Abstract
Detailed description of the brain size, rhinencephalon and hippocampal formation of the Bactrian camel is presented in our study. The brain weight of the Bactrian camel is 626 g averagely, and the encephalization quotient (EQ) value 1.3, indicating a high level of intelligence. The rhinencephalon is mature and well developed, accordant with the good olfactory sense. The hippocampus is relatively large, concomitant with the good ability of spatial memory. These anatomical features agree with the corresponding adaptive behaviors of the Bactrian camel and provide a morphological evidence of the camel to adapt to the acrid and semi- acrid environment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Bactrian camel lives in the acrid and semi-acrid area of the northwest China and Mongolia characterized by dense desert distribution, tremendous temperature differences between day and night, frequent and strong wind and extraordinarily low humidity. The Bactrian camel has evolved effective strategies to survive such environments, such as the appropriate structure of the kidney to increase the reabsorption of water (Chen and Wang 2002), capability to adjust body temperature to accommodate the drastic change of environmental temperature, (Zhao 1995), the slow respiratory rate to decrease energy consumption and thick wall of capillary blood vessel to reduce the influence of environmental temperature on body temperature (Schwartz and Dioli 1992). Despite these studies, rare attention has been paid to the brain. Friant and Hoest (1945) report the morphology of telencephalon of the Bactrian camel. The pattern of sulci and gyri of the brain of the Bactrian camel has been described in detail (Xie and Wang 2006). Both studies focus on the morphology primarily. In our study, we describe the detailed morphology of the rhinencephalon and hippocampus formation of the Bactrian camel and analyze the relationship of these two structures with the adaptive behaviors of the camel. On the one hand, we expect to provide a morphological basis for the comparative neuroanatomy. On the other hand, we try to seek more evidence about the adaptation of the camel to the acrid and semi-acrid environments, thus providing a scientific basis to protect the wild Bactrian camel and breed the domestic camel.
Materials and methods
Six heads of the adult Bactrian camel were collected from the slaughterhouse of the Right Alasan Banner Food Company in Inner Mongolia Autonomous Region of China. Perfusion and brain extraction were performed according to Xie and Wang (2006). Specimens were soaked in neutral formalin solution for about one month in order for complete fixation. Afterwards, samples were weighed, measured and observed. The EQ value was calculated according to Jerison (1970).
Results
Brain weight and EQ
Averagely, the brain weight of the Bactrian camel was 626 g and EQ value was 1.3 (Table 1).
Rhinencephalon
Observed ventrally (Fig. 1), the rhinencephalon consisted of a pair of olfactory bulbs, peduncles, tracts and piriform cortices symmetrically located in both hemispheres. The rhinal sulci, along the lateral surface of both hemispheres, clearly delimited the rhinencephalon from the neocortex.
Ventral view of rhinencephalon. 1. Olfactory bulb; 2. Longitudinal fissure; 3. Medial olfactory tract; 4. Intermediate olfactory tract; 5. Lateral rhinal sulcus; 6. Anterior perforated substance; 7. Caudal piriform cortex; 8. Olfactory peduncle; 9. Neocortex; 10. Lateral olfactory tract; 11. Olfactory gyrus; 12. Olfactory tubercle; 13. Optic chiasma; 14. Diagonal gyrus; 15. Hypophysis; 16. Intercrural fossa; 17. Oculomotor nerve
Olfactory bulb, tracts and peduncle
The olfactory bulb was the most anterior component of the rhinencephalon. The diameter of the widest region was 0.9 cm. It bent rostrodorsally towards the orbital surface of the hemisphere. The olfactory peduncle was a bunch of wide fiber bundle about 0.9 cm long. It stretched rostrally to the bulb and split into two olfactory tracts caudally. The olfactory tract consisted of the lateral and medial branches. They were about 0.4 cm and 0.18 cm respectively in width. The lateral tract stretched caudally to define the lateral border of rostral piriform cortex, until reaching the diagonal gyrus (Fig. 1). Between the lateral olfactory tract and lateral rhinal sulcus was the lateral olfactory gyrus. It started from a layer of grey matter covering the dorsal surface of the peduncle, and extended into the caudal piriform cortex. The medial olfactory tract deviated from the lateral branch at an angle of about 50 degree and extended to the rostral commissure area (Fig. 2). The intermediate tract was present. It stretched out from the crotches formed by lateral and medial tracts and terminated in the rostral perforated substance.
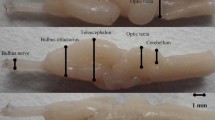
Medial view of the telencephalon. 1. Neocortex 2. Cingulated gyrus 3. Fasciolar gyrus 4. Fimbria 5. Parahippocampal gyrus 6. The convolution on the floor of hippocampal gyrus 7. Hippocampal sulcus 8. Dentate gyrus 9. Caudal piriform cortex 10. Medial olfactory tract 11. Corpus callosum 12. Diagonal gyrus
Rostral piriform cortex
The lateral and medial olfactory tracts and the diagonal gyrus surrounded a triangular area, called rostral piriform cortex or olfactory trigone. The diagonal gyrus, about 0.2 cm wide, lay rostrally to the optic chiasm and extended from the caudal end of lateral olfactory tract to the rostral commissure area (Fig. 2). The lateral half of the cortex surface, termed anterior perforated substance, was pierced by holes for the passage of blood vessels. The medial half of cortex surface was convex in most individuals, termed olfactory tubercle.
Caudal piriform cortex
The caudal piriform cortex was delimited by diagonal gyrus rostrally and rhinal sulcus laterally. The caudal border was undistinguishable from the neocortex. The surface of the caudal piriform cortex was divided by small fissures. Viewed medially, the caudal piriform cortex was contiguous with the parahippocampal and dentate gyrus at the ventral end (Fig. 2).
Hippocampus formation
The hippocampus formation of the Bactrian camel consisted of hippocampus, dentate gyrus and parahippocampal gyrus. It lay ventrally to the lateral ventricle and dorsally to the diencephalons. This location made it invisible from the outer surface of the brain.
Parahippocampal gyrus
After removal of the brainstem and partial diencephalons, the medial and ventral surface of the hippocampus formation was exposed. Viewed medially (Fig. 2), the hippocampal sulcus separated the parahippocampal gyrus from the dentate gyrus. The collateral sulcus bounded the parahippocampal gyrus caudally. This sulcus was in parallel with the hippocampal sulcus. It extended dorsally to the caudal splenial fissure. The ventrolateral end of parahippocampal gyrus abutted on the caudal piriform cortex and neocortex. Extending dorsally, the parahippocampal gyrus broadened and gave off a short rostral branch. This rostral branch was separated from the main trunk by the caudal callosal sulcus and finally joined the fasciolar gyrus (Fig. 2). The main trunk extended along the splenium to join the cingulated gyrus.
Dentate gyrus
The dentate gyrus was demarcated by the hippocampal sulcus caudally and formed a curved contour (Fig. 2). It was contiguous with the tubercle of the parahippocampal gyrus at the ventromedial end, was marked by three to four grooves transversely in the middle region and tapered gradually at the dorsolateral end to join the fasciolar gyrus. The diameter of its widest region was 0.4 cm.
Hippocampal fimbria
The fimbria was a smooth and flat white band attached to the concave surface of the hippocampus. It consisted of fibers from the alveus of hippocampus (Fig. 3). The fimbria was small at the ventral end but the size gradually increased when extending dorsally. The widest region was about 1.2 cm.
Hippocampus
The hippocampus was recessed into the lateral ventricle with a ‘C’ shape (Fig. 3). The whole length was about 6.0 cm and the widest region 0.9 mm. It was contiguous with the caudal piriform cortex at the ventrolateral end. Like the dentate gyrus, it also tapered as it extended towards the dorsolateral end. Its surface was covered by a layer of white fibers, called alveus. These fibers accumulated on the concave edge of the hippocampus and formed the fimbria.
Convolution on the floor of hippocampal sulcus
A tiny convolution was observed on the floor of the hippocampal sulcus which join the fasciolar gyrus at the dorsal end(Fig. 2).
Discussion
EQ and intelligence
Intelligence may be defined by the speed and success of how animals solve problems to survive in their natural and social environments (Roth and Dicke 2005). The brain is considered to be the carrier of intelligence. The EQ, defined as the ratio between actual brain size and expected brain size for a given body weight, has been employed to assess the intelligence (Jerison 1970). Since a majority of mammals are expected to have an EQ of 1, a higher EQ than 1 may be associated with more than average intelligence (Martin 1990; McHenry 1988).
The EQ of Bactrian camel is 1.3 (Table 1). It is higher than that of the ox, horse and sheep, but close to that of the dromedary camel and African elephant, implying that the Bactrian camel has a similar level of intelligence with the dromedary camel and African elephant, but higher than the ox, horse and sheep. Mammals with EQ near or above 2.0 like primates can make and use tools (Gordon 1966). Despite such observations in camels were rare, camels showe good spatial memory and excellent ability of orientation in the desert even during sandy storm (Yuan et al. 2001). Associate learning was also recorded by Yuan et al. (2001). It is observed that captured young camels would come to people for food once hearing the bell after several accidental practices to associate the bell ring with food.
Rhinencephalon and olfaction
The rhinencephalon of Bactrian camel is well developed and almost covers the whole ventral surface of the cerebrum as Madeleine and colleagues (1944) described, characteristic of macrosmatic mammals. The maturity of rhinencephalon is appropriately consistent with the acute olfaction of the camel. It is well-known that the camel could locate the water source from three miles away in the desert (Yuan et al. 2001), as is a unique survival skill for the camel. One volatile molecule that leads camels to water source has been identified (Gust et al. 2003). According to Chess et al. (1994), the receptor responding to the volatile molecule should be present in the sensory cells. Thus, it is very desirable to identify the receptor gene from the genome of the camel for the potential application of this gene.
Hippocampus and spatial memory
The hippocampus of the Bactrian camel is about 6.3 cm in length and 0.9 cm at its widest point. It is of approximately the same size with that of the human (Zhu 2002) and elephant (Shoshani et al. 2006). However, the relative size of hippocampus to the cerebrum in Bactrian camel is extraordinarily larger than in the human and elephant, suggesting a good ability of spatial memory. This is also in accordance with the report that food-hoarding birds using spatial memory to relocate their caches have a larger hippocampus than non-hoarding species (Garamszegi and Eens 2004). Other studies show the similar relationship of hippocampus size with spatial memeory (Krebs et al. 1990; Healy and Krebs 1992, 1996; Hampton et al. 1995; Basil et al. 1996). Spatial memory is very important for orientation, especially in the desert. Behavioral reports also suggest the good spatial memory of Bactrian camel. It has been reported that abandoned camels succeed to return home from 600 Km away (Yuan et al. 2001). In another report, a camel leads a girl home during sandy storm at night (New Capital Newspaper 2006). In all, these observations have demonstrated a good spatial memory of the camel.
Hippocampal formation and IG
The IG is an extremely thin, narrow layer of gray substance on the dorsal surface of the corpus callosum and extends to the fasciolar gyrus (McClure 1983). It is usually considered as a poorly developed portion of the hippocampus and a nonfunctional structure of the brain (Künzle 2004). In our observation, the parahippocampal gyrus, dentate gyrus and the convolution at the bottom of hippocampal sulces are connected with IG by the fasciolar gyrus. In mouse, the entorhinal area and olfactory bulb has direct projection to the IG (Adamek et al. 1984). In hedgehog tenrec, the IG receives specific afferents from the entorhinal cortex, anterior and posterior piriform cortices and a few fibers from the olfactory bulb and the dentate hilus (Künzle 2004). And projection from hippocampus to IG is also identified in rats (Jinno et al. 2007). These studies, on the one hand, agree with our observation of the connection between hippocampal formation and the IG. On the other hand, they suggest a active functional activities of the IG. More attention should be paid to studying this neglected area.
References
Adamek G D, Shipley M T, Sanders M S (1984) The indusium griseum in the mouse: architecture, Timm’s histochemistry and some afferent connections. Brain Res Bull 12(6):657–68 doi:10.1016/0361–9230(84)90147–3
Basil J A, Kamil A C, Balda R P, et al (1996) Differences in hippocampal volume among food storing corvids. Brain Behav Evol 47: 156–164 doi:10.1159/000113235
Chen Q Sh, Wang W H (2002). Morphological features of renal cell related to reabsorption in two- humped camel (in Chinese). Acta Zoologica Sinica 48 (2):245–250
Chess A, Simon I, Cedar H, Axel R (1994) Allelic inactivation regulates olfactory receptor gene expression. Cell 78: 823–834 doi:10.1016/S0092–8674(94)90562–2
Friant M and Hoëst D L (1945) The telencephalon of the camel: its interpretation through the study of a fetal stage. Ann Soc Roy Zool Belgique 75: 95–103
Garamszegi L Z, Eens M (2004) The evolution of hippocampus volume and brain size in relation to food hoarding in birds. Eco Lett 7: 1216–1224 doi:10.1111/j.1461–0248.2004.00685.x
Gordon J A (1966) Elephants do think. Arf Wildl 20: 75–79.
Gust B, Greg L, Fowler C K, Kieser T, Chater K F (2003) PCR-targeted Streptomyces gene replacement identifies a protein domain needed for biosynthesis of the sesquiterpene soil odor geosmin. PNAS 100(4): 1541–1546 doi:10.1073/pnas.0337542100
Hampton R R, Sherry D F, Shettleworth S J, et al (1995) Hippocampal volume and food-storing behavior are related in parids. Brain Behav Evol 45: 54–61 doi:10.1159/000113385
Healy S D, Krebs J R (1992) Food storing and the hippocampus in corvids - amount and volume are correlated. Proc R Soc Lond Ser (B) 248: 241–245
Healy S D, Krebs J R (1996) Food storing and the hippocampus in Paridae. Brain Behav Evol, 47: 195–199 doi:10.1159/000113239
Jerison H J (1970) Gross brain indices and the analysis of rossil endocasts. In: Noback C R, Montagna W (eds) The primate brain. Appleton- Century Crofts Educational Division/ Meredith Corporation, New York, pp: 225–443
Jinno S, Klausberger T, Marton L F (2007) Neuronal diversity in GABAergic long-range projections from the hippocampus. J Neurosci 27(33):8790–804 doi:10.1523/JNEUROSCI.1847–07.2007
Krebs J R, Healy S D, Shettleworth S J (1990) Spatial memory of Paridae: comparison of a storing and a non-storing species, the coal tit, Parus ater, and the great tit, P. major. Anim Behav 39: 1127–1137. doi:10.1016/S0003–3472(05)80785–7
Künzle H (2004)The hippocampal continuation (indusium griseum): its connectivity in the hedgehog tenrec and its status within the hippocampal formation of higher vertebrates. Anat Embryo, 208: 183–213
Martin R D (1990) Primate Origins and Evolution: a phylogenetic Reconstruction. Princeton University Press, Princeton
McClure R C, Dallman M J, Garrett P D (1973) In: Cat Anatomy: an Atlas, Text, and Dissection Guide, Lea and Febiger, Philadephia, PA
McHenry H (1988) New estimates of body weight in early hominids and their significance to encephalization and megadontia in “robust” Autralopithecines. In: Grine, F. (Ed.): Evolutionary History of the “robust” Australopithecines, Gruynter, New York, pp 133–148
New Capital Newspaper (2006) Competition between camel and sheep in camels’ hometown- the Bactrian camel species in Inner Mongolia is seriously endangered (in Chinese). Beijing
Roth G, Dicke U (2005) Evolution of the brain and intelligence. Trends Cog Sci 9(5): 250–257 doi:10.1016/j.tics.2005.03.005
Schwartz H J, Dioli M (1992) The one- humped camel in eastern Africa. Verlag Josef Margraf, Germany, pp 137–140
Shoshani J, Kupsky W J, Marchant G H (2006) Elephant brain part 1: Gross morphology, functions, comparative anatomy, and evolution. Brain Res Bull 70: 124–157 doi:10.1016/j.brainresbull.2006.03.016
Xie Z H and Wang J L (2006) Morphological study on the cerebrum of the Bactrian camel with particular reference to sulci. JCPR 13(1): 61–66
Yuan G Y, Zhang L, Yuan L (2001) A kind of world new species mammal 2000- wild Bactrian camels (in Chinese). Xinjiang juvenile publishing house, China
Zhao X X (1995) Ecophysiology and reproduction of the camel (In Chinese). Gansu Science and Technology Press, China, pp 50–52
Zhu C G (2002) The hippocampal formation, first ed (In Chinese). In: Zhu, C. G. (Ed): Neuroanatomy. People’s health press, pp. 7123–718
Acknowledgements
We are grateful to Dr. Wenling Ye and Lei Zhu for the collection of specimens, and Jinghong Ma, Xianyan Peng, Hongju Wang and Guoqiang Yuan for their assistance during the perfusion. Our study was funded by National Natural Science Foundation of China (39300097), and Open Foundation of Chinese Educational Department Key Laboratory of Arid and Grassland Agroecology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, J., Bai, Z., Gao, C. et al. Morphology of Rhinencephalon and Hippocampal formation of the Bactrian Camel (Camelus bactrianus) with their adaptive features. Vet Res Commun 33, 25–32 (2009). https://doi.org/10.1007/s11259-008-9068-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11259-008-9068-4