Abstract
Oncolytic viruses have been extensively used in cancer treatment due to their tropism, selective replication only in tumor cells, and possible synergic interaction with other therapeutics. Different researchers have demonstrated that bovine herpesvirus 4 (BoHV-4), a member of the gammaherpesviridae family, has oncolytic potential in some human-origin cancer cell lines like glioma through the selective replication strategy. Using four apoptosis detection methods, namely MTT, LDH, TUNEL, and Annexin V assays, we evaluated the apoptotic effect of BoHV-4 Movar33/63 reference strain along with a recombinant BoHV-4 expressing EGFP in U87 MG cells (human glioblastoma cell line), MDA MB-231 (human breast cancer cell line), and MCF10a (non-tumorigenic human mammary epithelial cell line). Our findings indicate that this virus can replicate and induce apoptosis in these cell lines and hinder in vitro proliferation in a dose-dependent manner. In conclusion, BoHV-4 has in vitro potential as a novel oncolytic virus in human cancer therapy. However, its replication potential in the MCF10a cells as a non-tumorigenic human mammary epithelial cell line is a concern in using this virus in cancer therapy, at least against human mammary tumors. Further studies must therefore be conducted to examine the specific apoptotic pathways induced by this virus to move on to further experiments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, oncolytic viruses have offered a new therapeutic tool to fight different kinds of cancers in both in vitro and in vivo experiments. These viruses can be used in a wild or recombinant form to express the oncolytic proteins derived from viral genes or foreign DNAs, which are involved in different oncolytic pathways [1].
Various mammalian viruses and their recombinant forms have been categorized in the oncolytic virus groups, including adenoviruses, poxviruses, HSV-1, coxsackieviruses, poliovirus, measles virus, Newcastle disease virus (NDV), and reovirus. Their main advantage is their specificity in targeting cancer cells rather than healthy ones [2].
Bovine herpesvirus 4 (BoHV-4) is a member of the Rhadinovirus genus and gammaherpesvirinae subfamily. Although this virus has a worldwide distribution in cattle [3], no studies have yet demonstrated a relationship between infection and clinical signs in infected animals [4]. There is also no clear evidence that it can infect humans [6].
In laboratory experiments, BoHV-4 shows the potential to replicate and have cytopathologic effects (CPE) in a broad range of primary established cell culture systems [5]. Several features make this virus a potential candidate for gene transfer in vaccination and cancer therapy. First, unlike other gammaherpesviruses, no signs of transformation have been documented in the infected cells. Second, this virus has a less complex genome than other herpesviruses, which makes its manipulation easier [6]. Third, BoHV-4 can remain persistent in monocytes and macrophages in the natural host, which can be considered as a positive sign in eliminating the need for the booster dose [7, 8]. Fourth, in contrast to other gammaherpesviruses, BoHV-4 can replicate in different cell lines, especially human originated tumorigenic cells [9]. To prove its tumorigenic potential, one study demonstrated its selective replication in glioma cells of the rat model [10] while its replication has been proven in several human carcinoma cell lines in vitro, including Human OVCAR-3 ovary adenocarcinoma and A549 lung carcinoma cells [9].
In the present study, we evaluated the replication and apoptosis potential of Movar33/63 as the European reference strain of BoHV-4 and a recombinant BoHV4-loxp-BAC-CMV-EGFP-loxp in U87 MG cells (human glioblastoma cell line), MDA MB-231 (human breast cancer cell line), and MCF10a (non-tumorigenic human mammary epithelial cell line). MCF10a cell lines were included to determine the virus’s replication potential non-tumorigenic human mammary epithelial cells.
Materials and methods
Viruses and cells
Three cell lines used in this study were MDA-MB 231(ATCC® HTB-26™; Human Mammary Carcinoma), U87 MG (ATCC® HTB-14™; Uppsala 87 Malignant Glioma), and MCF10a (ATCC® CRL-10317™; normal-like breast epithelial cell line). MDA-MB 231 and MCF-10a cells were maintained in ATCC-formulated Leibovitz’s L-15 Medium and MEBM (Lonza/Clonetics Corporation, MEGM, and Kit Catalog No. CC3150), respectively. For the U87 MG cells, we used EMEM media (Sigma, USA). The media of all three cells were supplemented by 10% fetal bovine serum (FBS) (Biological Industries, Israel), 2 mM L-glutamine (Biological Industries, Israel), 100 U penicillin, and 0.1 mg/ml streptomycin (Biological Industries, Israel) for cell maintenance. To conduct the TCID50 assay, Madin Darby bovine kidney (MDBK) cells were cultured in DMEM medium. All the cells were obtained from the cell culture stock of the Virology Department of Ankara University, Ankara, Turkey, tested for Mycoplasma contamination by 103 EZ-PCR Mycoplasma Test Kit (Biological Industries, Kibbutz Beit-Haemek, Israel), and subcultured at a ratio of 1:2 to 1:4 twice a week. The BoHV-4 Movar33/63 reference strain and BoHV4-loxp-BAC-CMV-EGFP-loxp viruses were provided from the same department’s virus collection. The BoHV4-loxp-BAC-CMV-EGFP-loxp virus was created by inserting the BAC-CMV-EGFP cassette between the ORF2 (Bo2) and ORF3 (Bo3) genes of the Movar33/63 strain via in vitro homologous recombination as previously described [11, 12].
Virus inoculation
For virus inoculations, the cells were cultured in 6-well plates for 24 h before inoculation with Movar33/63 and BoHV4-loxp-BAC-CMV-EGFP-loxp viruses at different moi. Briefly, the media were removed, the cells were washed by 1 × PBS, the diluted viruses were added in a total volume of 500 µl, and the cells were incubated at 37 °C for 1 h with frequent shaking every 15 min. The virus was then removed before adding cell-specific media supplemented by 1% FBS. The infected cells were then observed for 72–92 h to determine virus proliferation based on green fluorescence protein (GFP) expression and cytopathic effects (CPEs) for BoHV4-loxp-BAC-CMV-EGFP-loxp and presence of CPEs for Movar33/63.
Annexin V-FITC/PI staining (Movar 33/63 infected cells)
Movar33/63 infected MDA MB-231, MCF10a, and U87 MG cells were collected 72 h post-infection and apoptosis assay was performed using Annexin V-FITC kit (Finetest, China) as described previously [13]. Briefly, the infected cells were scraped and suspended in 400 µl of Annexin binding buffer before adding Annexin V-FITC and propidium iodide (PI). The cells were incubated at room temperature for 10 min in darkness before being visualized under a fluorescence microscope (Axio Vert A1 Microscope, Ziess, Germany).
TUNEL assay (Movar 33/63 infected cells)
For in situ apoptosis detection in Movar33/63 infected cells, we conducted TUNEL assay using ApopTag In situ Apoptosis Detection Kit (Chemicon International, USA) as described previously [14]. Briefly, 72 h after Movar33/63 inoculation of each of the cells cultured on glass slides, 1% paraformaldehyde (ThermoScientific, USA) was used to fix the cells before post-fixation step using ethanol-acetic acid solution. Then, 2:1 ratio of equilibration buffer and working strength TdT enzyme was added to the cells before incubating them for 1 h at 37 °C. Finally, the reaction was stopped by adding the stop solution, the cells were mounted with SlowFade Gold Antifade Mountant (ThermoScientific, USA) containing propidium iodide, and visualized under a fluorescence microscope (Axio Vert A1 Microscope, Ziess, Germany).
LDH assay
Movar33/63 and BoHV4-loxp-BAC-CMV-EGFP-loxp viruses were inoculated at moi of 0.1, 1, 10, and 100 to evaluate cytotoxicity effects in MDA MB-231, MCF10a, and U87 MG cells at 90% confluency. LDH assay (Pierce LDH Cytotoxicity Assay Kit; ThermoFisher, USA) was performed 72 h post inoculation in 96-well plates as previously described [15].
Each reaction was done in triplicate. Seventy-two hours after virus inoculation, 10 × lysis buffer was added to Maximum LDH Activity Control triple wells, and the cells incubated at 37 °C for 45 min. To perform the assay, 50 µl of the medium from all wells was transferred to a new 96-well plate and mixed with an equal amount of reaction buffer. The plate was incubated at room temperature for 30 min in the dark before the reaction was stopped using stop solution and read at 490 nm by an ELISA reader (Titertek Multiskan, Finland).
MTT assay
MDA MB-231, MCF10a, and U87 MG cells at 90% confluency were infected by Movar33/63 and BoHV4-loxp-BAC-CMV-EGFP-loxp viruses at moi of 0.1, 1, 10, and 100. The MTT assay (Vybrant® MTT Cell Proliferation Assay Kit; ThermoFisher, USA) was conducted 72 h post-inoculation in a 96-well plate as previously described [16].
All the experiments were done in triplicate. Briefly, 10 µl of 12 mM MTT stock solution was added to each well, which were incubated for 4 h at 37 °C/5% CO2 before adding 100 µl of SDS-HCl solution to each well. After incubation for 16 h at 37 °C, the plate was read at 570 nm by an ELISA reader (Titertek Multiskan, Finland).
Virus titration assay
To determine the virus titer produced from MDA MB-231, MCF10a, and U87 MG cells, Movar33/63 and BoHV4-loxp-BAC-CMV-EGFP-loxp viruses were inoculated at moi of 0.1 in T25 cell culture flasks containing each cell at 90% confluency. The viruses were collected 96 h post-infection and subjected to virus titration assay in a 96-well plate. The titers were calculated by TCID50 method [17]. Briefly, the viruses were serially diluted (log2) and added to MDBK cells. Each experiment was repeated four times. After 1 h of virus adsorption at 37 °C / 5% CO2, virus growth media containing 1% FBS was added to the infected cells, which were further incubated for 5 days to develop the complete CPEs in the virus control wells.
Statistical analysis
The data were analyzed by SPSS 18 using the homogeneity of variances test as well as multiple comparisons and ANOVA tests. A p value of 0.05 was considered statistically significant. Each data point represents the mean ± SD of a triplicate.
Results
Virus proliferation
After virus inoculation at a moi of 0.1, cytopathic effects (CPE) were initially detected in infected cells after 24 h before peaking at 72 h post infection. CPEs were observed as detached and rounding cells in both Movar33/63 and BoHV4-loxp-BAC-CMV-EGFP-loxp viruses. In addition to CPEs, the expression of green fluorescence protein (GFP) was detected in BoHV4-loxp-BAC-CMV-EGFP-loxp infected cells with the same pattern described for CPE. Movar33/63`s CPE was documented in MCF10a (Fig. 1a), MDA MB-231 (Fig. 1e), and U87 MG (Fig. 1i) cells at 72 h post infection. We also demonstrated the replication of BoHV4-loxp-BAC-CMV-EGFP-loxp in MCF10a (Fig. 1b, c), MDA MB-231 (Fig. 1f–g), and U87 MG (Fig. 1j, k) cells. Interestingly, viral CPEs which are caused by both Movar33/63 and BoHV4-loxp-BAC-CMV-EGFP-loxp in U87 MG cells were not detected until day 2, which was slower than for MDA MB-231 and MCF10a. Generally, CPEs in U87 MG were restricted to morphology changes and not detached cells. MDA MB-231 and MCF10a cells showed cell detachment and cloudy media 48 h post inoculation.
Cytopathic effects in Movar33/63 and BoHV4-loxp-BAC-CMV-EGFP-loxp infected MDA MB-231, MCF10a, and U87 MG cells. To evaluate virus proliferation, we inoculated the cells with 0.1 moi of Movar33/63 and BoHV4-loxp-BAC-CMV-EGFP-loxp. CPEs were fully detected after 72 h. EGFP expression in the infected MCF10a (a fluorescence and b phase contrast), MDA MB-231 (e fluorescence and f phase contrast), and U87 MG i fluorescence and j phase contrast) cells was considered as demonstrating virus replication potential in the cells. Movar33/63 strain also caused massive CPEs in MCF10a (c), MDA MB-231 (g), and U87 MG (k) cells according to morphological changes. Mock infected cells included MCF10a (d), MDA MB-231 (h), and U87 MG (l) cells
Annexin V-FITC assay
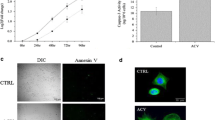
Apoptotic and necrotic cells were detected in the Movar33/63 infected MCF10a (Fig. 2a), MDA MB-231 (Fig. 2b), and U87 MG (Fig. 2c) cells at 72 h post infection.
Annexin V and TUNEL assay in MDA MB-231, MCF10a and U87 MG cells at 72 h post infection by Movar33/63 virus. Movar33/63 caused apoptosis in MCF10a (a), MDA MB-231 (b), and U87 MG (c) cells based on Annexin V assay. Apoptotic and necrotic cells are represented by blue and red arrays in the infected cells, respectively. Apoptosis was also detected in Movar33/63 infected MCF10a (d), MDA MB-231 (e), and U87 MG (f) cells 72 h post inoculation when examined by TUNEL assay
TUNEL assay
Movar33/63 caused apoptosis in MCF10a (Fig. 2d), MDA MB-231 (Fig. 2e), and U87 MG (Fig. 2f) cells 72 h post inoculation based on detection of TUNEL + cells among infected cells.
MTT assay
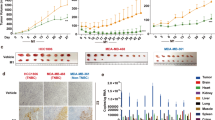
U87 MG, MDA MB-231, and MCF10a cells were inoculated at moi 0.1, 1, 10, and 100 and incubated for 72 h to perform MTT assay. In U87 MG cells, both viruses had the potential to hinder cell proliferation in vitro in different doses (Fig. 3a). At moi 0.1 and 1, BoHV4-loxp-BAC-CMV-EGFP-loxp virus was more lytic than the Movar33/63 strain at the same moi, although the difference was not significant. Analysis of MDA MB-231(Fig. 3b) and MCF10a (Fig. 3c) cells also demonstrated that both viruses interfered with cell proliferation at different moi. However, Movar33/63 was more infectious than BoHV4-loxp-BAC-CMV-EGFP-loxp in these 2 cells. In all three cells, the proliferation rate decreased in a dose-dependent manner, with moi 100 having the lowest rate of the viral titers.
a MTT assay in U87 MG cells: based on the MTT assay, both viruses impeded cell proliferation in a dose-dependent manner. b MTT assay in MCF10a cells: Movar33/63 and BoHV4-loxp-BAC-CMV-EGFP-loxp hindered cell proliferation. There was no significant difference between the two viruses. c MTT assay in MDA MB-231 cells: both viruses caused significantly more MTT responses than in mock-infected cells (p < 0.05). d LDH assay in U87 MG cells: based on LDH release, both viruses had clear dose-dependent cytotoxic effects in U87 MG cells. e LDH assay in MCF10a cells: both Movar33/63 and BoHV4-loxp-BAC-CMV-EGFP-loxp viruses had almost identical and significant cytotoxic effects at all moi compared to mock-infected cells (p < 0.05). f LDH assay in MDA MB-231 cells: high cytotoxicity levels were documented in both viruses and in a titer-dependent way. All experimental tests were repeated three times, with the results shown as mean ± SD; *p < 0.05; **p < 0.01; ***p < 0.001
LDH assay
Lactate dehydrogenase levels were analyzed 72 h after virus inoculation at four different moi (0.1, 1, 10, and 100). In U87 MG cells, both viruses caused significant dose-dependent cytotoxicity compared to mock-infected cells while the highest rate was at moi 100. In this cells, Movar 33/63 caused a slightly higher amount of cytotoxicity in 0.1 and 100 moi. In 1 and 10 moi, the difference between two viruses are not significant and is almost at the same levels (Fig. 3d). Both viruses had relatively high toxic effects on MDA MB-231 and MCF10a cells. In MCF10a cells, BoHV4-loxp-BAC-CMV-EGFP-loxp virus has slightly more cytotoxic effect in moi of 1 and 10 (Fig. 3f). On the other hand, Movar33/63 is more toxic in moi of 0.1, 1, and 10 in MDA MB-231 cells in comparison to BoHV4-loxp-BAC-CMV-EGFP-loxp virus (Fig. 3e). The mock-infected cells showed no cytotoxicity in this experiment.
TCID50 assay
Both viruses replicated in MDA MB-231, MCF10a, and U87 MG cells and developed CPEs. Collected BoHV4-loxp-BAC-CMV-EGFP-loxp from all three cells showed a higher titer in MDBK cells than the reference Movar33/63. For both viruses, the highest titer was obtained from MDA MB-231 infected cells whereas the lowest titer was from U87 MG infected cells (Fig. 4).
Titer determination of Movar33/63 and BoHV4-loxp-BAC-CMV-EGFP-loxp viruses when collected from infected MDA MB-231, MCF10, and U87 MG cells by TCID50 assay in MDBK cells. MDA MB-231 cells were more permissive to both viruses. In support of our other findings, the lowest titer was from U87 MG infected cells. All experimental tests were repeated four times; data are shown as mean ± SD
Discussion
Oncolytic viruses are classified into two main groups of natural and recombinant viruses. Genetically modified viral vectors have been designed to transfer oncolytic and onco-suppressor genes, such as wild-type p53 tumor suppressor and human granulocyte–macrophage colony-stimulating factor (GM-CSF) genes. These kinds of viral vectors also have the potential to improve antitumor immunity induction [18, 19]. On the other hand, some wild type viruses have oncolytic potential to selectively replicate in transformed cells without damaging normal tissues [20]. The best example of this group are reoviruses, which only replicate in cells with an activated Ras signaling pathway and specifically target Ras‐activated cancer cells [21,22,23,24].
BoHV-4 is a member of gammaherpesvirinae subfamily, which was previously demonstrated to be an oncolytic virus that can only replicate in certain human and animal tumorigenic cells [5]. In vitro experiments show that this virus has no potential to infect non-tumorigenic human cell lines, except for two embryonic lung cell lines: MRC-5 and Wistar-38. In lung carcinoma and ovary adenocarcinoma cells, the apoptosis potential of BoHV-4 virus relies on immediate-early and/or early gene expression in vitro [5, 9]. This virus carries a gene that expresses a protein that inhibits Fas- and TNFR1-induced apoptosis by interacting with caspase-8 [25].
Gliomas are the most frequent primary tumors of the central nervous system. Because they can repair their damaged DNA, there are currently no therapeutic agents available [26]. These tumors comprise both neoplastic and non-neoplastic cells. The main non-neoplastic cells in the glioma environment are tumor-associated macrophages (TAMs), which originate from residual (CD45low) or recruited microglia (CD45high). The majority of macrophages in glioma have a CD45high background [27]. Glioma treatment based on oncolytic viruses like HSV-1, modified human Ad5, and Newcastle viruses have attracted significant interest. Following many previous glioma studies, we used U87 MG cells as the human glioblastoma cell model. The recent sequencing of the complete U87 MG genome revealed many indels, copy number variations, and translocations. In a previous attempt, the replication of recombinant BoHV-4 in rat F98 glioma cells was documented both in vivo and in vitro [10].
The other innovation of the present study was to investigate the replication of BoHV-4 in a human breast cancer cell (MDA MB-231). Breast cancer remains one of the most common causes of cancer-related deaths in women. Currently, the main therapies include surgery, chemotherapy, and radiotherapy, which carry high risks while the metastatic characteristics of breast cancer remain a major concern [28]. Here, we used MDA MB-231 cells as the in vitro cancer model and MCF10a cells as the model of non-tumorigenic human breast epithelial cells.
To analyze the replication and apoptotic potential of BoHV-4, we used Movar33/63 (European) and BoHV4-loxp-BAC-CMV-EGFP-loxp viruses. The BoHV4-loxp-BAC-CMV-EGFP-loxp recombinant virus was created by inserting the loxp-BAC-CMV-EGFP-loxp cassette between the ORF2 and ORF3 genes of the Movar33/63 strain by in vitro homologous recombination to detect virus proliferation in the infected cells by observing green fluorescence protein (GFP) expression [11, 12].
We showed that BoHV-4 virus can replicate in U87 as a human glioblastoma model and MDA MB-231 as a human breast cancer model. These findings were achieved by detecting cytopathic effects and green fluorescence protein expression in the cells, which can be inferred as virus replication. One drawback of this experiment is that we did not use the virus specific promoter to drive the expression of the GFP in the infected cells. So, it is still a possibility that the GFP expression comes from cytomegalovirus promoter independent from virus replication. However, our other data, especially from Movar 33/63, are demonstrating that these viruses can replicate in these cells. The next step will be to design a recombinant BoHV-4 which can express the marker gene from a virus specific promoter. Our data from MTT, LDH, TUNEL, and Annexin V assays also confirmed the presence of apoptosis, which can originate from virus replication. In contrast, we did not detect high levels of apoptosis in the mock-infected cells.
In addition to the direct oncolytic effect of BoHV-4 virus on glioma cells, demonstrated both in this study and previous work, this virus can remain persistent in macrophage and monocyte cells. This characteristic can be considered as offering an effective strategy to target glioblastomas as a macrophage enriched environment in vivo [29]. However, more experiments must be conducted, especially in vivo, to verify this claim.
The most important finding in our study is that BoHV-4 replicated in MCF10a cells as a non-tumorigenic human breast epithelial cell line. Previous studies have demonstrated that this virus cannot replicate in non-tumorigenic human cells [5, 6]. However, our results indicate a significant toxicity rate in virus infected MCF10a cells. This was confirmed by both the developed CPE and the MTT and LDH assays. It will be essential to use the human primary cells to confirm all the data obtained in this experiment in the future studies.
In conclusion, we have demonstrated that BoHV-4 virus has the potential to replicate and possibly modulate apoptosis in human glioblastoma and breast cancer cells. However, our study confirms that it can only be used against certain human cancers like gliomas. The next step will be to determine which apoptosis pathway is stimulated by this virus in tumorigenic cells. It is also critical to determine the replication potential of this virus in primary human-originated cells to investigate its safety in future human trials.
References
Kaufman HL, Kohlhapp FJ, Zloza A (2015) Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov 14:642–662. https://doi.org/10.1038/nrd4663
Howells A, Marelli G, Lemoine NR, Wang Y (2017) Oncolytic viruses—interaction of virus and tumor cells in the battle to eliminate cancer. Front Oncol. https://doi.org/10.3389/fonc.2017.00195
Donofrio G, Herath S, Sartori C, Cavirani S, Flammini CF, Sheldon IM (2007) Bovine herpesvirus 4 is tropic for bovine endometrial cells and modulates endocrine function. Reproduction 134:183–197. https://doi.org/10.1530/REP-07-0065
Morán PE, Pérez SE, Odeón AC, Verna AE (2015) Bovine herpesvirus 4 (BoHV-4): general aspects of the biology and status in Argentina. Rev Argent Microbiol 47:155–166. https://doi.org/10.1016/j.ram.2015.02.007
Egyed L (1998) Replication of bovine herpesvirus type 4 in human cells in vitro. J Clin Microbiol 36:2109–2111
Gillet L, Minner F, Detry B, Farnir F, Willems L, Lambot M, Thiry E, Pastoret PP, Schynts F, Vanderplasschen A (2004) Investigation of the susceptibility of human cell lines to bovine herpesvirus 4 infection: demonstration that human cells can support a nonpermissive persistent infection which protects them against tumor necrosis factor alpha-induced apoptosis. J Virol 78(5):2336–2347
Donofrio G, Cavirani S, van Santen VL (2000) Establishment of a cell line persistently infected with bovine herpesvirus-4 by use of a recombinant virus. J Gen Virol 81:1807–1814
Donofrio G, Van Santen VL (2001) A bovine macrophage cell line supports bovine herpesvirus-4 persistent infection. J Gen Virol 82:1181–1185. https://doi.org/10.1099/0022-1317-82-5-1181
Gillet L, Daix V, Donofrio G, Wagner M, Koszinowski UH, China B, Ackermann M, Markine-Goriaynoff N, Vanderplasschen A (2005) Development of bovine herpesvirus 4 as an expression vector using bacterial artificial chromosome cloning. J Gen Virol 86:907–917. https://doi.org/10.1099/vir.0.80718-0
Redaelli M, Mucignat-Caretta C, Cavaggioni A, Caretta A, D’Avella D, Denaro L, Cavirani S, Donofrio G (2010) Bovine herpesvirus 4 based vector as a potential oncolytic-virus for treatment of glioma. Virol J. https://doi.org/10.1186/1743-422X-7-298
Donofrio G, Franceschi V, Capocefalo A, Cavirani S, Sheldon IM (2009) Isolation and characterization of bovine herpesvirus 4 (BoHV-4) from a cow affected by post partum metritis and cloning of the genome as a bacterial artificial chromosome. Reprod Biol Endocrinol RB&E 7:83. https://doi.org/10.1186/1477-7827-7-83
Aligholipour Farzani T, Földes K, Hanifehnezhad A, Yener Ilce B, Bilge Dagalp S, Amirzadeh Khiabani N, Ergünay K, Alkan F, Karaoglu T, Bodur H, Ozkul A (2019) Bovine herpesvirus type 4 (BoHV-4) vector delivering nucleocapsid protein of crimean-congo hemorrhagic fever virus induces comparable protective immunity against lethal challenge in IFNα/β/γR-/- mice models. Viruses 11(3):237. https://doi.org/10.3390/v11030237
Chang P, Kuchipudi SV, Mellits KH, Sebastian S, James J, Liu J, Shelton H, Chang KC (2015) Early apoptosis of porcine alveolar macrophages limits avian influenza virus replication and pro-inflammatory dysregulation. Sci Rep 5:17999. https://doi.org/10.1038/srep17999
Daidoji T, Koma T, Du A, Yang CS, Ueda M, Ikuta K, Nakaya T (2008) H5N1 avian influenza virus induces apoptotic cell death in mammalian airway epithelial cells. J Virol 82(22):11294–11307. https://doi.org/10.1128/JVI.01192-08
Baba C, Yanagida K, Kanzaki T, Baba M (2005) Colorimetric lactate dehydrogenase (LDH) assay for evaluation of antiviral activity against bovine viral diarrhoea virus (BVDV) in vitro. Antivir Chem Chemother 16(1):33–39. https://doi.org/10.1177/095632020501600104
Müller JA, Harms M, Schubert A, Mayer B, Jansen S, Herbeuval JP, Michel D, Mertens T, Vapalahti O, Schmidt-Chanasit J, Münch J (2017) Development of a high-throughput colorimetric Zika virus infection assay. Med Microbiol Immunol 206(2):175–185. https://doi.org/10.1007/s00430-017-0493-2
Burleson F.G., Chambers T.M., Wiedbrauk D.L. (1992). 12—TCID50. In: Virology, ***pp 58–61.https://doi.org/10.1016/B978-0-12-144730-4.50015-1.
Wilson DR (2002) Viral-mediated gene transfer for cancer treatment. Curr Pharm Biotechnol 3:151–164. https://doi.org/10.2174/1389201023378445
Hu JCC, Coffin RS, Davis CJ, Graham NJ, Groves N, Guest PJ, Harrington KJ, James ND, Love CA, McNeish I, Medley LC, Michael A, Nutting CM, Pandha HS, Shorrock CA, Simpson J, Steiner J, Steven NM, Wright D, Coombes RC (2006) A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin Cancer Res 12:6737–6747. https://doi.org/10.1158/1078-0432.CCR-06-0759
Atherton MJ, Lichty BD (2013) Evolution of oncolytic viruses: novel strategies for cancer treatment. Immunotherapy 5:1191–1206. https://doi.org/10.2217/imt.13.123
Bartee E, Bartee MY, Bogen B, Yu XZ (2016) Systemic therapy with oncolytic myxoma virus cures established residual multiple myeloma in mice. Mol Ther Oncolytics. https://doi.org/10.1038/mto.2016.32
Zamarin D, Palese P (2012) Oncolytic Newcastle disease virus for cancer therapy: old challenges and new directions. Fut Microbiol 7:347–367. https://doi.org/10.2217/fmb.12.4
Thirukkumaran C, Morris DG (2015) Oncolytic viral therapy using reovirus. Methods Mol Biol 1317:187–223. https://doi.org/10.1007/978-1-4939-2727-2_12
Reddy PS, Burroughs KD, Hales LM, Ganesh S, Jones BH, Idamakanti N, Hay C, Li SS, Skele KL, Vasko AJ, Yang J, Watkins DN, Rudin CM, Hallenbeck PL (2007) Seneca Valley virus, a systemically deliverable oncolytic picornavirus, and the treatment of neuroendocrine cancers. J Natl Cancer Inst 99:1623–1633. https://doi.org/10.1093/jnci/djm198
Wang GH, Bertin J, Wang Y, Martin DA, Wang J, Tomaselli KJ, Armstrong RC, Cohen JI (1997) Bovine herpesvirus 4 BORFE2 protein inhibits Fas- and tumor necrosis factor receptor 1-induced apoptosis and contains death effector domains shared with other gamma-2 herpesviruses. J Virol 71:8928–8932
Zemp FJ, Corredor JC, Lun X, Muruve DA, Forsyth PA (2010) Oncolytic viruses as experimental treatments for malignant gliomas: using a scourge to treat a devil. Cytokine Growth Factor Rev 21(2010):103–117. https://doi.org/10.1016/j.cytogfr.2010.04.001
Badie B, Schartner JM (2000) Flow cytometric characterization of tumor-associated macrophages in experimental gliomas. Neurosurgery 46(2000):957–962. https://doi.org/10.1097/00006123-200004000-00035
Siegel RL, Miller KD, Jemal A (2016) Cancer statistics. CA Cancer J Clin 66:7–30. https://doi.org/10.3322/caac.21332
Hambardzumyan D, Gutmann DH, Kettenmann H (2015) The role of microglia and macrophages in glioma maintenance and progression. Nat Neurosci 19:20–27. https://doi.org/10.1038/nn.4185
Acknowledgements
This study was partially supported by a grant from scientific research projects Ankara University (BAP: Project Number: 17B0239004).
Author information
Authors and Affiliations
Contributions
TAF, HG, AO and SBD designed the study; TAF conducted the experiments; TAF and FD analyzed data and prepared the figures; TAF, SBD, FD and FA wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that there are no financial or non-financial competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
All authors agreed with the content of this manuscript and they have given explicit consent to submit and publish it.
Additional information
Edited by Zhen F. Fu.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aligholipour Farzani, T., Bilge Dagalp, S., Ozkul, A. et al. Assessment of replication of bovine herpesvirus type 4 in human glioblastoma and breast cancer cells as a potential oncolytic virus. Virus Genes 57, 31–39 (2021). https://doi.org/10.1007/s11262-020-01802-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11262-020-01802-z