Abstract
Many examples of associational resistance have been reported, in which a plant’s neighbors reduce the rate of damage by herbivores that it experiences. Despite 30 years of interest and hundreds of examples of associational resistance, we still know very little about how plants avoid their herbivores. This lack of mechanistic understanding prevents us from predicting when or where associational resistance will be important or might affect species’ distributions. I demonstrate here that the plant neighborhoods that surrounded focal mule’s ears (Wyethia mollis) individuals affected the damage they received. In particular, distance between a focal mule’s ears individual and its nearest sagebrush neighbor (Artemisia tridentata) was a good predictor of how much leaf area the mule’s ears would lose to herbivores over 2 years. Mule’s ears close to sagebrush suffered less loss than those with more distant nearest sagebrush neighbors. Mule’s ears with near sagebrush neighbors suffered half the leaf loss as mule’s ears with sagebrush experimentally removed. This associational resistance was probably not caused by sagebrush attracting or increasing populations of predators of generalist herbivores. Sagebrush is known to emit chemicals that are feeding deterrents to generalist grasshoppers and these deterrents were probably involved here. Volatile chemicals emitted by damaged sagebrush have been found to induce resistance in neighboring plants of several species. However, I found no evidence for such eavesdropping here as mule’s ears gained associational resistance from sagebrush neighbors whether or not those sagebrush neighbors had been experimentally damaged. Understanding the mechanisms responsible for associational resistance is critical to predicting where and when it will be important.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past century, the dominant paradigm in community ecology has changed dramatically. Many early workers viewed plant communities as groups of species that were highly dependent on one another (Clements 1916). This view was replaced largely by the individualist concept of plant community organization championed by Gleason (1926) and subsequent workers. According to this worldview, the distribution and abundance of plant species are independent of other species. Support for this paradigm came from examination of numerous patterns of species’ distribution along environmental gradients. Plant species extended their ranges independently, rather than moving across the landscape in closed communities (Whittaker 1967).
This individualistic view stood in marked contrast with the working hypotheses of ecologists who studied the distribution and abundance of plant damage caused by herbivores (Tahvanainen and Root 1972; Feeny 1976; Atsatt and O’Dowd 1976). These workers reported that, rather than being independent, plants were strongly influenced by their neighbors. The likelihood that a plant will be eaten by herbivores often depends not only on its own inherent qualities, but also on the chemistry, morphology, distribution, and abundance of the guild of plants that it grows with. In a seminal paper, Atsatt and O’Dowd (1976) outlined three mechanisms by which plants gain “associational resistance” from neighbors. (1) Neighbors may be hosts for predators that attack the herbivores of the focal plant individual. (2) Neighbors may attract herbivores away from the focal individual. (3) Neighbors may allow focal individuals to avoid detection or attack by herbivores. In the years following Atsatt and O’Dowd’s influential review there have been hundreds of published papers describing this phenomenon (see reviews by Karieva 1983; Andow 1991; Milchunas and Noy-Meir 2002; Shelton and Badenes-Perez 2006).
There are several possible ways to reconcile the conflicting views of ecologists working on plant communities and those working on plant–insect interactions. Some workers argued that herbivores may not strongly influence plant distribution, abundance, and defense (Jermy 1984, 1993). This view seems untenable with what many studies have found (e.g., Louda 1982; Marquis 1992; Hamback et al. 2000; Maron and Vila 2001). Although we cannot necessarily assume that any particular herbivore will influence its host plant, the many examples showing that herbivory can limit plant numbers and distributions erode the credibility of this explanation.
Recently many plant ecologists have recognized that plant communities may be less individualistic than they have been viewed for much of the past century (Callaway 1997). This current shift has been accompanied by a realization that facilitation plays an important role in community organization, along with competition (Callaway 1995; Bruno et al. 2003; Gomez-Aparicio et al. 2004; Lortie et al. 2004). Recent work indicates that many plant species benefit by germinating and growing near other plants, particularly in environments that are physically stressful or have high pressure from herbivores (Bertness and Callaway 1994; Callaway et al. 2002).
It is also possible that both the community ecologist’s individualist concept and the observations from plant–herbivore interactions about the importance of plant neighborhood may both be partly correct because they make predictions at different scales. Community ecologists are explaining patterns at the scale of species’ ranges while ecologists studying plant–herbivore interactions are explaining patterns at the scale of interactions among individuals. Paradoxically our lack of knowledge about the mechanisms responsible for “associational resistance” prevents us from understanding why, when, and where these interactions will be important and will produce community level patterns (Kareiva 1983). We really know very little about how plants might “hide” from their herbivores. This prevents us from understanding which local interactions between herbivores and their hosts are likely to scale up to affect plant distributions and abundances.
The current study was motivated by the observation that individuals of Mule’s Ears (Wyethia mollis) suffer less leaf loss to herbivores when they grow in close association with Big Sagebrush (Artemisia tridentata). Both of these plants are dominant community members on volcanic soils on the east side of the Sierra Nevada mountains of California and Nevada. This situation is particularly intriguing since recent results indicated that sagebrush plants within 60 cm of experimentally clipped conspecific neighbors experienced less damage by their herbivores (Karban et al. 2004, 2006). A volatile cue was required for one plant to respond when its neighbor had been damaged. This eavesdropping was not restricted to individuals of sagebrush. Wild tobacco plants within 10–15 cm of clipped sagebrush experienced reduced damage by herbivores and increased production of flowers and seeds compared to tobacco plants near unclipped sagebrush neighbors (Karban and Maron 2002). However, several other plant species that grew in association with sagebrush did not show reductions in herbivore damage when neighboring sagebrush had been experimentally clipped (Karban et al. 2004). In summary, other plants seem capable of eavesdropping on damaged sagebrush although not all do; understanding how and when eavesdropping occurs will be essential in formulating a general theory of plant–plant communication.
My goals in this current study were first, to determine whether herbivore damage to Wyethia mollis was truly reduced in association with sagebrush and second, to evaluate whether eavesdropping between plants may have caused this result. I asked the following specific questions: (1) Does neighborhood affect herbivore damage to W. mollis? (2) Is this effect related to bare ground, coverage by woody plants generally, or is it specific to A. tridentata? (3) How frequently are W. mollis and A. tridentata neighbors? (4) How does the distance between W. mollis and A. tridentata affect herbivore damage levels? (5) Is volatile eavesdropping involved? In other words, does A. tridentata need to be experimentally clipped before herbivore damage is reduced for a neighboring W. mollis?
Natural history
Mule’s Ears (Asteraceae: Wyethia mollis) is a common and widespread herbaceous perennial found on both sides of the Sierra Nevada mountains but especially on the east side. It has a long-lived root and forms rosettes of large (20–40 cm) leaves that die back to ground level each winter. It forms dense patches in shallow volcanic soils that are too dry for many deeper-rooted chaparral species (Parker and Yoder-Williams 1989; Graf 1999). Dense patches of W. mollis are attributed to overgrazing by sheep and have persisted for 100 years without tree establishment (Kennedy and Doten 1901; Parker and Yoder-Williams 1989).
Sites with these edaphic and climatic conditions are also dominated by sagebrush (Asteraceae: Artemisia tridentata) (Graf 1999). Sagebrush is the most common, and the defining, plant of the Great Basin biome of western North America. Sagebrush is a long-lived woody shrub that thrives in many ecological situations and has become particularly abundant in areas that have experienced heavy grazing and fire suppression (Pickford 1932; Young et al. 1988). Sagebrush foliage releases volatile organic compounds that include monoterpenes, sesquiterpene lactones, coumarins, and flavonoids (Kelsey et al. 1978; Personius et al. 1987; Preston et al. 2001). These volatile compounds are known to deter generalist mammalian and insect herbivores (Personius et al. 1987; Bray et al. 1991; Karban and Baxter 2001). Many of these compounds exhibit quantitative changes following herbivory (Preston et al. 2001). These observations motivated the hypothesis that chemicals emitted by sagebrush may reduce herbivory on neighboring mule’s ears individuals (see above).
Both plants are attacked by herbivores of many feeding guilds (Weins et al. 1991; Karban personal observation). Although both plant species are hosts for diverse specialist herbivores, the primary leaf-feeders at my study site were generalist grasshoppers, especially Cratypedes neglectus, Trimerotropis fontana, and Camnula pellucida, and deer, Odocoileus hemionus.
Methods
Distance to nearest sagebrush and leaf damage to mule’s ears
Since field observations suggested that individual mule’s ears that were growing close to sagebrush experienced less leaf damage than those without close sagebrush neighbors, I first attempted to quantify this perceived trend. On 29 July 2004, I randomly selected 60 mule’s ears individuals by walking along an E–W transect south of lower Sagehen Creek (just upstream from Stampede reservoir, 39°26.48 N 120°11.25 W−39°26.67 N 120°11.05 W at an elevation of approximately 1850 m). Every 20 m, I selected the closest W. mollis individual and recorded the distance from that focal plant to its nearest sagebrush neighbor. This gave an estimate of the mean distance between nearest neighbor pairs of these two plant species. For each focal W. mollis plant, I excised the youngest expanded leaf, the oldest leaf that was not senescent, and the leaf that was intermediate between these two in terms of phyllotaxis. Leaves were excised at the petiole and immediately pressed in the field. Once the leaves had dried they were photocopied and the total leaf area and leaf area removed by herbivores was calculated for each leaf using a compensating polar planimeter (Los Angeles Scientific Instruments Model N-40). I calculated the percentage of leaf area removed by herbivores for each plant using these estimates; to normalize the distribution, the arcsine transformation was applied to these data before statistical analyses, although figures show untransformed data. The distribution of nearest neighbor distances between mules’ ears and sagebrush was also unusual. Many of the nearest pairs of these two species were quite close (less than 20 cm) and many were quite distant (greater than 20 m). Due to of this wide spread, I transformed these nearest neighbor distances to logs and analyzed the log-transformed data.
I repeated this procedure by selecting 60 mule’s ears along an E–W transect south of lower Sagehen Creek on 9 August 2005 (39°26.67 N 120°11.05 W–39°26.85 N 120°10.78 W at an elevation of approximately 1840 m). As in the previous year, I measured the distance between each individual mule’s ears and its nearest sagebrush neighbor. I estimated the percentage leaf area removed by herbivores as described above for each focal mule’s ears plant. In order to quantify the immediate neighborhood around each focal plant, I visually estimated the percentage cover of bare ground and other plants that grew within a 0.5 m radius around each mule’s ears individual.
In 2005 I evaluated the effect of neighborhood on damage to mule’s ears individuals by including percent bare ground, percent cover by grasses, sedges, and small forbs, and percent cover by woody plants (including sagebrush and mule’s ears) as predictor variables in a regression with percentage of leaf area removed by herbivores as the response variable. I measured both the percent cover of sagebrush within a 0.5 m radius of each focal mule’s ears plant and the distance between the focal mule’s ears and its nearest sagebrush neighbor. I included both of these measures in a regression to evaluate which one best predicted the percentage of leaf area removed by herbivores. Since distance between mule’ ears and sagebrush was measured for a sample of plants in 2004 and 2005, I used data from both years to test the specific hypothesis that mule’s ears individuals that grew in close proximity to sagebrush experienced less leaf loss than mule’s ears without sagebrush neighbors. I used a model with year as a categorical effect and distance between plants as a continuous effect. All statistical analyses were performed using JMP version 5.1.
These procedures all rely on observation and correlation. To further investigate the possibility that sagebrush neighbors reduce herbivory to mule’s ears, I conducted a sagebrush removal experiment in 2006. I selected 60 mule’s ears individuals at the study site that had one or more sagebrush neighbors within 20 cm. I randomly assigned half of these plants to have their sagebrush neighbor removed. On 14 June, I cut the sagebrush stems at ground level and removed all above-ground sagebrush biomass from a radius of 0.5 m around each mule’s ears assigned to this removal treatment. Control pairs of mule’s ears and sagebrush were marked, but not otherwise disturbed. I estimated percent leaf area removed by herbivores on 19 August for each mule’s ears plant, as described above. Effects of experimental removal of sagebrush on leaf damage to neighboring mule’s ears was evaluated with ANOVA.
Is eavesdropping involved in associational resistance?
Since mule’s ears close to sagebrush experienced less leaf loss than individuals without close sagebrush neighbors, I next evaluated whether this effect required the sagebrush neighbor to be damaged. I selected 60 individuals of mule’s ears with a sagebrush neighbor whose canopy grew within 10–15 cm on 9 June 2005. Plants grew along the north side of lower Sagehen Creek (39°26.92 N 120°11.14 W at an elevation of 1817 m). At this date, mule’s ears plants were approximately 15–20 cm tall, had not initiated flowering stalks, and had received little leaf damage. I clipped the distal edge of the sagebrush foliage for the branch closest to the focal mule’s ears with scissors on 9 June and 4 July, 2005. I collected three leaves from each focal mule’s ears plant on 9 August 2005 and estimated percentage of leaf area lost to herbivores as described above. I compared the damage received for mule’s ears individuals with clipped sagebrush neighbors versus those with unclipped sagebrush neighbors using ANOVA. I conducted a post-hoc power analysis (Cohen 1988) to evaluate the probability that I may have missed a real effect of clipping sagebrush on levels of leaf loss experienced by mule’s ears.
Results
The immediate neighborhood (0.5 m) that surrounded a focal mule’s ears individual had a significant effect on how much leaf loss the focal plant experienced. A multiple regression that included percent cover by bare ground, percent cover by grasses, sedges, and small forbs and percent cover by woody perennials (including other mule’s ears individuals and sagebrush) explained 21% of the variance in the percent of leaf area lost to herbivores (P = 0.004, Table 1). Although the overall neighborhood model explained a significant amount of variance in leaf loss to herbivory, none of the individual components of the model (percent cover by bare ground, grasses, or woody perennials) were significant.
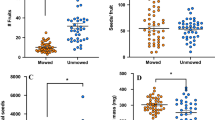
Since my initial hypothesis was that sagebrush, in particular, affects herbivory on neighboring mule’s ears, I conducted two additional analyses. I evaluated the correlation between sagebrush cover within 0.5 m radius of focal mule’s ears and the leaf loss that the focal plant experienced. This analysis was weakly suggestive that greater local sagebrush cover resulted in less damage to mule’s ears (Percent of mule’s ears leaf area lost to herbivores = −0.14 sagebrush cover +24.4, R 2 = 0.04, F 1,58 = 3.20, P = 0.08). When distance between mule’s ears to the nearest sagebrush was also included in the model, distance between the two plant species was a far better predictor of damage to mule’s ears than was percent cover by sagebrush (Table 2A). Plant cover was only measured in 2005. The strong relationship between distance separating mule’s ears and sagebrush neighbors and herbivory on mule’s ears was found for both 2004 and 2005 (Table 2B, Fig. 1). Individual mule’s ears that grew close to sagebrush experienced less loss of leaf tissue than individuals that grew farther from sagebrush. Overall levels of leaf damage were greater in 2005 than 2004 (year was significant in the model, Table 2B). However, the effect of distance on leaf loss was similar in both years (the interaction between distance and year was not significant).
Mule’s ears with sagebrush neighbors lost approximately half the leaf area to herbivores compared to mule’s ears with sagebrush neighbors removed for a radius of 0.5 m (Fig. 2, One-way ANOVA: F 1,58 = 5.92, P = 0.02). These experimental results from 2006 were consistent with the patterns observed in 2004 and 2005.
Mule’s ears individuals that were close to sagebrush suffered less damage than those that were farther from sagebrush neighbors (Fig. 1). However, damage to mule’s ears was not significantly affected by whether the sagebrush had been experimentally clipped or not (Fig. 3, One-way ANOVA: F 1,58 = 0.51, P = 0.48). Leaf loss for these plants, all of which grew within 10–15 cm of a sagebrush neighbor, was uniformly low at approximately 6–7%. This analysis was based on an experiment with 30 replicates of each of two treatments (n = 30, α = 0.05, u = 1), which provides relatively low statistical power to accept the null hypothesis and conclude that there is no effect of clipping on damage (Cohen 1988). I can be only 50–80% confident that I have not missed a real effect caused by experimental clipping of neighboring sagebrush that produced an effect of approximately the same size as those significant effects of neighborhood on damage to focal mule’s individuals (for R 2 = 0.14 as in the model in Table 2A then power = 0.54 and β = 0.46, for R 2 = 0.24 as in Table 2B then power = 0.81 and β = 0.19 following Cohen 1988).
Discussion
The plants that surround a focal mule’s ears individual are associated with its likelihood of suffering damage by herbivores. This result was consistent with my prior intuition based on field observations and was consistent with measurements taken over 2 years (Fig. 1). These observations were also corroborated by the experimental removal of sagebrush in 2006 (Fig. 2). Taken together, these results provide strong support that sagebrush neighbors reduce leaf losses by herbivores to mule’s ears. This phenomenon is biologically relevant; most mule’s ears individuals had sagebrush neighbors (the median nearest neighbor distance between these two species was 60 cm). Demonstrations of “associational resistance” are common in the literature (see “Introduction”), although we know little more now about why some plants reduce herbivory to their neighbors than we did 30 years ago when this phenomenon became widely appreciated by ecologists.
Three ecological mechanisms for associational resistance were proposed by Atsatt and O’Dowd (1976) and these can be applied to this case.
-
(1)
Sagebrush may increase the local population of predators and parasites of the herbivores of mule’s ears. This effect could decrease rates of herbivory because of reduced herbivore populations or because predators cause herbivores to feed less. This mechanism seems unlikely in this system for several reasons. The generalist herbivores (grasshoppers and deer) that removed most of the leaf tissue from mule’s ears experience relatively little predation and parasitism. For instance, excluding avian predators with cages had few demonstrable effects on arthropod densities on sagebrush (Wiens et al. 1991).
-
(2)
Sagebrush may attract herbivores away from mule’s ears. This mechanism seems unlikely in this system as well. Herbivores feed on sagebrush early and late in the season when other plants are not available (Currie and Goodwin 1966; Karban et al. 2003). During summer, generalist herbivores have access to foliage of annuals and species like mule’s ears that are functionally deciduous. At these times, they switch and concentrate their feeding on the plants that are only seasonally available. Sagebrush attracts few generalist herbivores during summer compared to mule’s ears.
-
(3)
Sagebrush may make detection of mule’s ears less likely. This could occur in a wide variety of ways. Some plants physically hide others. For example, neighbors that were large and dense reduced the likelihood that visually orienting pipevine swallowtail butterflies would discover their pipevine hosts (Rausher 1981). Some plants hide others by making it difficult for herbivores to reach the protected focal plant. For example, dense spiny canopies protected seedlings of many species in the Sonoran desert from consumption by mammalian herbivores (McAuliffe 1986, 1988). These mechanisms are unlikely to apply since sagebrush rarely physically covered mule’s ears in this system. If this mechanism were operating, all near woody neighbors of focal mule’s ears would be expected to reduce discovery and bare ground would be expected to increase discovery; neither of these patterns was found (Table 1).
Some plants may chemically hide others or deter feeding herbivores by chemical means (Visser 1986; Bernays and Chapman 1994). Previous results indicate that sagebrush emits odors that deter feeding by generalist herbivores. Several volatile compounds isolated from the headspace of sagebrush reduced the acceptability for mule deer of hay to which the chemicals had been experimentally added (Bray et al. 1991). Similarly, generalist grasshoppers that were offered a choice between feeding on excised lettuce leaves close to sagebrush (3 cm) or farther from sagebrush (30 cm) removed more leaf area from leaves far from the sagebrush neighbor (Karban and Baxter 2001). In this experiment, the grasshoppers made their feeding decisions without physically contacting the sagebrush neighbors.
In addition to the three mechanisms proposed by Atstatt and O’Dowd (1976), an additional one seems possible. Sagebrush that has been experimentally damaged emits elevated levels of volatile compounds (Karban et al. 2000; Preston et al. 2001). These volatile compounds could potentially serve as cues causing neighboring plants to respond by increasing their resistance to herbivores. Cues released by damaged sagebrush neighbors could provide induced associational resistance in neighboring plants. This mechanism could allow neighboring plants to elevate levels of defenses only under conditions when generalist herbivores were locally abundant and their risk of attack was high. At other times when generalist herbivores were less locally abundant levels of plant defenses could remain low.
Results from this system were consistent with the hypothesis that volatiles emitted by sagebrush were feeding deterrents but were inconsistent with the hypothesis that mule’s ears eavesdrop on cues emitted by damaged sagebrush to adjust their defenses against herbivory. Mule’s ears with close sagebrush neighbors experienced less leaf loss than mule’s ears with more distant sagebrush neighbors (Fig. 1, Table 2B). This effect was specific for sagebrush neighbors; mule’s ears with other neighbors did not experience a reduction in leaf damage (Table 1). Similarly, mule’s ears with sagebrush neighbors lost less leaf area than plants with sagebrush neighbors removed (Fig. 2). It is likely that this associational resistance was caused, at least in part, by generalist herbivores choosing to feed less near sagebrush (Karban and Baxter 2001). In the current study it mattered little whether the sagebrush had been experimentally damaged (Fig. 3). These results are consistent with the earlier findings that generalist grasshoppers chose not to feed near sagebrush, whether that sagebrush had been experimentally damaged or not (Karban and Baxter 2001).
This study attempts to elucidate the ecological mechanisms of associational resistance provided by neighboring sagebrush. It would be very informative to understand the physical and chemical mechanisms with which mule’s ears defends itself against herbivores. Unfortunately, I am unaware of any previous work that has considered defenses of this plant species. Such an investigation is beyond the scope of this present study.
In summary, sagebrush appears to provide associational resistance from herbivory for neighboring mule’s ears. Close proximity to sagebrush foliage deters feeding by generalist grasshoppers (Karban and Baxter 2001) and probably reduces leaf loss to these herbivores on neighboring plants. This occurs with or without damage to the sagebrush. In contrast, plant species that are attacked primarily by specialist herbivores may be less likely to gain associational resistance from growing close to sagebrush. Knowledge of the effect size and mechanism of this associational resistance allow us to begin to understand when and where it will be important. More generally, an understanding of the mechanisms of associational resistance between different plant species will allow us to predict which species will be favored as facilitation receives more notice by ecologists (Callaway 1995; Bruno et al. 2003; Gomez-Aparicio et al. 2004; Lortie et al. 2004).
References
Andow DA (1991) Vegetational diversity and arthropod population response. Annu Rev Entomol 36:561–586
Atsatt P, O’Dowd D (1976) Plant defense guilds. Science 193:24–29
Bernays EA, Chapman RF (1994) Host-plant selection by phytophagous insects. Chapman and Hall, New York
Bertness MD, Callaway RM (1994) Positive interactions in communities. Trends Ecol Evol 9:191–193
Bray RO, Wambolt CL, Kelsey RG (1991) Influence of sagebrush terpenoids on mule deer preference. J Chem Ecol 17:2053–2062
Bruno JF, Stachowicz JJ, Bertness MD (2003) Inclusion of facilitation into ecological theory. Trends Ecol Evol 18:119–125
Callaway RM (1995) Positive interactions among plants. Bot Rev 61:306–349
Callaway RM (1997) Positive interactions in plant communities and the individualistic-continuum concept. Oecologia 112:143–149
Callaway RM et al (2002) Positive interactions among alpine plants increase with stress. Nature 417:844–848
Clements FE (1916) Plant succession. Carnegie Institute of Washington, Publication #242
Cohen J (1988) Statistical power analysis for the behavioral sciences, 2nd ed. Lawrence Earlbaum, Hillsdale, New Jersey
Currie PO, Goodwin DL (1966) Consumption of forage by black-tailed jackrabbits on salt-desert ranges of Utah. J Wildl Manage 30:304–311
Feeny P (1976) Plant apparency and chemical defense. In: Wallace JW, Mansell RL (eds) Biochemical interactions between plants and insects. Recent advances in phytochemistry, vol 10. Plenum, New York, pp 1–40
Gleason HA (1926) The individualistic concept of the plant association. Bull Torr Bot Club 53:7–26
Gomez-Aparicio L, Zamora R, Gomez JM, Hodar JA, Castro J, Baraza E (2004) Applying plant facilitation to forest restoration: a metaanalysis of the use of shrubs as nurse plants. Ecol Appl 14:1128–1138
Graf M (1999) Plants of the Tahoe Basin. University of California Press, Berkeley
Hamback PA, Agren J, Ericson L (2000) Associational resistance: insect damage to purple loosestrife reduced in thickets of sweet gale. Ecology 81:1784–1794
Jermy T (1984) Evolution of insect/host relationships. Am Nat 124:609–630
Jermy T (1993) Evolution of insect–plant relationships – a devil’s advocate approach. Entomol Exp Appl 66:3–12
Karban R, Baldwin IT, Baxter KJ, Laue G, Felton, GW (2000) Communication between plants: induced resistance in wild tobacco plants following clipping of neighboring sagebrush. Oecologia 125:66–71
Karban R, Baxter KJ (2001) Induced resistance in wild tobacco with clipped sagebrush neighbors: the role of herbivore behavior. J Insect Behav 14:147–156
Karban R, Maron J (2002) The fitness consequences of interspecific eavesdropping between plants. Ecology 83:1209–1213
Karban R, Maron J, Felton GW, Ervin G, Eichenseer H (2003) Herbivore damage to sagebrush induces wild tobacco: evidence for eavesdropping between plants. Oikos 100:325–333
Karban R, Huntzinger M, McCall AC (2004) The specificity of eavesdropping on sagebrush by other plants. Ecology 85:1845–1852
Karban R, Shiojiri K, Huntzinger M, McCall AC (2006) Damage-induced resistance in sagebrush: volatiles are key to intra- and interplant communication. Ecology 87:922–930
Kareiva P (1983) Influence of vegetation texture on herbivore populations: resource concentration and herbivore movement. In: Denno RF, McClure MS (eds) Variable plants and herbivores in natural and managed systems. Academic Press, New York, pp 259–289
Kelsey RG, Stevenson TT, Scholl JP, Watson TJ, Shafizadeh F (1978) The chemical composition of the litter and soil in a community of Artemisia tridentata spp. vaseyana. Biochem Syst Ecol 6:193–200
Kennedy PB, Doten SB (1901) A preliminary report on the summer ranges of western Nevada sheep. Nevada State University, Agricultural Experiment Station, Reno, NV Bulletin #51, 57 pp
Lortie CJ, Brooker RW, Choler P, Kikvidze Z, Michalet R, Pugnaire FI, Callaway RM (2004) Rethinking plant community theory. Oikos 107:433–438
Louda SM (1982) Limitation of recruitment of the shrub Haplopappus squarrosus (Asteraceae) by flower- and seed-feeding insects. J Ecol 70:43–53
Maron JL, Vila M (2001) When do herbivores affect plant invasion? Evidence for the natural enemies and biotic resistance hypotheses. Oikos 95:361–373
Marquis RJ (1992) The selective impact of herbivores. In: Fritz RS, Simms EL (eds) Plant resistance to herbivores and pathogens. University of Chicago Press, Chicago, pp 301–325
McAuliffe JR (1986) Herbivore-limited establishment of a Sonoran desert tree, Cercidium microphyllum. Ecology 67:276–280
McAuliffe JR (1988) Markovian dynamics of simple and complex desert plant communities. Am Nat 131:459–490
Milchunas DG, Noy-Meir I (2002) Grazing refuges, external avoidance of herbivory and plant diversity. Oikos 99:113–130
Parker VT, Yoder-Williams MP (1989) Reduction of survival and growth of young Pinus jeffreyi by an herbaceous perennial, Wyethia mollis. Am Midl Naturalist 121:105–111
Personius TL, Wambolt CL, Stephens JR, Kelsey RG (1987) Crude terpenoid influence on mule deer preference for sagebrush. J Range Manage 40:84–88
Pickford GD (1932) The influence of continued heavy grazing and of promiscuous burning on spring-fall ranges in Utah. Ecology 13:159–171
Preston CA, Laue G, Baldwin IT (2001) Methyl jasmonate is blowing in the wind but can it act as a plant–plant airborne signal?. Biochem Syst Ecol 29:1007–1023
Rausher MD (1981) The effect of native vegetation on the susceptibility of Aristolochia reticulata (Aristolochiaceae) to herbivore attack. Ecology 62:1187–1195
Shelton AM, Badenes-Perez FR (2006) Concepts and applications of trap cropping in pest management. Annu Rev Entomol 51:285–308
Tahvanainen JO, Root RB (1972) The influence of vegetational diversity on the population ecology of a specialized herbivore, Phyllotreta cruciferae (Coleoptera: Chrysomelidae). Oecologia 10:321–346
Visser JH (1986) Host odor perception in phytophagous insects. Annu Rev Entomol 31:121–144
Whittaker RH (1967) Gradient analysis of vegetation. Biol Rev Cambridge Philos Soc 42:207–264
Wiens JA, Cates RG, Rotenberry JT, Cobb N, Van Horne B, Redak RA (1991) Arthropod dynamics on sagebrush (Artemisia tridentata): effects of plant chemistry and avian predation. Ecol Monogr 61:299–321
Young JA, Evans RA, Major J (1988) Sagebrush steppe. In: Barbour MG, Major J (eds) Terrestrial vegetation of California, 2nd ed. California Native Plant Society Special Publication #9, pp 763–769
Acknowledgments
I thank Mikaela Huntzinger and Claire Karban for help in the field, and Claire Karban, Jesse Karban, Aaron Combs, and Louie Yang for helping measuring leaf damage. Andy McCall, Louie Yang, and Truman Young improved the manuscript. I benefitted from facilities at the UC Sagehen Creek field station and Jeff Brown facilitated this research in numerous ways. This work was conducted in the Tahoe National Forest adjacent to the field station.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karban, R. Associational resistance for mule’s ears with sagebrush neighbors. Plant Ecol 191, 295–303 (2007). https://doi.org/10.1007/s11258-006-9243-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11258-006-9243-z