Abstract
Wetlands are effective in the treatment of polluted surface water. A semi-natural wetland pilot plant was established to verify the pollutant abatement effectiveness of the Venice Lagoon inlet water. The unique conditions of this brackish environmental site are: (1) a high concentration of carbonate and low concentrations of sulphides, and (2) the abundance of organic matter in sediments. The goal of this study was to examine how these characteristics influence the metal mobility in the wetland and to compare our results to published literature. The data collected were limited with respect to statistical analysis because the metal concentrations were often below the detection limits. Therefore, we chose to perform non-parametric analyses. To analyse the relationships among heavy metal concentrations and the physical and chemical wetland variables, and to investigate the processes of metal removal, we performed a multivariate statistical analysis and a Spearman correlation analysis. The results indicated that the reduced and basic conditions of the sediments seen in the Venice Lagoon environment facilitated the removal of metals due to the formation of insoluble compounds with sulphides and carbonates.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The Venice Lagoon is a highly valuable ecosystem that faces problems of water pollution and eutrophication. In response to these increasing environmental threats, the Veneto Region authorities have established a master plan for pollution control that includes the use of wetlands among other non-point source pollution control tools (Regione Veneto, 2000). An experimental water treatment plant in the form of a constructed wetland that mimics the wastewater characteristics and pedo-climatic conditions of this brackish-water area was established to examine the efficacy and efficiency of semi-natural wetlands to improve water quality.
Numerous recent papers have investigated the ability of wetlands to reduce metal concentrations in polluted watersheds (e.g., Bates et al., 2002; Buykx et al., 2002; DeVolder et al., 2003; Fox & Doner, 2003; Green et al., 2003; ITRC, 2003; Kadlec & Knight, 1996; Karpiscak et al., 2001; Knox et al., 1999; Shutes et al., 2001; Scholz, 2003; Willow & Cohen, 2003; Ye et al., 2001). The accumulation process involves the progressive storage of pollutants in the sediment, which is the most significant component of water ecosystems with respect to metal accumulation (Buykx et al., 2002; Wen & Allen, 1999; Willow & Cohen, 2003; Ye et al., 2001). The process involves complex physical and chemical mechanisms including adsorption, precipitation, phytoaccumulation and degradation, which are dependant on the nature of the sediment matrix and the properties of the adsorbed compounds (Elder, 1988; Machate et al., 1999; Quian et al., 1999).
Eh and pH are the most important variables governing heavy metal solubility (Guo et al., 1997). Sediment redox conditions can vary from slightly reduced ( + 300 mV) to highly reduced (−300 mV). Bacteria use a variety of electron acceptors in the following order: O2, NOx, Mn4+, Fe3+, SO4 2−, CO2 (Guo et al., 1997). The major redox thresholds are given by the Mn oxide dissolution (Eh < 300/450 mV; Green et al., 2003), the Fe oxide dissolution (Eh < 100/120 mV; Green et al., 2003) and the creation of sulphides (Eh < −100/−160 mV; DeVolder et al., 2003).
The most common ligands that form insoluble complexes with metals in the sediment are organic matter (Guo et al., 1997; Linge & Oldham, 2002; Willow & Cohen, 2003; Zhang et al., 2003), carbonates (Green et al., 2003; Guo et al., 1997; Stewart et al., 2003; Wijnja & Schulthess, 2000; Zhang et al., 2003) and insoluble sulphides (Guo et al., 1997; O’Day et al., 2004). Anaerobic conditions promote the growth of sulphate-reducing bacteria, which generate hydrogen sulphide. Most of the heavy metals react with hydrogen sulphide, leading to the formation of highly insoluble metal sulphides (Sheoran & Sheoran, 2006). The total sulphur concentration in wetland soils ranges from 0.2 to 16% of the dry weight (0.06 to 5.0 mmol g−l dry weight; Howarth et al., 1992).
pH influences heavy metal stability by modifying mobility. For example, the solubility of Cd, Pb, Cu and Zn increases with decreasing pH (Ryu et al., 2003; Sukreeyapongse et al., 2002; Zhang et al., 2003). Furthermore, cation exchange and the formation of organic matter complexes are enhanced under acidic conditions, whereas a basic pH promotes reactions such as adsorption and precipitation (Voegelin et al., 2003).
Another relevant binding reaction involves precipitation with carbonates. The relative stability of metal-carbonate minerals is Pb > Zn > Cu (Green et al., 2003).
Lead, copper, nickel and zinc are bivalent cations, which are soluble under acid conditions (Ryu et al., 2003; Sukreeyapongse et al., 2002; Zhang et al., 2003). These metals form complexes with carbonates, sulphides, chlorides and oxides and have a strong affinity for organic matter (Fendorf et al., 2004; Green et al., 2003; Guo et al., 1997; Shen et al., 2002; Willow & Cohen, 2003). A study conducted in the Venice Lagoon reported that Zn, Cu, Pb, and Cd did not show a strong affinity for sulphides; however, they were found in clay, which had a high metal concentration (Bertolin et al., 1995). The researchers suggested that this was a result of deposition with organic matter.
Mercury has three oxidation forms: Hg (0), which is volatile, Hg2 2+ (I), which is relatively insoluble, and Hg2+ (II), which forms stable compounds with Cl− and carbonates and is bound in sediments with organic matter, silt or clay (Knox et al., 1999). Arsenic is a metalloid with two oxidation numbers: AsO3 3− (III) and AsO4 3− (V). The most important variable affecting As accumulation in lakes over the long term is thought to be pH, with a threshold pH value of 6.0 (Yang et al., 2002). In a basic environment, As is soluble under any redox conditions due to the dissolution of Fe oxides (Masscheleyn et al., 1991).
The wetland created in this study was designed to mimic the unique characteristics of the Venice watershed. Firstly, it had a high concentration of carbonate in the sediment rather then sulphides. Secondly, there was an abundance of organic matter in the sediment. Finally, the wetland consisted of brackish water. The goal of this study was to examine how these characteristics influence metal mobility and to verify whether the relationships described in the literature hold for these environmental conditions.
2 Materials and Methods
2.1 Study Area
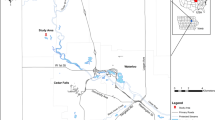
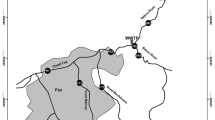
An experimental free water surface (FWS) wetland (Canale Novissimo, Ramo Abbandonato) was constructed in the Venice Lagoon watershed near Chioggia, Venice, Italy, in 2002 to investigate the effectiveness of such a system in the treatment of water entering the lagoon. The wetland was created in a reclaimed lowland delta that is currently below sea level using an abandoned channel parallel to the Brenta River (Fig. 1). The main hydraulic factors such as water stages and volumes in the wetland, as well as detention time were controlled using S7 SIMATIC SIEMENS 300 software. An integrated remote control allowed for the acquisition of data and modification of software settings.
The water entered the system from a reclaimed agricultural channel, which drained a 135 ha sub-basin (80% crops, 20% urban and industrial land use). The inflow of water was characterised by non-point source agricultural and urban pollution, and the system was brackish because of the influence of the Venice Lagoon. The wetland was 50 m wide and 4,140 m long, and was divided into three subsystems that differed in their morphology and vegetation. The first section (1,480 m) was a riparian-swamp ecosystem, the second section (1,040 m) consisted of a riparian and wet ecosystem and the third section (1,620 m) contained a marsh ecosystem.
A regional 1.5 m soil survey profile by the Regional Agency for Environmental Protection (ARPAV, 2004) classified the soils of this area as fine-silty, mixed, calcareous, mesic and cumulic Humaquept varieties. These soils have a high content of organic carbon, low permeability (10−5–10−6 cm s−1) and a neutral pH (between 7.3 and 7.9). The carbonate content was between 43 and 264 g kg−1. During wetland construction, we sampled the first 20 cm of soil, and during piezometer installation, we performed a stratigraphic analysis of the first 5 m below the soil surface (five probes for each subsystem). Our stratigraphy and soil sampling were in accordance with the regional classification. The organic carbon content changed from the inlet soils, where it was the highest (about 8%), to the outlet soils, which were more hydric (about 2% organic matter). Peat layers were found at variable depths. The sand content increased from 50% in the first subsystem to 90% in the third. An alloctone sandy loam/silty loam soil with 2% organic matter soil was used to redesign the wetland berms in the first subsystem and partially in the second.
2.2 Experimental Design
The organisation of the pump and water level control system, and the design measures for the wetland morphology allowed us to control the main hydraulic parameters. The inlet and outlet pumps released the required amount of water, and a salinometer at the inlet pump measured water conductivity and stopped the pump when it reached a fixed threshold (6 mS cm−1 for this study). A hydrological year was estimated to follow the mean hydraulic regime of the reclaim basin. Data collection commenced in October 2002 and continued until October 2006. We collected free water samples at the entrance point, the exit point, and the two intermediate sections of the wetland with polyethylene bottles every 3 months. Every 6 months we collected a sediment sample at two footbridges with a 2.5 m long steel corer (4.2 cm internal diameter). Sediment samples were homogenised and stored in a glass bottles immediately. Water and sediments samples were stored under ice and were submitted to the Labcontrol s.n.c., San Martino di Venezze (Rovigo, Italy) for analysis of pH, Eh, total organic carbon (TOC), total sulphur, sulphides (S2−) and metals. Water were analyzed also for suspended solids (SS) and water conductivity (EC).
A calibrated portable pH meter (WTW pH 90) connected to a pH electrode (WTW Type E50) was used to measure pH of the water and sediments. Redox potential was measured using a calibrated platinum electrode coupled to a calomel reference electrode (Sentek Ag/AgCl, Type H 93). Both were connected to an mV redox meter (WTW pH90).
Total sulphur was analysed by a gas-chromatographic internal method (MPI.034/05) with an Electron Capture Detector (ECD). Sulphides were measured by an indirect titration of sulphide ions with iodine and a back titration of the excess iodine with thiosulphate (MIPAF, Ministero delle Politiche Agricole e Forestali, 1999). Metals were identified by an atomic absorption procedure following EPA methods: 7470 (Hg), 7210 (Cu), 7950 (Zn), 7420 (Pb), 7062 (As), 7190 (Cr), and 7520 (Ni).
2.3 Statistical Analysis
For the sediment analyses we used the mean values of the data collected at the two sampling points, and for the inner free water analyses we used the mean values of the data collected at the intermediate sections and at the outlet pump stations.
The dataset available for the statistical analyses was limited because the metal concentrations were often below the detection limits. To evaluate the main relationships among metal concentrations and physical parameters, we chose to perform non-parametric analyses, which do not require a normal distribution of the dataset.
To evaluate the relationships among heavy metals and other physical and chemical variables, we performed a non-parametric Spearman correlation analysis. We used multidimensional scalar analysis (MDS) to illustrate the relationships among parameters and to highlight the main heavy metal dynamics in the wetland free water. MDS analysis was only used for parameters showing values at least 1/3 above the detection limits. When the concentrations of metals were not detectable, we substituted the value of the limit of detection of the analytical method to maintain a conservative approach. To evaluate goodness of fit we referred to the Stress index, which evaluates how far the reduced-space configuration is from being monotonic to the original distance matrix. A Stress index < 0.1 is considered a sufficient fit (Legendre & Legendre, 1998; Statsoft Inc., 2001).
Statements of statistical significance were based on p < 0.05, unless otherwise stated. We performed the statistical analysis with STATISTICA ® version 6.0 software, (Statsoft, Inc. 2001).
3 Results
The metal concentrations of the incoming wetland water and inner water were very low, often below the detection limits. The mean, minimum and maximum concentration values detected in sediment and water are presented in Tables 1 and 2.
3.1 Water
The results from the non-parametric Spearman correlation in the wetland free water indicated the presence of a relationship among Zn, Cr and Cu (r = 0.54, r = 0.63). Zn and Cr were also related to the sulphides (r = 0.61 and r = 0.63, respectively), whereas Cu was related to the water conductivity (r = 0.62). Pb was negatively related to pH (r = −0.52) and positively related to the suspended solids (r = 0.64).
The opposition between Pb and pH characterized the first extracted dimension of the MDS analysis (Fig. 2). The same dimension also represented the relationship between Pb and the suspended solids. Along the second dimension, As showed a significant negative correlation with total S, while Cu, Zn and Cr clustered in the central area of the plot.
In the incoming water we observed a relationship among Zn, TOC and water conductivity (r = 0.66, r = 0.58) and between Zn and Hg (r = 0.53); also, Pb was linked to suspended matter (r = 0.54). The MDS analysis revealed a clear link between Zn, TOC and water conductivity. Furthermore, the MDS supported the opposition of As, TOC and S along the second dimension (Fig. 3).
3.2 Sediment
In the Canale Novissimo wetland, the sediments redox conditions were always below the oxide dissolution thresholds, ranging around the S stability threshold (−50/−250 mV). The wetland sediments were neutral with respect to pH (6.8–7.4).
Sulphide concentrations started off low, then gradually increased throughout the monitoring period. In addition, a gradual increase of some metals (Cu, Zn, Ni, Hg) was detected in the sediment of the wetland (Fig. 4).
We recorded a three orders of magnitude increase in S concentration in the sediment in 1 day. This was likely a result of the dumping of building material in the wetland. We omitted this outlier from the statistical analysis.
The correlation analysis examining the chemical and physical parameters in the sediments highlighted the presence of a correlation among Cr and Cu (r = 0.86), Cr and Ni (r = 0.96), Cr and Zn (r = 0.89), Cu and Ni (r = 0.89), Cu and Zn (r = 0.96), and Ni and Zn (r = 0.86). Moreover, the correlation analysis indicated that As and Zn were positively correlated with sulphides (r = 0.85 and r = 0.82, respectively), whereas Pb was negatively correlated with total S.
MDS analysis confirmed the above relationships. Specifically, Zn, Cu, Cr and Ni were linked to each other, and Zn and As were close to S in the MDS area. In the second dimension, total S was set against Pb, whereas along the first dimension Hg was set against TOC. The opposite relationship between Eh and metals was clear along the second dimension (Fig. 5).
3.3 Interactions Between Water and Sediment
The correlation analysis between chemical and physical parameters in the sediments highlighted the presence of a correlation between Pb in the sediment and TOC in the water (r = −0.86), S in sediment and pH in the water (r = −0.76), and sulphide in the sediment and Zn in the water (r = −0.84).
The negative correlation between Pb, sulphide and S in the sediments, and pH, TOC and Zn in the water characterised the first extracted dimension in the MDS analysis (Fig. 6). Along this dimension, with the exception of the sediment concentration of Hg, which was positively correlated to organic C in the sediment, all sediment metals clustered in the left part of the graph with sulphides. S in water did not show any significant correlations. Eh and pH in the sediment were linked to Pb in free water in the upper part of graph.
4 Discussion
In the Canale Novissimo wetland, the redox conditions of sediments were always below the oxide dissolution thresholds, and pH conditions were neutral. Therefore, we expected the solubility of Pb, Cu and Zn to be near zero (Ryu et al., 2003; Sukreeyapongse et al., 2002) and the As dissolubility to be increased (Masscheleyn et al., 1991).
The neutrality and the limited variations in the pH of the sediment along all the experimental periods were dependent on the abundance of carbonates, which is a specific characteristic of this area. Therefore, the creation of metal complexes with carbonates is expected to occur in this system (Green et al., 2003).
In the incoming water, the correlation among Zn, TOC and water conductivity suggested that these three variables are linked to suspended solids entering in the wetland. The correlation between suspended solids and Pb could represent the contribution of road runoff (Knox et al., 1999).
In the wetland free water, the correlation among Zn, Cr and Cu indicated that the three metals had a similar dynamic, based on the formation of highly insoluble sulphides (Sheoran & Sheoran, 2006). The correlation between Pb and suspended solids was maintained.
The exchange of heavy metals between surface water and sediment was mostly dependent on sediment Eh conditions, as suggested by the multidimensional analysis. Indeed, the reduced conditions enhanced the build-up of metals and sulphides in the sediments (DeVolder et al., 2003; Guo et al., 1997; Linge & Oldham, 2002; O’Day et al., 2004; Willow & Cohen, 2003; Zhang et al., 2003). Furthermore, the correlation between metals in the sediment and sulphides supported the hypothesis of a complex formation (Fendorf et al., 2004; Green et al., 2003; Guo et al., 1997; Shen et al., 2002; Willow & Cohen, 2003). In contrast to Bertolin et al. (1995), we found that the precipitation of metals with sulphides was the main process allowing for the removal of metals.
The correlation between Hg concentrations and pH in the sediment could represent the formation of insoluble \({\text{CO}}^{{{\text{2 - }}}}_{{\text{3}}} \) complexes (Knox et al., 1999). MDS analysis revealed a strong relationship between sediment Hg concentrations and TOC, suggesting the presence of adsorption processes (Sheoran & Sheoran, 2006).
Given the redox sediment conditions, we expected that pH would be the main driving variable for the As concentration in the sediment (Dixit & Hering, 2003; Fox & Doner, 2003); however, As appeared to be linked to sulphides in the sediment.
5 Conclusions
Despite the low metal concentrations, our results indicated that the most relevant factors for the removal of heavy metals from the watershed were: (1) sediment pH, confirming the low metal solubility in basic environments, and (2) the formation of insoluble sulphides under anoxic conditions, as was indicated for metals in both the sediment and the water. In particular, Zn, Cr, Cu and Ni seemed to have the same chemical dynamics and were linked with sulphides.
Another potential process detected by our analyses was the formation of insoluble carbonates, given their abundance in the soils of this area. At the beginning of this research we did not detect sulphide levels that are normally present in this type of system (Howarth et al., 1992). However, the wetland did appear to be removing metals, likely by means of the formation of carbonates (unpublished data). Following the summer of 2004, we began to detect the presence of sulphides and AVS (acid-volatile sulphide), albeit in low concentrations compared to values reported in the literature (Van Den Berg et al. 1998). The removal of metals from the wetland appeared to increase during this period. Therefore, we suggest that the co-precipitation of metals with carbonates could complement co-precipitation with sulphides as binding processes to remove metal contamination from wetlands.
The presence of high concentrations of organic matter, sulphides and carbonate in the wetland soils led us to believe that the heavy metals were bound and were no longer bioavailable (Stewart et al., 2003; Wood & Shelley, 1999).
Despite our limited data set, we detected the presence of removal processes such as absorption and precipitation, which suggest that the unique characteristics of the Venice Lagoon environment promote the removal of heavy metals from the system. To confirm these preliminary results, we are collecting data for an additional two experimental years. From this, we hope to better estimate the metal removal efficiency of this semi-natural wetland.
References
ARPAV, (2004). Carta dei suoli del bacino scolante in laguna di Venezia. Osservatorio Regionale Suolo, Castelfranco Veneto (TV). Italy
Bates, A. L., Orem, W. H., Harvey, J. W., & Spiker, E. C. (2002). Tracing sources of sulfur in the Florida Everglades. Journal of Environmental Quality, 31, 287–299.
Bertolin, A., Frizzo, P., & Ramazzo, G. (1995). Sulphide speciation in surface sediments of the Lagoon of Venice: A geochemical and mineralogical study. Marine Geology, 123, 73–86.
Buykx, S. E. J., van den Hoop, M. A. G. T., & Loch, J. P. G. (2002). Dissolution kinetics of heavy metals in Dutch carbonate- and sulfide-rich freshwater sediments. Journal of Environmental Quality, 31, 573–580.
DeVolder, P. S., Brown, S. L., Hesterberg, D., & Pandya, K. (2003). Metal bioavailability and speciation in a wetland tailings repository amended with biosolids compost, wood ash and sulfate. Journal of Environmental Quality, 32, 851–864.
Dixit, S., & Hering, J. G. (2003). Comparison of arsenic (V) and (III) sorption onto iron oxide minerals: Implications for arsenic mobility. Environmental Science & Technology, 37, 4182–4189.
Elder, J. F. (1988). Factors affecting wetland retention of nutrients, metals, and organics. In: J.A. Kusler and G. Brooks (eds.), Proceedings for the national wetland symposium: wetland hydrology (p. 178–184). 16–18 Sept. 1987. Chicago, IL.
Fendorf, S., La Force, M. J., & Li, G. (2004). Temporal changes in soil partitioning and bioaccessibility of arsenic, chromium, and lead. Journal of Environmental Quality, 33, 2049–2055.
Fox, P. M., & Doner, H. E. (2003). Accumulation, release and solubility of arsenic, molybdenum and vanadium in wetland sediments. Journal of Environmental Quality, 32, 2428–2435.
Green, C. H., Heil, D. M., Cardon, G. E., Butters, G. L., & Kelly, E. F. (2003). Solubilization of manganese and trace metals in soils affected by acid mine runoff. Journal of Environmental Quality, 32, 1323–1334.
Guo, T., Delaune, R. D., & Patrick, W. H. Jr. (1997). The effect of sediment redox chemistry on solubility/chemically active forms of selected metals in bottom sediment receiving produced water discharge. Spill Science and Technology Bulletin, 4, 165–175.
Howarth, R. W., Stewart J. W. B., & Ivanov, M. V. (1992). Sulphur cycling on the continents. Wetlands, terrestrial ecosystems and associated water bodies. Paris, France: Scientific Committee on Problems of Environment (SCOPE).
ITRC (Interstate Technology & Regulatory Council). (2003). Technical and Regulatory Guidance Document for Constructed Treatment Wetlands. WTLND-1. Washington, D.C.: Interstate Technology & Regulatory Council, Mitigation Wetlands Team. Retrieved from: http://www.itrcweb.org
Kadlec, R. H., & Knight, R. L. (1996). Treatment Wetlands. Boca Raton, FL, USA: Lewis.
Karpiscak, M. M., Whiteaker L. R., Artiola J.F., & Foster K. E. (2001). Nutrient and heavy metal uptake and storage in constructed wetland systems in Arizona. Water Science and Technology, 44, 455–462.
Knox, A. S., Gamerdinger, A. P., Adriano, D. C., Kolka, R. K., & Kaplan, D.I. (1999). Sources and practices contributing to soil contamination. American Society of Agronomy. Bioremediation of contaminated soils, Agronomy Monograph no. 37.
Lasat, M. M. (2002). Phytoextraction of toxic metals: A review of biological mechanisms. Journal of Environmental Quality, 31, 109–120.
Legendre, P., & Legendre, L. (1998). Numerical Ecology. Amsterdam, The Netherlands: Elsevier Scientific Publishing Company.
Linge, K. L., & Oldham, C. E. (2002). Arsenic remobilization in a shallow lake: The role of sediment resuspension. Journal of Environmental Quality, 31, 822–828.
Machate, T., Heuermann, E., Schramm, K., & Kettrup, A. (1999). Purification of fuel and nitrate contaminated ground water using a free water surface constructed wetland. Journal of Environmental Quality, 28, 1665–1673.
Masscheleyn, P. H., Delaune, R. D., & Patrick, W. H. (1991) Arsenic and selenium chemistry as affected by sediment redox potential and pH. Journal of Environmental Quality, 20, 522–527.
MIPAF (1999). Decreto Ministeriale 13 settembre 1999. Approvazione dei “Metodi ufficiali di analisi chimica del suolo.” Gazzetta Ufficiale n. 248 del 21–10–1999.
O’Day, P. A., Vlassopoulos, D., Root, R., & Rivera, N. (2004). The influence of sulfur and iron on dissolved arsenic concentrations in the shallow subsurface under changing redox conditions. PNAS, 101, 13703–13708.
Quian, J., Zayed, A., Zhu, Y., Yu, M., & Terry, N. (1999). Phytoaccumulation of trace elements by wetland plants: III. Uptake and accumulation of ten trace elements by twelve plant species. Journal of Environmental Quality, 28, 1448–1455.
Regione Veneto (2000). Piano per la prevenzione dell’inquinamento e il risanamento delle acque del bacino idrografico immediatamente sversante nella laguna di Venezia. Venice, Italy.
Ryu, H. W., Moon, H. S., Lee, E. Y., Cho, K. S., & Choi H. (2003). Leaching characteristics of heavy metals from sewage sludge by Acidithiobacillus thiooxidans MET. Journal of Environmental Quality, 32, 751–759.
Scholz, M. (2003). Performance predictions of mature experimental constructed wetlands which treat urban water receiving high loads of lead and copper. Water Research, 37, 1270–1277.
Shen, Z. G., Li, X. D., Wang, C. C., & Chen, H. M. (2002). Lead phytoextraction from contaminated soil with high-biomass plant species. Journal of Environmental Quality, 31, 1893–1900.
Sheoran, A. S., & Sheoran, V. (2006). Heavy metal removal mechanism of acid mine drainage in wetlands: A critical review. Minerals Engineering 19, 105–116.
Shutes, R. B. E., Revitt, D. M., Scholes, L. N. L., Forshaw, M., & Winter, B. (2001). An experimental constructed wetland system for the treatment of highway runoff in the UK. Water Science and Technology, 44, 571–578.
StatSoft, Inc. (2001). STATISTICA for Windows [Computer program manual]. Tulsa, OK.
Stewart, M. A., Jardine, P. M., Barnett, M. O., Mehlhorn, T. L., Hyder, L. K., & McKay, L. D. (2003). Influence of soil geochemical and physical properties on the sorption and bioaccessibility of chromium(III). Journal of Environmental Quality, 32, 129–137.
Sukreeyapongse, O., Holm, P. E., Strobel, B. W., Panichsakpatana, S., Magid, J., & Hansen, H. C. B. H. (2002). pH-dependent release of cadmium, copper and lead from natural and sludge-amended soils. Journal of Environmental Quality, 31, 1901–1909.
Van Den Berg, G. A., Loch, J. P. G., Van Der Heijdt, L. M., & Zwolsman, J. J. G. (1998). Vertical distribution of acid-volatile sulfide and simultaneously extracted metals in a recent sedimentation area of the river Meuse in The Netherlands. Environmental Science & Technology, 17, 758–763.
Voegelin, A., Barmettler, K., & Kretzschmar, R. (2003). Heavy metal release from contaminated soils: Comparison of column leaching and batch extraction results. Journal of Environmental Quality, 32, 865–875.
Wen, X., & Allen, H. E. (1999). Mobilization of heavy metals from Le An River sediment. The Science of the Total Environment, 227, 101–108.
Wijnja, H., & Schulthess, C. P. (2000). Interaction of carbonate and organic anions with sulfate and selenate adsorption on an aluminum oxide. Environmental Science & Technology, 64, 898–908.
Willow, M. A., & Cohen R. R. H. (2003). pH, dissolved oxygen and adsorption effects on metal removal in anaerobic bioreactors. Journal of Environmental Quality, 32, 1212–1221.
Wood, T. S., & Shelleym, M. L. (1999). A dynamic model of bioavailability of metals in constructed wetland sediments. Ecological Engineering, 12, 231–252.
Yang, J.-K., Barnett, M. O., Jardine, P. M., Basta, N. T., & Casteel, S. W. (2002). Adsorption, sequestration and bioaccessibility of As(V) in soils. Environmental Science & Technology, 36, 4562–4569.
Ye, Z. H., Whiting, S. N., Quian, J. H., Lytle, C. M., Lin, Z. Q., & Terry, N. (2001). Trace elements removal from coal ash leachate by a 10-year-old constructed wetland. Journal of Environmental Quality, 30, 1710–1719.
Zhang, M., He, Z., Calvert, D. V., Stoffella, P. J., & Jiang, X. (2003). Surface runoff losses of copper and zinc in sandy soils. Journal of Environmental Quality, 32, 909–915.
Acknowledgements
The project was funded by the Venice Water Authority (Ministero Infrastrutture e Trasporti) and coordinated by its concessionaire Consorzio Venezia Nuova (CVN). We gratefully acknowledge the assistance of Alberto Giulio Bernstein, Head of the Environmental Engineering Department of CVN. We also thank Protecno srl (Italy) for the provision of the hydraulic data and raw analysis, and Labcontrol snc (Italy) for assisting with the chemical analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mattiuzzo, E., Favero, L., Zennaro, F. et al. Heavy Metal Behaviour in an Experimental Free Water Surface Wetland in the Venice Lagoon Watershed. Water Air Soil Pollut 183, 143–151 (2007). https://doi.org/10.1007/s11270-007-9363-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-007-9363-7