day (Tables 2 and 3).
3.3 Biochemical Composition of Experimental Plants Grown in Aquaculture
Accumulation of mercury and arsenic in plant tissues deteriorated the concentration of N, P and K in plants. Table 4 shows total-nitrogen (Total-N) content in roots of E. crassipes decreased from 41.5 ± 2.3 to 26.2 ± 1.2 mg g−1. Leaves of E. crassipes also showed similar trend (decrease in total-N from 41.2 ± 2.1 to 24.3 ± 1.0 mg g−1). Total-N, P and K contents were highest in E. crassipes followed by L. minor and S. polyrrhiza. Reduction in the N, P and K contents in plant tissues may be associated with the higher accumulation of metals. Correlation coefficient between total-N, P and K in root and leaves of plant and metal in the mining effluent used for study were found highly significant (p < 0.05; Table 4).
Analysis for chlorophyll and protein content in foliage of experimental plants exhibited highest in E. crassipes as 0.86 ± 0.05 and 6.5 ± 0.2 mg g−1 fresh weight respectively (Table 5). Analysis at regular intervals demonstrated a decrease in chlorophyll and protein content in plants.
4 Discussion
4.1 Mercury and Arsenic Uptake by Macrophytes
Variations in the metal up take may be associated with the difference in the rate of plant growth and efficiency towards metal absorption. Whereas, a little increase in the mercury and arsenic concentration at the 25th day analysis reveals decaying of plant tissues from which metals are again released to the effluent. This happens when up take mechanisms break down, due to overload of the regulatory mechanism. When this phenomenon occurs the plants show toxicity symptoms and biomass production is reduced (Wenzl et al. 2001). Higher reduction in Hg and As content in the effluent containing E. crassipes as compared to the experimental sets containing L. minor and S. polyrrhiza indicates higher affinity of E. crassipes towards the mercury and arsenic up take. Analysis of variance (Dunnett t test) revealed significant variations (p < 0.001) in the removal of metals in different experimental set. The larger root system of E. crassipes removed higher metal content. Large root system and increased numbers of fine roots oxidize the rhizosphere to a great extent and increase the availability of metal uptake (Ravit et al. 2003). Higher accumulation of mercury and arsenic in the roots may be due to the presence of greater anionic sites in the cell wall. This fact makes the roots as the primary sites of exposure to toxic metals present in the surrounding medium. The depth to which plant roots can penetrate is limited and this restricts the uptake of contaminants and rhizosphere actions to shallower level (William 2002; Skinner et al. 2007). Analysis of variance (Dunnett t test) revealed significant difference (p < 0.001) between the accumulation of mercury and arsenic in different plant parts for all the three hydrophytes selected. Correlation coefficient between mercury and arsenic concentration in root, leaf and coalmine effluent were negatively significant (p < 0.05; Table 1). Polynomial regression (Figs. 4 and 5) has shown the significant mercury and arsenic removal by the macrophytes from the coalmine effluent and accumulation in root and leaves. The three plants showed different removal pattern for mercury and arsenic from effluent. Data presented here indicate that metals accumulated by the macrophytes were largely retained in roots, as shown by general translocation factor value less than one. Accumulation and exclusion are two basic strategies by which plants respond to elevated concentration of heavy metals (Vogel-Mikus et al. 2005). De et al. (1985) concluded that mercury accumulation into the roots was about four times higher than the shoots at lower concentrations and about twice as high at 20 mg l−1. Kamal et al. (2004) established removal rate of mercury depends on its concentration in the medium.
Translocation factor values varied with metal content in the effluent. The more abundant the metal in effluent, the lower was the translocation factor of the metal. Decrease in translocation factor on 25th day analysis revealed shortfall in the translocation due to decaying of tissues. Extent of translocation within plants also depended on the metal and plant species concerned. Translocation factor varied among the plant species grown
4.2 Biochemical Effects of Hg and As Removal
Mercury has been reported to inhibit biosynthesis of chlorophyll through targeting –SH groups of δ-aminolaevulinic acid dehydratase (ALAD) in seedlings of bajra (Prasad and Prasad 1987). De et al. (1985) observed mercury decreased chlorophyll, protein, RNA, dry weight, catalase and protease activity. A similar trend of decline with increasing Hg dose was observed in the case of protein. Higher activity of protease and other catabolic enzymes activated by mercury may degrade the protein content of the cell. Mercury inhibits Nitrate Reductase (NR) activity. NR is a cysteine rich enzyme and mercury has strong affinity for thiol (–SH) groups. Binding of metals with –SH groups reduces NR activity (Pandey and Srivastava 1993).
Three major biochemical effects of arsenic are inhibition of ATP production, coagulation of protein and complexation with coenzymes. Arsenic has many properties of heavy metals and its toxic effects resemble lead and mercury. The availability of arsenic for uptake varies with species of arsenic. Dimethyl arsonic acid has lowest availability, followed by monomethyl arsonic acid as As (V), and with As (III) having the highest bioavailability (Carbonell et al. 1998). Reduction in chlorophyll content may be attributed to impaired uptake of essential elements, damage of photosynthetic apparatus or due to chlorophyll degradation by increased chlorophyllase activity (Sharma and Dubey 2005). Similarly reduction in protein may be due to degradation of proteases. Arvind and Prasad (2005) reported reduction of protein content in Ceratophyllum demersum due to higher concentrations of lead and cadmium. Carbonell-Barrachina et al. (1998) stated that regardless of the chemical form of arsenic both root and shoot concentrations significantly increases with increasing levels of arsenic in the medium. Arsenic, because of its chemical similarity to phosphorus interferes with some biochemical reactions involving phosphorus-biochemical generation of energy yielding substance adenosine triphosphate (ATP). In these reactions, arsenolysis occurs instead of phosphorylation and no ATP is formed. As (III), in the form of arsenite ion reacts with sulphydryl groups on the enzyme, thus inhibiting its activity. Correlation coefficient between chlorophyll and protein content in foliage and metal concentration in mining effluent was highly significant (Table 5). Chlorophyll and protein content decreased in plant tissues due to accumulation of mercury and arsenic by the selected macrophytes. Different plant species have different capacity for metal accumulation. Roots are the prime sites for accumulation of metals. These are effective traps for immobilizing metals from the contaminated waters. As metal accumulation in the tissues had reached maximum after 20 days, plant decay occurred as indicated by a loss of N, P, K, and a release of metals to the medium. Analysis revealed that when macrophytes are grown in captivity gives best possible results of metal removal.
5 Conclusion
Present study provides an approach for the removal mercury (Hg) and Arsenic (As), two important toxic heavy metals from the coal mining effluent. Three species of aquatic macrophytes E. crassipes, L. minor, and S. polyrrhiza proved highly effective in removing these two metals from the coal mining effluent during 25 days experiment. Maximum removal of metals was recorded on 20th day of exposure. The macrophytic species removed appreciable amounts of the Hg and As. Nevertheless, these metals have led their toxic effects by reducing N, P, K, chlorophyll and protein content of the experimental plants. Roots of the macrophytes proved better accumulator of the metals as they always contained higher amount of Hg and As in comparison to the leaves. Translocation factor also revealed low transportation of metals from root to leaves; its values were always less than one. Based on these results selected plants can be used on large scale for removal of mercury and arsenic.
References
Antunes, A. P. M., Watkins, G. M., & Duncan, J. R. (2001). Batch studies on removal of gold (III) from aqueous solution by Azolla filiculoides. Biotechnology, 23, 249–251.
Arnon, D. E. (1949). Copper enzyme in isolated chloroplast, polyphenol oxidase in Beta vulgaris. Plant Physiology, 24, 1–15.
Arvind, P., & Prasad, M. N. V. (2005). Cadmium–zinc interactions in a hydroponic system using Ceratophyllum demersum L.: Adaptive ecophysiology, biochemistry and molecular toxicology. Brazilian Journal of Plant Physiology, 17, 3–20.
Axtell, N. R., Sternberg, P. K., Steven, N., & Claussen, K. (2003). Lead and nickel removal using Microspora and Lemna minor. Bioresource Technology, 89, 41–48.
Bissen, M., & Frimmel, F. H. (2000). Speciation of As(III), As(V), MMA and DMA in contaminated soil extracts by HPLC-ICP/MS. Fresenius’ Journal of Analytical Chemistry, 367, 51–55.
Brooks, R. R., & Robinson, B. H. (1998). Aquatic phytoremediation by accumulator plants. In R. R. Brooks (Ed.), Plants that Hyperaccumulate Heavy Metals: their Role in Archaeology, Microbiology, Mineral Exploration, Phytomining and Phytoremediation (pp. 203–226). Wallingford: CAB International.
Brown, B. T., & Rattigan, B. M. (1979). Toxicity of soluble copper and other metal ions to Elodea canadensis. Environmental Pollution, 20, 303–314.
Carbonell, A. A., Aarabi, M. A., DeLaune, R. D., Gambrell, R. P., & Patrick, W. H. Jr (1998). Arsenic in wetland vegetation: availability, phytotoxicity, uptake and effects on plant growth and nutrition. Science of the Total Environment, 217, 189–199.
Carbonell-Barrachina, M. A., Aarabi, M. A., DeLaune, R. D., Gambrell, R. P., & Patrick, W. H. Jr (1998). The influence of arsenic chemical form and concentration on Spartina patens and Spartina alterniflora growth and tissue arsenic concentration. Plant Soil, 198, 33–43.
Cohen-Shoel, N., Barkay, Z., Ilzycer, D., Gilath, L., & Tel-Or, E. (2002). Biofilteration of toxic elements by Azolla biomass. Water, Air and Soil Pollution, 135, 93–104.
De, A. K., Sen, A. K., Modak, D. P., & Jana, S. (1985). Studies of toxic effects of mercury II on Pistia stratiotes. Water, Air and Soil Pollution, 24, 351–360.
Dikshit, A. K., Pallamreddy, K., Reddy, L. V. P., & Saha, J. C. (2000). Arsenic in ground water and its sorption by kimberlite tailings. Journal of Environmental Science and Health, 35, 65–85.
Ensley, H. E., Sharma, H. A., Barber, J. T., & Polito, M. A. (1996). Metabolism of chlorinated phenols by Lemna gibba, duckweed. In E. L. Kruger, T. A. Anderson, J. R. Coats, & A. C. Society (Eds.) Phytoremediation of soil and water contaminants (pp. 238–253). Orlando, FL: American Chemical Society.
European Standard EN (1483, 1997). European Standard EN 1483, 1997. Water quality. Determination of mercury. Bruxelles: European Committee for Standardization.
Indiana Geological Survey. (2004). Indiana University, www.igs.indiana.edu/geology.
International Standard Organization ISO 11969 (1996). Water quality. Determination of arsenic. Atomic absorption spectrometric method (hydride technique). Geneva: International Organization for Standarization.
Jackson, M. L. (1962). Soil chemical analysis pp. 183–190. Englewood Cliffs, NJ, USA: Prentice Hall.
Kamal, M., Ghalya, A. E., Mahmouda, N., & Cote, R. (2004). Phytoaccumulation of heavy metals by aquatic plants. Environment International, 29, 1029–1039.
Kelly, C., Mielke, R. E., Dimaquabo, D., Curtis, A. J., & Dewitt, J. G. (1999). Adsorption of Eu (III) into roots of water hyacinth. Environmental Science and Technology, 33, 1439–1443.
Landberg, T., & Greger, M. (1996). Difference in uptake and tolerance to heavy metal in Salix from unpolluted and polluted areas. Applied Geochemistry, 11, 175–180.
Lasat, M. M. (2002). Phytoextraction of toxic metals: A review of biological mechanism. Journal of Environmental Quality, 31, 109–120.
Lin, T.-H., Huang, Y.-L., & Wang, M.-Y. (1998). Arsenic species in drinking water, hair, fingernails, and urine of patients with blackfoot disease. Journal of Toxicology and Environmental Health, Part A, 53, 85–93.
Lowry, O. H., Rosebraugh, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with folin-phenol reagent. Journal of Biological Chemistry, 193, 265–275.
Manahan, S. E. (1997). Environmental science and technology. New York: Lewis Publishers.
Matsui, M., Nishigori, C., Toyokuni, S., Takada, J., Akaboshi, M., Ishikawa, M., et al. (1999). The role of oxidative DNA damage in human arsenic carcinogenesis: Detection of 8-hydroxy-2¢-deoxyguanosine in arsenic-related Bowen’s disease. Journal of Investigative Dermatology, 113, 26–31.
Mishra, V. K., Upadhyaya, A. R., Pandey, S. K., & Tripathi, B. D. (2008a). Concentrations of heavy metals and nutrients in water, sediments and aquatic macrophytes of GBP Sagar an anthropogenic lake affected by coal mining effluent. Environmental Monitoring and Assessment, DOI 10.1007/s10661-007-9877-x (in press).
Mishra, V. K., Upadhyaya, A. R., Pandey, S. K., & Tripathi, B. D. (2008b). Heavy metal pollution induced due to coal mining effluent on surrounding aquatic ecosystem and its management due through naturally occurring aquatic macrophytes. Bioresource Technology, 99, 930–936.
Muramoto, S., & Oki, Y. (1983). Removal of some heavy metals from polluted water by water hyacinth (Eichhornia crassipes). Bulletin of Environmental Contamination and Toxicology, 30, 170–177.
Noraho, N., & Gaur, J. P. (1996). Cadmium adsorption and intracellular uptake by two macrophytes, Azolla pinnata and Spirodela polyrhiza. Archiv fuer Hydrobiologie, 136, 135–144.
Pandey, M., & Srivastava, H. S. (1993). Inhibition of nitrate reductase activity and nitrate accumulation by mercury in maize leaf segment. Journal of Environmental Health, 35, 110–114.
Peach, K., & Tracey, M. V. (1956). Modern methods of plant analysis, Vol.-1. Berlin: Springer.
Pickering, I. J., Prince, R. C., George, M. J., Smith, R. D., George, G. N., & Salt, D. E. (2000). Reduction and coordination of arsenic in Indian mustard. Plant Physiology, 122, 1171–1177.
Prasad, D. D. K., & Prasad, A. R. K. (1987). Altered d-aminolaevulinic acid metabolism by lead and mercury in germinating seedlings of bajra (Pennisetum typhoideum). Journal of Plant Physiology, 127, 241–249.
Rahmani, G. N. H., & Sternberg, S. P. K. (1999). Bioremoval of lead from water using Lemna minor. Bioresource Technology, 70, 225.
Ravit, B., Ehrenfeld, J. G., & Haggblom, M. M. (2003). A comparison of sediment microbial communities associated with Phragmites australis and Spartina alterniflora in two brackish wetlands of New Jersey. Estuaries, 26, 465–474.
Sharma, P., & Dubey, R. S. (2005). Lead toxicity in plants. Brazilian Journal of Plant Physiology, 17, 35–52.
Skinner, K., Wright, N., & Porter-Goff, E. (2007). Mercury uptake and accumulation by four species of aquatic plants. Environmental Pollution, 145, 234–237.
Spearot, R. M., & Peck, J. R. (1984). Recovery process for complexed copper bearing rinse waters. Environmental Progress, 3, 124–129.
Standard Methods for Examination of Water and Wastewater (1995). American Public Health Association, American Water Works Association, and Water Pollution Control Federation, Washington, DC.
US Environmental Protection Agency. (2006). www.epa.gov.
Vogel-Mikus, K., Drobne, D., & Regvar, M. (2005). Zn, Cd and Pd accumulation and arbuscular mycorrhizal colonization of pennycress Thlapi praecox Wulf (Brassicaceae) from the vicinity of a lead mine and smelter in Slovenia. Environmental Pollution, 133, 233–42.
Wang, W., & Lewis, M. A. (1977). Metal accumulation by aquatic macrophytes. In W. Wang, J. W. Gorsuch, & J. S. Hughes (Eds.) Plants for environmental studies (pp. 367–416). New York: CRC Lewis Publishers.
Wenzl, P., Patino, G. M., Chaves, A. L., Mayer, J. E., & Rao, I. M. (2001). The high level of aluminum resistance in signal grass is not associated with known mechanisms of external aluminum detoxification in root apices. Plant Physiology, 125, 1473–1484.
William, J. B. (2002). Phytoremediation in wetland ecosystem: progress, problems and potential. Critical Reviews in Plant Sciences, 21, 607–635.
Zaranyika, M. F., & Ndapwadza, T. (1995). Uptake of Ni, Zn, Fe, Co, Cr, Pb, Cu and Cd by water hyacinth in Mukuvisi and Manyame rivers, Zimbabwe. Journal of Environmental Science and Health, Part A, 30, 157–169.
Acknowledgement
Authors are thankful to Mining Authorities; Northern Coal fields limited, Singrauli for their permission and co-operation during research and work Council of Scientific and Industrial Research, New Delhi for financial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mishra, V.K., Upadhyay, A.R., Pathak, V. et al. Phytoremediation of Mercury and Arsenic from Tropical Opencast Coalmine Effluent Through Naturally Occurring Aquatic Macrophytes. Water Air Soil Pollut 192, 303–314 (2008). https://doi.org/10.1007/s11270-008-9657-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-008-9657-4