Abstract
Many ant–plants have pubescent leaves, but the extent to which leaf trichomes enhance the defensive capabilities of ant–plants is poorly known. Here, we examined the influence of foliar trichomes and ants on herbivory in the Neotropical ant–plant Tococa guianensis. We performed a feeding preference test and a field experiment in which we manipulated the presence of ants and trichomes in three distinct populations of T. guianensis; two associated with obligate mutualistic ants and one associated with opportunistic ants. We found that both mutualistic ants and leaf trichomes act as mechanisms of defense against herbivores in T. guianensis. However, the relative importance of each of these two mechanisms was context dependent. The magnitude of the effect of trichomes (as measured by the effect size) was relatively low and varied little among plant populations. The effect of ants on foliar herbivory was up to seven times stronger than the effect of the trichomes. Nevertheless, because the magnitude of ant effects was spatially variable—depending on whether mutualistic ants were present or not, and probably also on the local abundance of herbivores—the relative contribution of ants and trichomes for plant defense was also spatially variable. These findings indicate that mechanical defenses (leaf trichomes) alone cannot replace the biotic defenses of T. guianensis. However, trichomes can play a comparatively more important role in plant defense at times or places where mutualistic ants are not present.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A large number of plants rely on predatory ants as a mechanism of defense against herbivores (Rosumek et al. 2009; Trager et al. 2010). Protective ant–plant mutualisms are particularly common in tropical habitats, where many plant species produce nectar or other types of food rewards to attract ants to their leaves (Heil and McKey 2003; Oliveira and Freitas 2004; Rico-Gray and Oliveira 2007). Most of these interactions are facultative to both plants and ants. However, some species of ants and plants have engaged in obligate mutualistic interactions. The most classical examples include a suite of independently evolved plant species—known as myrmecophytes or ant–plants—which, in addition to food resources, provide nesting space for their ant partners (Davidson and McKey 1993). In exchange, many of the ant species that live on ant–plants repel the herbivores that attack their host-plants and often increase plant fitness (Rosumek et al. 2009; Trager et al. 2010).
Direct (chemical or physical) and indirect (biotic) defenses are known or assumed to be costly and, therefore, it is expected that plants should avoid investing in redundant defenses (Strauss et al. 2002; Koricheva et al. 2004). Therefore, one would expect that myrmecophytes would invest comparatively less in direct defenses than non-myrmecophytes (Janzen 1966). Furthermore, one would expect a lower investment in direct defenses by myrmecophytes in the presence of mutualistic ants. Although several studies have found support to this hypothesis (Seigler and Ebinger 1987; Eck et al. 2001; Dyer et al. 2001; Latteman et al. 2014), the existence of a trade-off between direct and indirect defenses in ant–plants is still matter of debate (reviewed in Heil et al. 2002). One possible explanation for the absence of a trade-off between direct and indirect defenses is that direct antiherbivore defenses can play other roles (e.g., phenolics have anti-pathogenic properties), and thus reducing the investment in such defenses may not always be advantageous to myrmecophytes (Heil et al. 2002). In addition, direct defenses can have a complementary role in the anti-herbivore defense system of myrmecophytes. For instance, in the ant–plant Piper cenocladum mutualistic ants (Pheidole bicornis) are effective against specialist herbivores, whereas direct defenses (amides) are effective against generalist orthopterans and leaf-cutter ants (Dyer et al. 2001).
Many ant–plants have pubescent leaves (Davidson et al. 1989), but the extent to which leaf trichomes enhance the defensive capabilities of ant–plants is poorly known (but see Piovia-Scott 2011). Here, we examined the influence of foliar trichomes and ants on herbivory in the Neotropical ant–plant Tococa guianensis (Melastomataceae). T. guianensis is the most widespread species of the genus Tococa, ranging from southern Mexico to Bolivia (Michelangeli 2003).Throughout most of its range T. guianensis is associated with one or more species of obligate plant–ants, mostly species of the genera Azteca and Allomerus (Bizerril and Vieira 2002; Michelangeli 2003; Frederickson 2005; Dejean et al. 2006). However, in some marginal populations T. guianensis either has no ants or is associated with opportunistic ants that presumably provide less protection than its obligate ant associates (Moraes and Vasconcelos 2009).Furthermore, previous studies indicate that at least one putative direct defensive trait of T. guianensis—the density of non-glandular leaf trichomes—can vary among plant populations (Michelangeli 2003).
We asked the following questions: (i) Do leaf trichomes act as a physical anti-herbivore defense in T. guianensis? (ii) If so, what is the magnitude of this effect compared to the effect of indirect defenses (ants)? (iii) Are the effects of trichomes and ants on leaf herbivory in T. guianensis additive or interactive? (iv) Are any of these effects context dependent? To answer these questions, we performed a feeding preference test and a field experiment in which we manipulated the presence of ants and trichomes in three distinct populations of T. guianensis. We predicted that (i) trichomes would have a lesser effect on herbivory, compared to the effects of ants, and that (ii) the magnitude of effect of trichomes and ants—and thus their relative importance—would be spatially variable depending on the characteristics of the ant, plant, and herbivore populations.
Methods
Study sites
We performed the experiments with three populations of T. guianensis. These plant populations were found near the towns of Uberlândia (hereafter UB), Aragarças (AR), and the village of Cachoeira da Fumaça (CF) (Table 1). All these three sites have a seasonal climate, characterized by a dry winter from May to September and a rainy summer from October to April. The habitat characteristics and local density of T. guianensis were similar among the three populations studied (Table 1). Individuals from these populations were found adjacent to a small stream or to the stream headwater in the understory of a gallery forest. Plants in AR and CF were associated with Allomerus octoarticulatus, an obligate plant–ant. This ant species does not occur in UB where plants are colonized by a suite of opportunistic ants from the genera Brachymyrmex, Camponotus, Cephalotes, Crematogaster, Linepithema, Pheidole, Solenopsis, Tapinoma and Wasmannia (Moraes and Vasconcelos 2009).
Density of trichomes
To quantify the density of leaf trichomes in plants from AR, CF, and UR we randomly selected three young and three mature leaves from 15 adult individuals in each population. Leaves were classified as young or mature depending on differences in leaf color (young leaves have a light green color whereas mature leaves a dark green color) and toughness (young leaves are much less tough). The number of trichomes was counted in six randomly selected 0.25 cm2 sections of each leaf (avoiding the mid-vein), of which three were located in the upper surface and three in the lower surface of the leaf.
Laboratory bioassay
A laboratory test was performed to determine the influence of trichomes on the consumption of leaves of T. guianensis by one unidentified generalist species of caterpillar from the family Noctuidae. The caterpillars (2nd–4th instars) were collected in the field and maintained in plastic boxes. Two leaf disks (of 9 cm2 each), removed from the same young leaf, were presented to each caterpillar. Leaves used in this experiment were collected in UB. One leaf disk was intact (control) whereas the other had its trichomes removed using an electric shaver. After 72 h, the area consumed from each disk was measured using the software ImageJ v. 1.42 (Rasband 2014). The test was replicated 50 times, and each test was done using leaves from a different plant and a different caterpillar.
Experimental removal of leaf trichomes and resident ants
Within each population, we located and marked a total of 44 plant individuals (>1.5 m in height). These plants were randomly allocated to one of the following treatments: (i) Unmanipulated controls (ants and leaf trichomes present), (ii) ants and trichomes removed, (iii) ants removed and trichomes maintained, and (iv) ants maintained and trichomes removed. Only one treatment was applied to each plant individual. The treatments were applied to all emerging new leaves (located in up to three different branches) that had no prior damage at the beginning of our experiment. The treatments were applied to leaves from only a few branches per plant because some plant individuals were fairly large, and thus would have been very difficult to apply our removal treatments to the entire plant.
To remove the resident ants we applied a few drops of a pyrethroid insecticide (Cypermethrin® at a concentration of 0.5 ml per liter of water) inside the domatia (Fig. 1a). Ant recolonization was prevented by applying a stick resin (Tanglefoot®) at the base of the branch (Fig. 1b) and by blocking the entrance of the domatia with a small cotton ball. Leaf trichomes were removed from the upper and lower surfaces of each leaf using an electric shaver (Fig. 1c). Care was taken as to not cause any damage to the leaf surfaces. At the beginning of the experiment we marked all undamaged, emerging leaves from our focal branch using colored plastic wires. We also measured the maximum length and width of each marked leaf in order to estimate their total leaf areas using the following equation: leaf area = −0.325 + 0.732 × (leaf width × leaf length) (R 2 = 0.984, P < 0.001, n = 111) (Moraes and Vasconcelos 2009). The area of each leaf damaged by herbivores was determined using a transparent plastic grid (with a precision of 1 cm2) (Fig. 1d). The percentage of damage was calculated as follows: (damaged area/total leaf area) × 100.
The experiment was run almost simultaneously at the three sites. Marked leaves were checked at approximately two-month intervals, during a total period of 6 months, in order to re-measure their sizes and amount of damage. In each sampling interval, we also marked and measured all leaves that emerged during the previous 2 months. The trichomes of these leaves were removed as described before.
Statistical analysis
Differences in the density of leaf trichomes among plants from different sites were assessed using one-way ANOVA. Randomized-block ANOVA was used to compare the leaf area consumed by individual caterpillars (blocks) from leaves with or without trichomes. Repeated measures ANOVA was employed to assess the influence of ants and trichomes on herbivory in plants from each of the three populations studied. The dependent variable (i.e., the repeated measures) represented the average amount of damage on leaves that were under observation for 2, 4, or 6 months. From four to 20 leaves (mean = 9.5) were marked in each experimental plant, and data on leaf herbivore damage in each sampling period was logit transformed (Warton and Hui 2011) prior to the statistical analyses. As a measure of the effect size of each individual factor (ants, trichomes, and their interaction) we calculated the Partial Eta Square (\(\eta_{P}^{2}\)) statistic as follows: SS effect/(SS effect + SS residuals) where SS is the sum of squares. In all analyses above, assumptions about data normality and homoscedasticity were checked using, respectively, the Lilliefors and Levene´s test.
Results
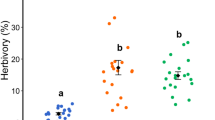
Plants from AR had a significantly higher density of trichomes on their leaves compared to plants from CF and UB, and such difference was detected both in young (F 2.42 = 5.8, P = 0.006) and mature leaves (F 2.42 = 3.3, P = 0.047) (Fig. 2). The removal of trichomes significantly increased the consumption of young leaves by caterpillars during our laboratory assay (Mean ± SD proportion of the leaf disks consumed: with trichomes = 0.15 ± 0.17, trichomes removed = 0.30 ± 0.27; F 1.36 = 9.4; P = 0.004). Our field experiment showed that, in the three plant populations studied, there was a trend towards finding higher herbivore damage in leaves whose trichomes were removed than in the control leaves (Fig. 3). However, this effect was statistically significant in only one plant population (the UB population) (Table 2). Ant removal had a strong positive and significant effect on herbivory in plants from the AR population, a marginally significant effect in the CF population, and no effect in the UB population (Table 2; Fig. 3).
Effects of ants and leaf trichomes on herbivore damage in leaves with ca. 2 and 6 months of age. (a–b) plants associated with Allomerus octoarticulatus in Aragarças, (c–d) plants associated with Allomerus octoarticulatus in Cachoeira da Fumaça, and (e–f) plants associated with opportunistic ants in Uberlândia
Discussion
Overall, the results of our study indicate that both A. octoarticulatus and leaf trichomes act as mechanisms of defense against herbivores in T. guianensis. However, we also found that the relative importance of each of these mechanisms can vary strongly among plant populations. In the AR population the magnitude of the effect of ants on leaf herbivory was much stronger (ca. 7 times greater) than the effect of the trichomes. This suggests that direct mechanical defenses (leaf trichomes) alone cannot replace the biotic defenses of T. guianensis. Similarly, studies with myrmecophytic Acacia have revealed that mechanical defenses (thorns) alone do not deter mammalian herbivores; however, thorns increased the effectiveness of resident ants in repelling these animals (Stapley 1998). In contrast, here, trichomes tended to reduce herbivory in both the presence and absence of ants, indicating that the effects of mutualistic ants and trichomes are additive and not interactive.
In contrast to the pattern observed in the AR population, the experimental removal of A. octoarticulatus ants from plants in CF did not result in a dramatic increase in herbivory. Rather, the effect of the ant treatment was weak (and only marginally significant) and similar in magnitude to the effect of trichomes. Similarly, studies with ant–plants of the genus Cecropia (Fáveri and Vasconcelos 2004) and with the extrafloral nectar-bearing plant Chamaecrista fasciculata (Barton 1986) have revealed that although mutualistic ants have a key role in the defense of these plants, there are sites in which ant removal has no effect on plant fitness. In both studies, herbivory abundance was found or assumed to be very low in the sites where the ant effects were null (Barton 1986; Fáveri and Vasconcelos 2004). Similarly, here, spatial variation in herbivore abundance may help explain the inter-populational difference in the outcome of the interaction between A. octoarticulatus and T. guianensis. Herbivory rates on ant-removed leaves were much greater in plants from the AR population, probably because herbivore abundance is much greater in the AR than in the CF population.
Several studies have shown or suggested that the outcome of ant–plant interactions is dependent on the local conditions in which the interaction occurs (e.g., Kersch and Fonseca 2005; Abdala-Roberts and Marquis 2007; Yamawo et al. 2014). Our findings reinforce this view by indicating that the magnitude of the protective effect of mutualistic ants on T. guianensis can range from weakly to strongly positive and that such variation appears to be related with the biotic context in which the interaction occurs.
Obligate mutualistic ants are not present in the UB population and the experimental removal of ants that forage or eventually nest opportunistically in plants from this population did not have any measurable impact on herbivory. This suggests—as also evidenced earlier (Bizerril and Vieira 2002)—that opportunistic ants do not engage in mutualistic interactions with T. guianensis. In contrast, the removal of leaf trichomes increased herbivory on plants from the UR population. This indicates that trichomes play a comparatively more important role in the defense of T. guianensis where mutualistic ants are not present, and therefore where direct defenses are presumably most needed. However, by comparing the density of leaf trichomes among plant populations (Fig. 2) we did not find evidence—as observed earlier (Moraes and Vasconcelos 2009)—that T. guianensis invest more in the production of leaf trichomes when not associated with mutualistic ants. In fact, plants from the UB population we studied had significantly fewer (and not more) trichomes than plants from the AR population. Plants from the AR population also presented a greater density of trichomes than those from the CF population in spite of the fact that plants from both populations were associated with mutualistic ants. However, we also found that plants from the AR population seem to experience higher levels of herbivory than plants from the other populations. This finding is consistent with those from another ant–plant system (Cordia nodosa × Allomerus octoarticulatus) in which plants respond to increased herbivory by investing in direct defensive traits regardless of the presence or absence of mutualistic ants (Frederickson et al. 2012).
In short, our study indicates that mutualistic ants (A. octoarticulatus) have a much more important role in the anti-herbivore defensive system of T. guianensis than leaf trichomes. However, where these ants do not occur (due to dispersal limitation or environmental constraints), the role of trichomes in plant defense becomes comparatively more prominent. Similarly, trichomes may be of importance for plant defense during the early stages of plant development when mutualistic ants have yet not colonized their host-plants. Furthermore, it may well be possible that the leaf trichomes of T. guianensis play other roles, such as reducing plant water loss (e.g., Woodman and Fernandez 1991). Although further studies are need to evaluate these hypotheses, they suggest that it is selectively advantageous for T. guianensis to produce trichomes, even though trichomes have a limited role in plant defense in the presence of mutualistic ants.
References
Abdala-Roberts L, Marquis RJ (2007) Test of local adaptation to biotic interactions and soil abiotic conditions in the ant-tended Chamaecrista fasciculata (Fabaceae). Oecologia 154:315–326
Barton AM (1986) Spatial variation in the effect of ants on extrafloral nectary plant. Ecology 67:495–504
Bizerril MXA, Vieira EM (2002) Aztecaants as antiherbivore agents of Tococaformicaria (Melastomataceae) in Brazilian Cerrado. Stud Neotrop Fauna Environ 37:145–149
Davidson DW, McKey D (1993) Ant plant symbioses: stalking the chuyachaqui. Trends Ecol Evol 8:326–332
Davidson DW, Snelling RR, Longino JT (1989) Competition among ants for myrmecophytes and the significance of plant trichomes. Biotropica 21:64–73
Dejean A, Delabie JHC, Cerdan P, Gibernau M, Corbara B (2006) Are myrmecophytes always better protected against herbivores than other plants? Biol J Linn Soc 89:91–98
Dyer LA, Dodson CD, Beihoffer J, Letourneau DK (2001) Trade-offs in antiherbivore defenses in Piper cenocladum: ant mutualists versus plant secondary metabolites. J Chem Ecol 27:581–592
Eck G, Fiala B, Linsenmair KE, Hashim RB, Proksch P (2001) Trade-off between chemical and biotic antiherbivore defense in the south east Asian plant genus Macaranga. J Chem Ecol 27:1979–1996
Engelbrecht BMJ, Herz HM (2001) Evaluation of different methods to estimate understory light conditions in tropical forests. J Trop Ecol 17:207–224
Fáveri S, Vasconcelos HL (2004) The Azteca-Cecropia association: are ants always necessary for their host plants? Biotropica 36:641–646
Frederickson ME (2005) Ant species confer different partner benefits on two neotropical myrmecophytes. Oecologia 143:387–395
Frederickson ME, Ravenscraft A, Hernandéz LMA, Booth G, Astudillo V, Miller GA (2012) What happens when ants fail at plant defence? Cordianodosa dynamically adjusts its investment in both direct and indirect resistance traits in response to herbivore damage. J Ecol 101:400–409
Heil M, McKey D (2003) Protective ant–plant interactions as model systems in ecological and evolutionary research. Ann Rev Ecol Evol Syst 34:425–553
Heil M, Delsinne T, Hilpert A, Schürkens S, Andary C, Linsenmair KE, Sousa SM, McKey D (2002) Reduced chemical defence in ant–plants? A critical re-evaluation of a widely accepted hypothesis. Oikos 99:457–468
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Inter J Climatol 25:1965–1978
Janzen DH (1966) Coevolution of mutualism between ants and acacias in Central America. Evolution 20:249–275
Kersch MF, Fonseca CR (2005) Abiotic factors and the conditional outcome of an ant–plant mutualism. Ecology 86:2117–2126
Koricheva J, Nykänen H, Gianoli E (2004) Meta-analysis of trade-offs among plant antiherbivore defenses: are plants jacks-of-all-trades, masters of all? Am Nat 163:64–75
Latteman TA, Mead JE, DuVall MA, Bunting CC, Bevington JM (2014) Differences in anti-herbivore defenses in non-myrmecophyte and myrmecophyte Cecropia trees. Biotropica 46:1–5
Michelangeli FA (2003) Ant protection against herbivory in three species of Tococa (Melastomataceae) occupying different environments. Biotropica 35:181–188
Moraes SC, Vasconcelos HL (2009) Long-term persistence of a Neotropical ant–plant population in the absence of obligate plant–ants. Ecology 90:2375–2383
Oliveira PS, Freitas AVL (2004) Ant–plant–herbivore interactions in the neotropical cerrado savanna. Naturwissenschaften 91:557–570
Piovia-Scott J (2011) Plant phenotype influences the effect of ant mutualists on a polymorphic mangrove. J Ecol 99:327–334
Rasband WS (2014) ImageJ: image processing and analysis. U. S. National Institutes of Health, Bethesda. http://imagej.nih.gov/ij/
Rico-Gray V, Oliveira PS (2007) The ecology and evolution of ant–plant interactions. University of Chicago Press, Chicago
Rosumek FB, Silveira FAO, Neves FS, Barbosa NPU, Diniz L, Oki Y, Pezzini F, Fernandes G, Cornelissen T (2009) Ants on plants: a meta-analysis of the role of ants as plant biotic defenses. Oecologia 160:537–549
Seigler DS, Ebinger JE (1987) Cyanogenic glycosides in ant-Acacias of Mexico and Central America. South West Nat 32:499–503
Stapley L (1998) The interaction of thorns and symbiotic ants as an effective defence mechanism of swollen-thorn acacias. Oecologia 115:401–405
Strauss SY, Rudgers JA, Lau JA, Irwin RE (2002) Direct and ecological costs of resistance to herbivory. Trends Ecol Evol 17:278–285
Trager MD, Bhotika S, Hostetler JA, Andrade GV, Rodriguez-Cabal MA, McKeon CS, Osenberg CW, Bolker BM (2010) Benefits for plants in ant–plant protective mutualisms: a meta-analysis. PLoS One 5:e14308
Warton D, Hui F (2011) The arcsine is asinine: the analysis of proportions in ecology. Ecology 92:3–10
Woodman RL, Fernandez GW (1991) Differential and leaf-hairs mechanical defense: herbivory, evapotranspiration, and leaf-hairs. Oikos 60:11–19
Yamawo A, Tagawa J, Hada Y, Suzuki N (2014) Different combinations of multiple defence traits in an extrafloral nectary-bearing plant growing under various habitat conditions. J Ecol 102:238–247
Acknowledgments
We wish to thank Eurico F. Rosa, Adriana Mohr, Fernando Pedroni, and Marcello M. Barbosa for their help during the field work. Thiago J. Izzo, Ricardo Campos and Kátia G. Facure provide valuable comments on earlier versions of this manuscript. Financial support was provided by the Brazilian Council for Scientific Development and Research (CNPq).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Stephen Brewer.
Rights and permissions
About this article
Cite this article
Bartimachi, A., Neves, J. & Vasconcelos, H.L. Geographic variation in the protective effects of ants and trichomes in a Neotropical ant–plant. Plant Ecol 216, 1083–1090 (2015). https://doi.org/10.1007/s11258-015-0491-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-015-0491-7