Abstract
An assessment of the environmental quality of sediments at several locations of the Ría de Pontevedra (NW of Spain) was performed by integrating toxicity data obtained from multiespecies bioassays, chemical data from analysis of mussels and sediment, and physical–chemical parameters of the sampled sites. Subsequently, a toxicity identification evaluation (TIE) method intended for characterization and identification of the toxic agents was applied to the most polluted location by using the Paracentrotus lividus sea urchin bioassay. Both metals and organic compounds seem to be the causative agents of toxicity in elutriates of the studied sediment. Finally, multivariate statistics were applied for a better interpretation of results. A factor analysis was developed to establish the relationship among variables and to derive local sediment quality guidelines (SQG) by linking chemical contamination to biological effects. When multidimensional scaling and cluster analysis were performed to group the locations according to either the chemistry or toxicity data, P3-site was always clearly broken up the others. The different approaches all supported the same conclusion: site P3 can be considered highly contaminated by both trace metals and PAHs resulting in high toxicity for all the tested species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
It has been demonstrated that there is not a simple direct relationship between chemical concentrations in sediments and ecological risk (O’Connor and Paul 2000). Presence of contaminants does not necessarily mean that they are bioavailable. Analogously, bioavailability does not imply that contaminants have effects on organisms, populations or communities. An integrative assessment using different approaches including the measurement of sensitive and ecologically relevant biological variables is necessary to allow a more complete understanding of the patterns of pollution (Chapman 2000; Carballeira 2003; Fernández et al. 2006a, b). In this study we have combined data from chemical analyses, bioaccumulation measurements and toxicity data from three embryo-larval bioassays. A local species of mussel (Mytilus galloprovincialis) was used to determine bioaccumulation because of their abundance in sites of concern, high filtration rate and demonstrated accumulation potential (O’Connor 1998). Also, the mussel M. galloprovincialis, an ascidia (Ciona intestinalis) and a sea-urchin (Paracentrotus lividus) were used to carry out the toxicity tests, because of their commercial and ecological relevance, representativeness of different phyla, and their sensitivity to several kinds of organic and inorganic microcontaminants (Bellas et al. 2001; Fernández and Beiras 2001; His et al. 1999b; Bellas et al. 2005). With the aim of characterizing chemical-groups causing toxicity in the most polluted sample site, a Toxicity Identification Evaluation (TIE) method was conducted based on the P. lividus embryo-larval bioassay. This method consists of assaying elutriate samples that have previously been subjected to physical and chemical treatments that selectively eliminate different groups of potentially toxic substances. When toxicity decreases or disappears after a treatment, the eliminated chemical group is assumed to be the causative agent of toxicity. The TIE method was first developed by the U.S Environmental Protection Agency to identify the substances causing toxicity in municipal and industrial effluents (Burgess et al. 1995; Deanovic et al. 1999). Later on, the use was extended to fresh- and marine waters (Boucher and Watzin 1998; Thomas et al. 1998) and to harbor and estuarine sediments (Ho et al. 1997, Anderson et al. 2007). In this work we have applied the TIE to determine the causal toxic agent(s) in intertidal estuarine sediments.
Finally, for a better interpretation of the results, a factor analysis was made with the physical, chemical and the biological data, linking contaminants to biological effects and deriving sediment quality guidelines (SQG) which are demonstrated to provide practical, reliable and predictive tools for assessing sediment quality (Long 1992; McDonald et al 1996; Chapman and Mann 1999, Wenning and Ingersoll 2002). These guidelines establish for each chemical two thresholds: the concentration below which adverse biological effects are not expected, and the concentration above which adverse biological effects are expected. Other multivariate analyses, the multidimensional scaling (MDS) and cluster, were applied to group locations according to the degree of contamination and toxicity.
All these approaches were compared in order to obtain complementary results conducting to a more complete assessment of the quality of sediments in the studied area.
2 Materials and Methods
2.1 Sampling Sites
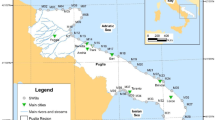
During 2000 and 2001 a more detailed assessment was undertaken in the Ría de Pontevedra, the estuary showing maximum ecological risk. This was based on the results of previous surveys of marine pollution in the four main Galician estuaries (Beiras et al. 2003a, b). The first year nine sites were sampled (P1–P9) and the next year three additional sites were included (P1.5; P3.5 and P8.5). Sampling locations are recorded in Fig. 1. At each site, general environmental parameters were measured in situ in the seawater and sediments (Table 1), samples of mussels were taken for analytical chemistry, and samples of sediment were collected for both analytical chemistry and toxicity bioassays.
Temperature, pH, salinity and dissolved oxygen were recorded in surface water by using Orion and Hanna electrodes. Phosphates \({\left( {{\text{PO}}^{{{\text{3 - }}}}_{{\text{4}}} } \right)}\) in water and biological oxygen demand (BOD5) were measured according to standard methods (APHA-AWWA-WPCF 1992) as described in Beiras et al. (2003b). At each site three samples of about 500 g of the surface layer (2 cm) of the intertidal sediments were taken at low tide, following the vertices of a triangle of 2 m side, using a wooden palette. Samples intended for bioassays were placed into sealed polyethylene bags, carried to the laboratory on ice, and stored at 4°C in the dark for a maximum of 3 days before the bioassay was conducted. Samples for chemical analysis were stored freeze-dried in glass flasks.
Redox potential of sediment was recorded in situ at 1, 3 and 5 cm depth using a Crison electrode, consisting in a pH-meter that measure mV jointly with a metallic electrode made of platinum. A redox standard solution made of FeSO4, Fe2(SO4)3, H2SO4 + H2O was used to check that the electrode was working well.
Organic matter content (OM) was measured from triplicate samples by drying the sediment at 80°C until constant weight and incineration in a furnace at 450°C for 24 h. Salt weight was discounted from the dry and ash weight of the sediment.
At each site, about 20 mussels (M. galloprovincialis) of approximately 4 cm length were collected into sealed polypropylene bags and stored at −80°C.
2.2 Analyses of Metals and Organics in Mussels and Sediment
Metals (Cu, Zn, Pb, Cd, As and Cr) in mussels were analyzed by atomic emission spectrometry with inductive coupled plasma (ICP-AES) (Perkin-Elmer), after liofilization with Alpha 1-4 Christ-Freeze Dryers and pressurized acid digestion of samples in MDS 2000 microwave oven (CEM Corporation). Refractory material (BCR-CRM 278R) was used for digestions. For analyses, ERM-CE278 (mussel tissue, European Reference Materials) and Merck-6 (multielemental standard) were used such as reference material. Recoveries were up to 95%.
Metals in sediments were determined by X-ray fluorescence (Siemens SRS 3000) in dry samples triturated in the agate mill down to < 200 μm grain size. Quantification was made by comparing with a calibration curve obtained with 25 international reference geological samples such as PACS-2 and MESS-3 (National Research Council, Canada).
All these analyses were conducted by the CACTI (Centre of Scientific–Technological support to the Research – University of Vigo–Spain).
∑12 priority PAHs (phenanthrene, fluoranthene, pyrene, benzo(a)anthracene, crysene, benzo(e)pyrene, benzo(b)fluoranthene, benzo(k)fluoranthene, benzo(a)pyrene, benzo (g,h,i)perilene, dibenzo(a,h)anthracene and indene(1,2,3,-c,d)pyrene) were also determined in sediments by High Performance Liquid Chromatography (HPLC). Extraction, preparing and quantification procedures are described in Pérez 2003. Instrumental calibration was conducted by using a commercial mixture of 12 PAHs, adding Benzo(e)pyreno (that was not included in the mixture) and 2-methil-crisene as internal standard. These analyses were conducted by the IEO (Spanish Oceanographic Institute). The quality of the chemical analyses at IEO-Vigo was controlled at two levels: (1) Internally by using certified reference materials along with each series of samples. For sediments, HS-1, BCSS-1 and BEST-1 reference materials were obtained from the National Research Council of Canada. (2) Externally, by participation in intercalibration exercises promoted by international institutions, namely the International Atomic Energy Agency (IAEA), (Coquery et al. 2000; Villeneuve and de Mora 2000), the QUASIMEME programme (Quality Assurance of Information for Marine Environmental Monitoring in Europe) from 1993 to 1996 (Wells 1996), QUASIMEME Laboratory Performance Studies from 1996 to date (QUASIMEME 2001), and QUASH (Quality Assurance of Sample Handling) from 1997 to 2000 (Smedes et al. 2000).
2.3 Elutriation and Metal Analysis in Elutriates
Elutriates were obtained according to methods previously described (Beiras 2002), consisting of 30 min rotatory mixing at 60 r.p.m. of 100 g sediment in 500-ml polypropylene flasks filled with 0.22 μm-filtered seawater (FSW) of oceanic characteristics. The flasks were filled to the brim, with no air inside, in order to mimic sediment resuspension in the sea floor. After overnight decanting at 20 ± 1°C, the supernatant was removed and then gently aerated for 10 min in order to prevent false positives caused by hypoxic conditions or residual H2S.
For chemical analysis, the elutriates were filtered at 0.45 μm with polycarbonate filters and then stored in polypropylene flasks at −20°C. Analysis of Zn, Cd, Pb and Cu were carried out by differential pulse anodic stripping voltammetry. Measurements were made with a GSTAT10 potentiostat (Ecochemie B.V.) coupled to a polarographic stand VA 663 (Metrohm). A hanging mercury drop electrode (HMDE) of 0.33 mm2 surface and a saturated calomel reference electrode were used. The limit of detection (LOD) was 0.02 μg/L, 0.08 μg/L, 0.30 μg/L, 0.43 μg/L for Pb, Cd, Cu and Zn respectively.
Analysis of mercury was made by cold vapour atomic absorption spectrometry technique (CV-AAS). The ionic mercury was reduced to its elemental form by using SnCl2 in acidic solution, and then it was volatilized and transported into the absorption cell where absorbance was measured at 253.65 nm wavelength. The CV-AAS was automated by means of FIAS system (Perkin-Elmer FIES 200) (Weltz and Shubert-Jacobs 1991), technique that permits lower determination limits and higher precision compared with other cold vapour techniques. The LOD was 0.35 μg/L.
2.4 Embryological Bioassays
At each site, the toxicity of the elutriates was assessed by using P. lividus sea-urchin embryos. Mature sea-urchins were collected by hand from a natural population in the outer part of the Ría de Vigo. Obtaining the gametes and conducting the in vitro fertilization was previously described in detail by Beiras et al. (2003b). Embryos were incubated at 20°C for 48 h and then fixed by adding two droplets of 40% formalin. Embryogenesis success, defined as percentage of 4-arm pluteus larvae, was recorded by direct observation under an inverted microscope (100 individuals per replicate).
In 2001, toxicity was additionally assessed by using the embryo-larval bioassays with the ascidian C. intestinalis and the bivalve M. galloprovincialis following the methods described by Bellas et al. (2001) and His et al. (1997) respectively.
Undiluted elutriates (200 g/l) and dilutions of 1/4 (50 g/l) and 1/10 (20 g/l) with 0.22 μm-filtered control seawater were tested. Five replicates per treatment and five blanks containing only control seawater were tested in 20 ml polypropylene vials. Temperature, salinity, pH and oxygen concentration were recorded prior to bioassay, to ensure optimal conditions of incubation (see Table 1). When the presence of hydrogen sulfide was suspected, its concentration was also measured by using a commercial colorimetric test (Merk, Aquaquant).
2.5 TIE Method
Toxicity Identification Evaluation (TIE) was based on Burgess et al. (1995) and adapted to P. lividus larvae. Elutriates obtained from toxic sediment sampled in 2001 at P3 site were manipulated in order to selectively remove potential toxic fractions. Toxicity of manipulated elutriates was tested following the bioassay previously described, and results were compared with a baseline test simultaneously conducted. All treatments were applied to undiluted elutriates and to the 1/4 and 1/10 dilutions. Five manipulations were tested: aeration, oxidant reduction, metal chelation, C18 solid phase extraction and combination of both chelation and C18 extraction.
Slight aeration intended for removing volatile and/or reducing substances was applied for 15 min by using an air pump. Oxidant reduction consisted of adding 30 mg/l of sodium thiosulphate (Na2S2O3) to the diluted and undiluted elutriates. The Na2S2O3 was added from a stock prepared in distilled water.
Metal chelation was achieved by adding 80 mg/l of ethylenediaminetetraacetic acid (EDTA) to the diluted and undiluted elutriates from a stock prepared in distilled water.
Previous toxicity tests with EDTA and Na2S2O3 were performed to ensure that concentrations had no effect on embryogenesis of P. lividus sea-urchin.
Solid phase extraction with chromatography column led to the sorption of non polar molecules or non polar functional groups to the column. Extraction was performed by passing diluted and undiluted elutriates through a C18 column. Elutriates were previously filtered through 0.4 μm polycarbonate filters and C18 columns were activated with HPLC-grade methanol prior to use.
Combinations of both C18 and chelation manipulations were also performed as follows: diluted and undiluted elutriates were passed after filtration through 0.4 μm and a C18 column previously activated as described above. Then EDTA was added to the manipulated elutriates at a concentration of 80 mg/l.
For each manipulation five replicates per dilution and five blanks consisting of filtered control seawater were tested. Percentage of embryogenesis success was recorded after the incubation period.
2.6 Statistical Analyses
Percentages of embryogenesis success (P) in each vial were corrected by the mean response in the control vials (Pc) following the Abbott’s formula: \({\text{P' = }}{\left( {{{\left( {{\text{P - Pc}}} \right)}} \mathord{\left/ {\vphantom {{{\left( {{\text{P - Pc}}} \right)}} {{\left( {100 - {\text{Pc}}} \right)}}}} \right. \kern-\nulldelimiterspace} {{\left( {100 - {\text{Pc}}} \right)}}} \right)} \times 100\). Resulting percentages (P′) and chemical concentrations were normalized respectively by arcsine and logarithmic transformation.
Contamination and toxicity data were treated with a factor analysis using principal components as extraction procedure. The main goal of this factor analysis was to derive a reduced number of new variables (Factors) as the linear combinations of original variables with the minimum loss of information. This allows to discriminate the most relevant variables in the study and to show the relationships among them.
We have also used these Factors to derive the Sediment Quality Guidelines (SQG) for each chemical, following the method described by DelValls and Chapman (1998). These SQG were defined by two values: the maximum concentration of a chemical that was not associated to biological effects, and the minimum concentration of the chemical that was associated to biological effects. To derive these values we have used the factor scores for each case (site) that quantify the prevalence of every factor for each station. These factor scores are directly obtained from the analysis. We have translated these values into a bar chart (Fig. 4). When the factor score of Factor x (which correlates chemicals with harmful effects) was negative or zero, the maximum concentration of the concerned chemical at those stations represented the maximum concentration not associated with adverse effects. Then, it was defined as concentration below which biological effects were low or minimal. In the other way, the minimal concentration of the chemical among the stations where Factor x-scores were positive was considered concentration causing adverse biological effects. The interval of uncertainly was defined between these two values.
Factor analysis was carried out by using the STATISTICA 6.0, and Muldimensional Scaling (MDS) and Cluster analysis were conducted with the SPSS 11.5 software.
3 Results
3.1 Analysis of Metals and Organics in Mussels and Sediment
Table 2 summarizes the results of chemical analysis in sediments and mussels sampled in 2000 and 2001 at different sites of the Ría de Pontevedra. P3 site showed in general maximum values for all contaminants measured in both matrices, particularly for Cu, Zn, Pb, Cr and PAH concentration in sediment. Also P2 and P4 sites show concentrations of contaminants slightly above the other stations. PAH concentration measured at P4 site was especially remarkable. Differences of contaminant concentrations among locations were much higher for the mineral matrix than for the biological matrix; however, both matrices agreed in indicating P3 as the most polluted site. Mussels sampled in P3 showed the highest concentrations of Cu, Zn, Pb and Sn.
3.2 Embryological Bioassays
Figure 2 shows the percentage of embryological success recorded after incubation of test organisms in the sediment elutriates. In all cases negative controls were suitable for acceptation of the tests. For P. lividus embryos similar pattern was observed in 2000 and 2001: elutriates of P3 showed a very high toxicity, preventing embryo development even when the elutriate was tenfold diluted, and P4 site showed moderate toxicity only for undiluted elutriate. In 2001 also P1 showed a moderate toxicity that disappeared when elutriates were diluted. All the other sites did not show toxicity. Bioassays performed with M. galloprovincialis showed similar results: high toxicity was observed for P3 and moderate toxicity at P1 and P4 sites, where toxicity disappeared with the dilution of the elutriates. The ascidian bioassay showed less sensitivity and only the most toxic site (P3) was detected to be toxic. In general, a good agreement among bioassays was observed.
Embryogenesis success for P. lividus, M. galloprovincialis and C. intestinalis fertilised eggs exposed to elutriates from sediments sampled at the Ría de Pontevedra in 2000 and 2001. No dilution (200 g/L), dilution 1/4 (50 g/L) and dilution 1/10 (20 g/L) are plotted in different colours. Data corresponding to the non toxic sites P7, P8 and P9 for P. lividus (2000) are not available
3.3 TIE Method
Figure 3 shows the percentage of embryogenesis success after incubation of fertilized eggs in the untreated elutriates (baseline) and after several manipulations intended for elimination of different potential toxic fractions. With the aim of interpreting the results of the TIE method we have also recorded a qualitative response (the embryological stage reached by embryos) together with the embryological success (recorded as percentage of larvae). Fertilized eggs of sea-urchin change into morulae, blastulae, gastrulae and prism embryos before reaching the larvae stage. The higher the toxicity, the earlier the development of the embryos stops. Elutriates tested after aeration and Na2S2O3 addition did not show any reduction of toxicity in comparison to the baseline bioassay (Fig. 3b–c), so toxicity was not caused neither by volatile nor oxidant substances. On the contrary, EDTA addition and C18 solid phase extraction showed effectiveness in reducing toxicity, only observed for diluted elutriates (Fig. 3d–e): the highest dilution treated with EDTA allowed embryo development to gastrula stage, although the undiluted elutriate arrested embryo development at morula stage. A similar pattern was observed for elutriates treated with a C18 column: higher dilutions allowed development to later embryological stages, while 100% of morula was observed for the undiluted elutriate. Furthermore, when EDTA and C18 manipulations were combined the toxicity was completely removed, and 100% of normal larvae were recorded after the incubation period already for the undiluted elutriate.
With the goal of identifying chemicals causing the toxicity of P3 elutriates, concentrations of Hg, Cu, Zn, Pb and Cd were measured in the elutriates and compared to the median effective concentrations (EC50) causing effects on P. lividus embryo development. Table 3 lists the metal concentrations measured in the elutriates from sediments sampled in 2001 at the Ría de Pontevedra. Also, the median effective concentrations (EC50) on P. lividus embryo development are recorded for each metal (Fernández and Beiras 2001). There were no significant differences in Cd and Hg concentrations among sites. Nevertheless, elutriates from P3 showed the maximum concentrations for Pb and especially for Zn and Cu. The toxic units (TU), defined as concentration/EC50, were calculated for each metal and site (Table 3). Assuming the toxicity of metals follow an additive model (Marking and Dawson 1975), then when ∑TU > 1 the elutriate should be toxic. According to this model, toxicity is predicted at P3 only (∑TU = 2.349, whilst for the remaining sites ∑TU < 1). This theoretical prediction agrees with the results of the different bioassays conducted.
3.4 Factor Analysis, Multidimensional Scaling (MDS) and Cluster Analysis
The original data set used in the factor analysis included five physical–chemical parameters, seven chemical concentrations in sediments, seven chemical concentrations in mussels, and three toxicity endpoints, corresponding to sampling of 2001. Toxicity endpoints were all expressed as percentage of unsuccessfully embryological development in order to establish direct correlations with concentration of contaminants. These original variables have been reduced to four new variables (Factors) that explain 81.40% of the total variance of the original data. Table 4 shows the significant factor loadings (after varimax rotation) for each original variable. These rotated factor loadings are coefficients correlating the original variables and the factors. According to DelValls and Chapman (1998) a factor loading ≥0.4 corresponds to an associated explained variance of over 65%. For this study we have selected a cut-off of 0.50 and then, each factor has been described according to the dominant group of variables associated. The first factor explains 39.04% of total variance and groups chemicals in sediments (except Ni and Cr), Cu and Zn in mussels and all the biological effects. The second factor explains 21.39% of the total variance and groups As, Cr and Pb in mussels, Ni in sediments and phosphates. The third factor explains 13.15% of the total variance; it groups effects on sea-urchin and mussel embryogenesis, Hg in mussels, Cr in sediments and two physical–chemical parameters (MO and salinity). Finally, the fourth factor explaining the 7.81% of total variance, groups pH, BOD5 and As in mussels. As and Hg in mussels as well as salinity have negative loading showing an inverse relationship with the other variables.
Figure 4 shows the prevalence of each factor in studied sites. The Factor 1, that links metals and PAHs in sediments to biological effects, presents the maximum score in P3 (3.10), followed by P4 (0.16). P2 shows prevalence in Factor 2 (3.10) that mainly represents metals in mussels and P1 shows a high score of Factor 3 (2.65), showing biological effects linked to Cr in sediment and physical–chemical parameters.
In this case, the most important factor is Factor1 that explains the majority of total variance and links chemicals in sediments to biological effects.
We have used this Factor to derive the Sediment Quality Guidelines (SQG) for each chemical. To derive these values we have used the factor scores represented in Fig. 4, estimated for each case (site). When the factor score of Factor 1 (which correlates chemicals with harmful effects) was negative or zero, the maximum concentration of the concerned chemical at those stations represented the maximum concentration not associated with adverse effects (e.i. concentration below which biological effects were low or minimal). The minimal concentration of the chemical among the stations where Factor 1-scores were positive was considered concentration causing adverse biological effects. The calculated SQGs for As, Pb, Zn, Cu and PAHs are shown in Table 5.
Figure 5 shows the spatial distribution of sampling sites obtained from multidimensional scaling and cluster analysis performed with the chemical data from sediment analysis (stress = 0.054), and with toxicity data from bioassays (stress = 0.087). Both analyses clearly segregated P3 from the other sites because of its high contamination and toxicity levels. MDS with chemical data remarked P2 and especially P4 (due to high concentration of PAH) and MDS with toxicological data also segregated P1 site probably due to a chemical that has not been considered.
4 Discussion
Chemical analyses showed maximal concentrations of inorganic and organic contaminants in P3 in 2000 and 2001, both in sediments and mussels. This location can be considered as highly contaminated by Cu, Zn and Pb, comparing to concentrations measured at non polluted Galician sites studied from 1997 to 1999 (Beiras et al. 2003a, b). A high variability is typical for PAH concentrations in the marine environment. The OSPAR Commission established the background values for PAHs in sediments of the North–East Atlantic within the range 100–1,000 μg/kg (dw). Nevertheless, in areas such as estuaries, bays, or locations of regular shipping or oil production, concentrations from 200 μg/kg dw to over 6,000 μg/kg dw are reported (OSPAR 2000). Baumard et al. (1998) in a study carried out in the Mediterranean Sea, considered low contamination when ∑12 PAH was below 100 μg/kg, while concentrations up to 1,000 μg/kg corresponded to chronically contaminated industrialized areas and harbors. In the present study ∑12 PAH at P3 site was about 6,800 μg/kg being two orders of magnitude higher than the other sampled sites and at P4 site this concentration was remarkably high. At both locations concentration of PAHs was above the threshold of natural concentrations reported in literature and can be considered as highly contaminated for these organic compounds. Concentrations of metals and PAHs measured at the other locations were similar to the reference concentrations and can be considered as non contaminated sites.
When toxicity of sediments was tested by using multiespecies bioassays, P3 was the most toxic site, preventing embryo development already at the tenfold diluted elutriate. Also P4 showed a slight toxicity when undiluted elutriate was tested. Elutriates of the other sites were non toxic, except P1 that in 2000 showed toxicity when undiluted elutriate was tested. In order to establish the correlation between contamination of sediments and toxicity, we have adapted the P. lividus bioassay to the Toxicity Identification Evaluation method (TIE), showing that bioassays can be used not only to detect toxicity but to characterize the toxic substances. Elutriates were manipulated to remove different chemical fractions and the standard P. lividus embryo bioassay was subsequently conducted. Finally, the results were compared to the baseline test performed with the non-manipulated toxic elutriate. Addition of sodium thiosulphate or aeration did not remove toxicity from the elutriates, so it was not caused by volatile or oxidant substances, such as chlorine. Nevertheless, when EDTA chelation or C18 solid phase extraction were applied, toxicity was slightly reduced, and when both manipulations were applied together toxicity completely disappeared, indicating that metal ions and non polar substances were both exerting toxicity. These results were confirmed by the chemical analysis. P3 elutriates showed the highest concentrations of Zn, Cu and Pb, exceeding the Zn and Cu median effective concentration (EC50) (Kobayashi 1981; Fernández and Beiras 2001), and supporting that these metals were toxic agents. There is no analysis of organics in the elutriates but sediment from P3 showed high concentration of PAHs, up to three orders of magnitude higher than the other sites. So, we can suspect that organic toxicity removed after C18 treatment is likely due to these compounds. Previous studies showed low toxicity of five individual PAHs on P. lividus embryo development (Fernández 2002) probably due to their low solubility in seawater. Nevertheless, when chemicals with similar mode of action act together additive or even synergistic effects were frequently observed (Kobayashi 1995; Vanegas et al. 1997; Fernández and Beiras 2001). Therefore, combination of several PAHs in the elutriates could lead to overall toxicity (Fernández et al. 2006a, b). Moreover, the possibility of other combination toxicity mechanisms between metals and PAHs different than concentration-addition, for instance synergy, must also be taken into account. When a theoretical addition model (Marking and Dawson 1975) was applied, P3 was predicted to be toxic, according to the results of the biological toxicity test. The toxicity detected in P4 elutriates may also be due to PAHs. The Sediment Quality Criteria Guidelines proposed by Swartz (1999) confirm this suspect: PAHs concentrations measured at P2, P3 and P4 sites are above effects range-median (ERM) values, concentrations above which probable toxicity on marine invertebrates is expected. Nevertheless, we cannot discard the presence of other compounds not measured such as the tributyl-tin oxide (TBT). This substance is an organometal used in the past as antifouling on ship paints and its extreme toxicity to several marine invertebrates has been well documented (His et al. 1999a; Ozretic et al. 1998; Kobayashi and Okamura 2002). P3 site is located near a shipyard and P4 site is placed in a harbour, so it is possible that sampled sediments contained TBT. But this is only a speculation that needs further analyses.
Toxicity in P1 site was already observed in a previous assessment (Beiras et al. 2003b) and was attributed to non analyzed organic compounds probably correlated with a fluvial input leading to this location.
The factor analysis conducted with physical–chemical and biological data supports the conclusion: the contents of chemicals in sediment (metals and PAHs) and the toxic effects on sea-urchins, ascidians and mussels were grouped in Factor 1, supporting the idea that metals and PAHs were probably responsible of the toxicity observed in developed bioassays. Cu and Zn contents in mussels were also grouped in Factor 1, indicating a relationship between sediment and mussel concentrations stronger than that for other metals, such as Pb and Cr whose values vary within a narrower range. In fact, a low degree of heterogeneity of chemical concentrations in mussels was observed among locations compared to sediments.
We have used these factor scores to obtain local sediment quality guidelines derived from the data obtained in this study. However, we must remark that site P3 is an outlier with regard to all the others sites. To obtain more precise local-SQGs, a range of different levels of contamination correlated with biological effects would be desirable. DelValls and Chapman (1998) also propose the inclusion of macrobenthic infaunal community structure data, to derive these SQGs. However, we have compared different sediment quality guidelines obtained from different approaches, with those derives from our data (Table 5). Using the criterion of McDonald et al. (1996), that considerer sediment quality guidelines (SQG) as comparable when they agreed within a factor of three, the four sets of guidelines were comparable except for PAH, where TEL/PEL and ERL/ERM were less restrictive than ours.
Finally, there was also a good agreement between these chemical and biological results as shown by MDS and cluster analysis. P3 location was discriminated from the other sites independently of the kind of data or sampling year. Only toxicity recorded at P1 in 2000 was not in agreement with chemical data as previously explained.
All the complementary approaches developed in this work have converged into the same conclusions. The necessity of complementary chemical and toxicological data for a good assessment of the biological quality of marine sediments has been widely expressed (DelValls et al. 1998; O’Connor and Paul 2000; Beiras et al. 2003a, b). In addition to this, it provides the possibility of deriving local SQG that could be used as a substitute for direct measures of potential adverse effects of contaminants in sediments on benthic organisms (Wenning and Ingersoll 2002). Therefore, training in the development of SQGs as one tool among several to assess the sediment quality is recommended.
References
Anderson, B., Hunt, J., Phillips, B., Thompson, B., Lowe, S., Taberski, K. (2007). Patterns and trends in sediment toxicity in the San Francisco Estuary. Environmental Research, 105(1), 145–155.
APHA-AWWA-WPCF (1992). Métodos normalizados para el análisis de aguas potables y residuales. Ediciones Díaz de Santos. S.A. Madrid.
Baumard, P., Budzinski, H., Michon, Q., Garrigues, P., Burgeot, T., & Bellocq, J. (1998). Origin and bioavailability of PAHs in the Mediterranean Sea from mussel and sediment records. Estuarine, Coastal and Shelf Science, 47, 77–90.
Beiras, R. (2002). Comparison of methods to obtain a liquid phase in marine sediment toxicity bioassays with Paracentrotus lividus sea urchin embryos. Archives of Environmental Contamination and Toxicology, 42, 23–28.
Beiras, R., Bellas, J., Fernández, N., Lorenzo, J. I., & Cobelo-García, A. (2003b). Assessment of coastal marine pollution in Galicia (NW Iberian Peninsula); metal concentrations in seawater, sediments and mussel (Mytillus galloprovincialis) versus embryo-larval bioassays using Paracentrotus lividus and Ciona intestinalis. Marine Environmental Research, 56, 531–553.
Beiras, R., Fernández, N., Bellas, J., Besada, V., González-Quijano, A., & Nunes, T. (2003a). Integrative assessment of marine pollution in Galician estuaries using sediment chemistry, mussel bioaccumulation and embryo-larval toxicity bioassay. Chemosphere, 52, 1209–1224.
Bellas, J., Beiras, R., Mariño, J. C., & Fernández, N. (2005). Toxicity of organic compounds to marine invertebrates embryos and larvae: A comparison between sea urchin embryogenesis bioassay and alternative test species. Ecotoxicology, 14, 337–353.
Bellas, J., Vázquez, E., & Beiras, R. (2001). Toxicity of Hg, Cu, Cd and Cr on early developmental stages of Ciona intestinalis (Chordata, Ascidiacea) with potential application in marine water quality assessment. Water Research, 35(12), 2905–2912.
Boucher, A. M., & Watzin, W. C. (1998). Toxicity identification evaluation of metal-contaminated sediments using an artificial pore water containing dissolved organic carbons. Environmental Toxicology and Chemistry, 18(3), 509–518.
Burgess, R. M., Ho, K. T., Tagliabue, M. D., Kuhn, A., Comeleo, R., Comeleo, P., et al. (1995). Toxicity characterization of an industrial and a municipal effluent discharging to the marine environment. Marine Pollution Bulletin, 30(8), 524–535.
Carballeira, A. (2003). Considerations in the design of a monitoring program of the biological effects of the Prestige oil spill. Ciencias Marinas, 29(1), 123–139.
Chapman, P. M. (2000). The sediment quality triad then, now and tomorrow. International Journal of Environment and Pollution, 13, 351–356.
Chapman, P. M., & Mann, G. S. (1999). Sediment quality guidelines (SQGs) and ecological risk assessment (ERA). Marine Pollution Bulletin, 38(5), 339–344.
Coquery, M., Azemard, S., & de Mora, S. J. (2000). World-wide intercomparison exercise for the determination of trace elements and methylmercury in estuarine sediment sample IAEA-405. Report IAEA/AL/127, IAEA/MEL/ 70, Monaco.
Deanovic, L., Connor, V. M., Knight, A. W., & Maier, K. J. (1999). The use of bioassay and Toxicity Identification Evaluation (TIE) procedures to assess recovery and effectiveness of remedial activities in a mine drainage-impacted stream system. Archives of Environmental Contamination and Toxicology, 36, 21–27.
DelValls, T. A., & Chapman, P. (1998). Site-specific sediment quality guidelines for the Gulf of Cádiz (Spain) and San Francisco Bay (USA), using the sediment quality triad and multivariate analysis. Ciencias Marinas, 24(3), 313–336.
DelValls, T. A., Forja, J. M., & Gómez-Parra, A. (1998). Integrative assessment of sediment quality in two litoral ecosystems from the Gulf of Cádiz, Spain. Environmental Toxicology and Chemistry, 17, 1083–1084.
Fernández, N. (2002). Evaluación biológica de la contaminación marina costera mediante bioensayos con embriones del erizo de mar Paracentrotus lividus. Ph. Thesis. Universidade de Vigo, Spain.
Fernández, N., & Beiras, R. (2001). Combined toxicity of dissolved mercury with copper, lead and cadmium on embryogenesis and early larval growth of the Paracentrotus lividus sea-urchin. Ecotoxicology, 10(5), 263–271.
Fernández, N., Cesar, A., González, M., & DelValls, T. A. (2006a). Level of contamination in sediments affected by the Prestige oil-spill and impact on the embryo development of sea-urchin. Ciencias Marinas, 32(2B), 421–427.
Fernández, N., Cesar, A., Salamanca, M. J., & DelValls, T. A. (2006b). Toxicological characterisation of the aqueous soluble phase of the Prestige fuel-oil using the sea urchin embryo bioassay. Ecotoxicology, 15(7), 593–599.
His, E., Beiras, R., & Seaman, M. N. L. (1999b). The assessment of marine pollution. Bioassays with bivalve embryos and larvae. In A. I. Southward, P. A. Tyler, & C. M. Young (Eds.) Advances in Marine Biology, Vol 37. London: Academic Press.
His, E., Heyvang, I., Geffard, O., & De Montaudouin, X. (1999a). A comparison between oyster (Crassostrea gigas) and sea urchin (Paracentrotus lividus) larval bioassay for toxicological studies. Water Research, 33(7), 1706–1718.
His, E., Seaman, M. N. L., & Beiras, R. (1997). A simplification of the bivalve embryogenesis and larval development bioassay method for water quality assessment. Water Research, 31(2), 351–355.
Ho, K. T., Mckinney, R. A., Khun, A., Pelletier, M. C., & Burgess, R. M. (1997). Identification of acute toxicants in New Bedford Harbor sediments. Environmental Toxicology and Chemistry, 16(3), 551–558.
Kobayashi, N. (1981). Comparative toxicity of various chemicals, oil extracts and oil dispersant extracts to Canadian and Japanese sea urchin eggs. Publications of Seto. Mar. Biol. Lab. XXVI (1/3), 123–133.
Kobayashi, N. (1995). Bioassay data for marine pollution using echinoderms. In P. N. Cheremisinoff (Ed.) Encyclopaedia of Environmental Control Technology, Vol. 9. Houston: Gulf Publ. Co.
Kobayashi, N., & Okamura, H. (2002). Effects of new antifouling compounds on the development of sea urchin. Marine Pollution Bulletin, 44, 748–751.
Long, E. R. (1992). Ranges in chemical concentrations in sediments associated with adverse biological effects. Marine Pollution Bulletin, 24(1), 38–45.
Marking, L. L., & Dawson, V. K. (1975). Method of assessment of toxicity or efficacy of mixtures of chemicals. US Fish Wildlife Service Investigation & Control, 67, 1–8.
McDonald, D. D., Carr, R. S., Calder, F. D., Long, E. R., & Ingersoll, C. G. (1996). Development an evaluation of sediment quality guidelines for Florida coastal waters. Ecotoxicology, 5, 253–278.
NOAA (1999). Sediment Quality Guidelines developed for the National Status and Trend Programs.
O’Connor, T. P. (1998). Mussel watch results from 1986 to 1996. Marine Pollution Bulletin, 37(1–2), 14–19.
O’Connor, T. P., & Paul, J. F. (2000). Misfit between sediment toxicity and chemistry. Marine Pollution Bulletin, 40(1), 59–64.
OSPAR Commission (2000). Quality Status Report 2000: Region IV–Bay of Biscay and Iberian Coast. OSPAR Commission, London.
Ozretic, B., Petrovic, S., & Krajnovic-Ozretic, M. (1998). Toxicity of TBT-based paint leachates on the embryonic development of the sea urchin Paracentrotus lividus Lam. Chemosphere, 37(6), 1109–1118.
Pérez, M. I. (2003). Estudio de los niveles de contaminación por hidrocarburos aromáticos policíclicos en la Ría de Pontevedra y evaluación de su toxicidad sobre la embriogénesis del erizo de mar Paracentrotus lividus. Master Tesis. Universidade de Vigo. Spain.
Riba, I., Zitko, V., Forja, J. M., & DelValls, T. A. (2003). Deriving sediment quality guidelines in the Guadalquivir estuary associated with the Aznalcóllar mining spill: A comparison of different approaches. Ciencias Marinas, 29(3), 1–14.
Smedes, F., Davies, I. M., Wells, D., Allan, A., & Besada, V. (2000). Quality Assurance of Sampling and Sample Handling (QUASH). Interlaboratory study on sieving and normalisation of geographically different sediments: QUASH Round 5 (sponsored by the EU Standards, Measurements and Testing Programme), Aberdeen.
Swartz, R. C. (1999). Consensus sediment quality guidelines for polycyclic aromatic hydrocarbon mixtures. Environmental Toxicology and Chemistry, 18(4), 780–787.
Thomas, K. V., Thain, J. E., & Waldock, M. J. (1998). Identification of toxic substances in United Kingdom estuaries. Environmental Toxicology and Chemistry, 18(3), 401–411.
Vanegas, C., Espina, S., Botello, A. V., & Villanueva, S. (1997). Acute toxicity and synergism of cadmium and zinc in white shrimp, Panaeus setiferus, juveniles. Bulletin of Environmental Contamination and Toxicology, 58, 87–92.
Villeneuve, J.-P., & de Mora, S. J. (2000). Report on the Worldwide and Regional intercomparison for the determination of organochlorine compounds in the fish homogenate IAEA- 406. IAEA-MEL69.
Wells, D. E. (1996). Preliminary review of the QUASIMEME Laboratory Testing Scheme 1993–1996. QUASIMEME Project Office. Aberdeen, UK.
Weltz, B., & Schubert-Jacobs, M. (1991). Evaluation of the flow injection system and optimization of parameters for hydride generation atomic absorption spectrometry. Atomic Spectroscopy, 12(4), 91–103.
Wenning, R. J., & Ingersoll, G. C. (2002). Summary of the SETAC Pellston Workshop on use of sediment quality guidelines and related tolls for the assessment of contaminated sediments; 17–22 August 2002; Fairmont, Montana, USA. Society of Environmental Toxicology and Chemistry (SETAC). Pensacola FL, USA.
Acknowledgments
The authors would like to express their thanks to CACTI (Centro de Apoio Científico Tecnolóxico á Investigación – Universidade de Vigo–Spain) and IEO (Instituto Español de Oceanografía) for their help in the chemical analyses. This work was funded by the Spanish “Comisión Interministerial de Ciencia y Tecnología” through grant AMB99-0946 and REN2000-0498MAR.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fernández, N., Bellas, J., Lorenzo, J.I. et al. Complementary Approaches to Assess the Environmental Quality of Estuarine Sediments. Water Air Soil Pollut 189, 163–177 (2008). https://doi.org/10.1007/s11270-007-9565-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-007-9565-z