Abstract
Objective
To evaluate the safety and effectiveness of an individualized regional citrate anticoagulation (RCA) protocol for hemodialysis.
Methods
In this single-center, retrospective study, blood coagulation in the extracorporeal circulation, adverse reactions, in vivo ionized calcium (iCa2+) concentrations, and the infusion dose of citrate during RCA in hemodialysis were observed in 98 patients from February 2021 to March 2022.
Results
A total of 98 patients underwent RCA during hemodialysis 362 times, and blood coagulation occurred in the extracorporeal circulation 29 times. Among the 29 cases of coagulation, most of the patients exhibited hypercoagulability, and among approximately 80% of the treatments, the deviation between the actual infusion rate of citrate in the extracorporeal circulation and the theoretical value was ± 10%. After hemodialysis, pH values and bicarbonate ion (HCO3−) levels were clearly improved, and online conductivity monitoring (OCM) values and blood coagulation scores in the extracorporeal circulation were identical to those measured in similar studies.
Conclusion
An individualized RCA protocol for hemodialysis is safe, effective, simple, and inexpensive and can meet the needs of individualized treatment; therefore, its application is worthy of promotion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The utilization of RCA technology during hemodialysis was first reported by Morita in 1961 [1]. This technology is highly effective for anticoagulation without affecting the whole blood coagulation state, and its superiority is reflected in its anti-inflammatory role and improved biocompatibility with dialyzers. However, the lack of standardized protocols, challenges in achieving individualized treatment, operational complexity, and high costs have hampered the wider application of this technology in clinical treatment. Previously, a single-center, prospective, randomized controlled clinical study conducted in our center [2] proposed and verified an individualized protocol of RCA for hemodialysis, and this protocol has since been routinely used in our center. In this study, the clinical data of patients undergoing RCA for hemodialysis from February 2021 to March 2022 were reviewed.
Methods
Research design and objects
In this single-center, retrospective clinical study, patients who underwent RCA for hemodialysis in our center from February 2021 to March 2022 were enrolled and routinely treated with an individualized RCA protocol for hemodialysis [2]. Doctors could adjust this regional anticoagulation protocol based on conventional treatment considering specific clinical conditions. In addition, they chose hemodialysis equipment, consumables, and vascular access and set hemodialysis parameters according to clinical needs. The inclusion criteria were as follows: (1) patients who underwent hemodialysis and (2) those who received RCA for active bleeding or highly suspected bleeding and other situations. The exclusion criteria were (1) severe liver function damage, with total bilirubin (TBIL) ≥ 60 μmol/L; (2) uncorrectable hygienic bubble trapping, with blood pressure (BP) < 90/60 mmHg; (3) hypoxemia, with a partial pressure of oxygen (PO2) < 60 mmHg; or (4) lactic acidosis, with lactic acid (Lac) > 3 mmol/L. The catheter was sealed with heparin sodium after dialysis. This study was approved by the Ethics Committee of our hospital (Court Judgment No. 2022.08).
Equipment and materials
The equipment and materials used in this study included the following: Fresenius 4008S Hemodialysis Machine (Fresenius Medical Care, Bad Homburg, Germany); Gambro AK96 Hemodialysis Machine (Gambro Lundia AB, Lund, Sweden); Wesley W-T2008-B Hemodialysis Machine (Wesley Biotech, Chengdu, China); Fresenius Hemoflow F6HPS Low-Flux Dialyzer with a surface area of 1.3 m2 and Fresenius FX80 High-Flux Dialyzer with a surface area 1.8 m2 (Fresenius Medical Care, Frankfurter, Germany); Delang B-16H, B-18H, and B-20H High-Flux Dialyzers with surface areas of 1.6 m2, 1.8 m2, and 2.0 m2, respectively [Bain Medical Equipment (Guangzhou) Co., Ltd., Guangzhou, China]; Sanxin SM-160H, SM-180H, and SM-200H High-Flux Dialyzers with surface areas of 1.6 m2, 1.8 m2, and 2.0 m2, respectively (Jiangxi Sanxin Medtec Co., Ltd., Nanchang, China); SXG-Y-A/B dialysate with 137 mmol/L sodium ion (Na+), 2.0 mmol/L potassium ion (K+), 1.5 mmol/L calcium ion (Ca2+), 0.5 mmol/L magnesium ion (Mg2+), 108 mmol/L chlorine ion (C1−), 31 mmol/L bicarbonate ion (HCO3−), and 4.0 mmol/L acetate ion (CH3COO−) (Jiangxi Sanxin Medtec Co., Ltd., Nanchang, China); 4% trisodium citrate (an anticoagulant, Chengdu Qinshan Likang Pharmaceutical Co., Ltd., Chengdu, China); and a Werfen GEM4000 Blood Gas Analyzer (Werfen Group, Barcelona, Spain).
RCA protocol
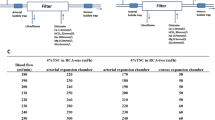
Per the individualized protocol for RCA [2], the required citrate infusion rate was calculated according to the systemic ionized calcium (iCa2+) concentration and blood flow velocity at the beginning of hemodialysis and at 1 h after hemodialysis initiation. Four-fifth was infused from the arterial segment of the extracorporeal circulation and one-fifth from the venous bubble trap on the extracorporeal circulation.
Laboratory inspection items
Blood samples were collected from the patients at the beginning of hemodialysis and at 1 h after hemodialysis began, and systemic iCa2+, HCO3−, and pH were detected using a blood gas analyzer. The analyzed items and times could be increased in light of specific clinical conditions.
Blood sampling methods
Blood sampling method at the beginning of hemodialysis: blood samples were collected from the internal fistula of patients who had been successfully punctured using an internal fistula needle without preflushing with normal saline. For patients who underwent central venous catheter placement, 20 mL of blood was extracted for blood sampling after the tube-sealing liquid in the catheter was drawn out. Blood sampling method during hemodialysis: at 3 min after termination of the infusion of trisodium citrate solution at the arterial end and the venous bubble trap, blood was sampled at the arterial blood collection point of the extracorporeal circulation. Blood sampling method at the end of hemodialysis: at the end of hemodialysis, the infusion of trisodium citrate solution was terminated, the dialysate bypass was closed, ultrafiltration was stopped, and blood samples were collected from the arterial blood collection point of the extracorporeal circulation 3 min later.
Evaluation of the effectiveness of anticoagulation
After hemodialysis, the semiquantitative method [3] was adopted by the hemodialysis nurse to evaluate the blood coagulation in the filter, arterial bubble trap, and venous bubble trap. A higher score indicated more effective anticoagulation. The scoring method for blood coagulation in the arterial and venous bubble traps was as follows: five points—no visible blood coagulation; four points—fibrin formation during coagulation; three points—formation of small blood clots (< 2 mL); two points—formation of large blood clots (≥ 2 mL); and one point—complete blood coagulation in the bubble traps. The scoring method for blood coagulation in the filter was as follows: five points— < 20 coagulated fibers; four points—20‒50 coagulated fibers; three points—51‒100 coagulated fibers; two points— > 100 coagulated fibers; and one point— > 20% coagulated fibers.
The definition and evaluation of circuit clotting
Our definition of clotting is that cardiopulmonary bypass cannot continue, and treatment needs to be interrupted. The main evaluation indicators were dialyzer coagulation consisting of three or more events and a continuous cross-molding pressure of more than 350 mmHg, as well as an assessment by the two senior nurses that the treatment could not continue.
Statistical methods
Statistical analysis was conducted using SPSS 24.0 software (IBM Inc., NC, USA), and measurement data are expressed as the mean ± standard deviation. The means of normally distributed data were compared between two groups by the independent samples t test, while the means of data without a normal distribution were compared by the Mann–Whitney U test. In addition, comparisons among multiple groups were performed by one-way analysis of variance, and intragroup comparisons were carried out by the least significant difference (LSD) test. Count data are expressed as the frequency distribution and corresponding percentage, and percentages were compared using the Chi-square test. Fisher's exact test was used if the Chi-square test conditions were not met. Moreover, the influences of multiple variables on coagulation in the extracorporeal circulation were examined via binary logistic regression analysis. P < 0.05 represented a statistically significant difference.
Results
The 98 enrolled patients received RCA a total of 362 times. All patients received at least 1 administration, and the maximum number of administrations was 42, with an average of 3.69. These patients were aged 23–87 years, with an average age of 56.78 ± 14.92 years. They underwent RCA mainly for active bleeding, highly suspected bleeding, trauma, recent surgery, idiopathic thrombocytopenia, and heparin-induced thrombocytopenia (HIT). Their demographic information and clinical features are shown in Table 1.
Among the 362 RCA administrations, blood coagulation in the extracorporeal circulation occurred 29 times, with an incidence of 8%. During RCA, lip numbness appeared in two cases and was relieved after terminating RCA in one case and after reducing the infusion dose of citrate in the other case. Moreover, the systemic iCa2+ in one case was lower than 0.85 mmol/L at 1 h after hemodialysis and increased after intravenous infusion of 10 mL of 10% calcium gluconate. One case developed hypotension and was relieved after reducing ultrafiltration. All treatments were divided into two groups according to the occurrence of extracorporeal circulation coagulation. The clinical characteristics of the two groups are shown in Table 2. Binary logistic regression analysis was conducted on the possible factors affecting coagulation in the extracorporeal circulation, and the results are shown in Table 3.
Comparison revealed that the actual infusion rate of citrate in nearly 80% of the patients deviated from the theoretical infusion rate within ± 10% (Table 4). Blood coagulation in most patients with a deviation greater than ± 10% was affected by clinical factors influencing the blood coagulation state, such as HIT, severe hypoproteinemia, tumors, severe infection, and long-term bed rest. Eighty-two of 83 treatments with deviations greater than ± 20% were associated with clinical factors affecting coagulation status, and 64 of 69 treatments with deviations < -20% and < -10% or 10% and < 20% were associated with clinical factors affecting coagulation status.
Discussion
In contrast to heparin anticoagulation, RCA has no influence on systemic coagulation function and can prevent inflammation and enhance filter biocompatibility [4, 5]. Consequently, RCA has become the optimal method of anticoagulation in continuous renal replacement therapy (CRRT) [6]. Although RCA is also conducive to hemodialysis patients, it is adopted only for a small number of hemodialysis patients with active bleeding or highly suspected bleeding. Its routine use in hemodialysis patients is mainly limited by operational complexity, the lack of standardized protocols, challenges in achieving individualized treatment, and the high cost.
During RCA, systemic iCa2+ is the primary factor influencing effectiveness and safety, and previous studies have shown that systemic iCa2+ is stable in this process [2]. After treatment, systemic iCa2+ increased compared with that before treatment (Table 1), which was caused by the higher rate of citrate infusion in some patients, a more obvious decrease in systemic iCa2+, and greater calcium diffusion from the dialysate into blood. As hemodialysis has a strong small-molecule solute exchange capacity, the fluctuation range of systemic iCa2+ gradually decreases and tends toward a steady state with treatment progression. Table 1 shows that the fluctuation range of systemic iCa2+ gradually decreases with increasing treatment time. Citrate removal during hemodialysis is mainly achieved by two procedures, namely, in vivo metabolism and filters. Reportedly, 2 h is required for citrate metabolism to plateau in critically ill patients treated with CRRT [7]. In this study, only noncritically ill patients were included, and the removal rate of citrate by in vivo metabolism in noncritically ill patients was higher than that in critically ill patients. Hemodialysis performs better than CRRT in citrate removal [8], resulting in speculation that approximately 1 h is required for the citrate concentration to stabilize in vivo. As systemic iCa2+ is basically stable during RCA in hemodialysis and citrate metabolism in vivo also becomes stable at approximately 1 h, measuring systemic iCa2+ at the beginning of hemodialysis and at 1 h after hemodialysis initiation can guarantee the safety of RCA. In this study, hypocalcemia occurred only in 3 of 362 administrations. After hemodialysis, blood pH and HCO3− were markedly improved (Table 1), with no evident acid–base imbalance, which indicated the safety of the protocol.
Calcium in blood consists of iCa2+, protein-bound calcium, and chelated calcium; iCa2+ has biological activity [9] and is needed during coagulation [10]. The principle of RCA is that citrate chelates iCa2+ in blood in the extracorporeal circulation to reduce its concentration and inhibit blood coagulation. The effectiveness of RCA can be affected by many factors, which are dominated by blood flow velocity and systemic iCa2+. In the traditional protocol of RCA for hemodialysis, the blood flow velocity is kept constant, and an empirical citrate infusion rate is set based on this velocity regardless of the influence of the systemic iCa2+ on the effectiveness of anticoagulation. When the concentration of systemic iCa2+ is lower or higher, a relatively large dose of citrate may lead to hypocalcemia, or a relatively small dose of citrate may result in coagulation in the extracorporeal circulation. Continuously monitoring systemic iCa2+ and iCa2+ in the dialyzer can clearly address this problem, but this process is complex and increases the cost accordingly. In some hemodialysis centers, systemic iCa2+ is not monitored at all, and the safety and effectiveness of RCA are ensured based on experience, which can be highly risky. According to the linear relationship between the concentration of citrate in whole blood in the extracorporeal circulation and systemic iCa2+, two parameters, blood flow velocity and systemic iCa2+, were included in this protocol to more accurately set the dose of citrate to determine the infusion rate of citrate. At 1 h after hemodialysis initiation, the effectiveness of anticoagulation could be adequately ensured by revising the citrate infusion rate according to the blood flow velocity and the systemic iCa2+, and the iCa2+ in the dialyzer was not detected. Blood coagulation occurred in the extracorporeal circulation in 29 of 362 administrations, corresponding to an incidence rate of 8%, which is close to the results of Lin et al. [11]. The coagulation score of each segment of the extracorporeal circulation circuit approaches was obtained in our previous research [2]. The scores of the coagulation group were all lower than those of the non-coagulation group (Table 2). The venous pot scored the lowest, due to the fact that the blood with low ionic calcium content chelated by citrate was passed through the dialyzer, calcium ions in the dialysate were added to the blood through the dialyzer, and the blood ionic calcium levels in the venous pot were restored. At the fourth hour of treatment, the changes in venous pressure and transmembrane pressure (TMP) in the coagulation group were significantly higher than those in the non-coagulation group, suggesting that more attention should be directed toward the changes in venous pressure and transmembrane pressure in the treatment process, especially in the later stage of treatment (Table 2). The adequacy of dialysis examined by online conductivity monitoring (OCM) can also indirectly reflect the effectiveness of anticoagulation. In this study, the OCM value was 1.06 ± 0.24 (Table 1), which is close to the value of 1.02 ± 0.15 measured by Khalid Al Saran et al. [12]. These results all demonstrated that this protocol exhibited a good anticoagulation effect with a simple operation and low cost. Binary logistic regression analysis was conducted on factors that may affect coagulation in the extracorporeal circulation, and vascular access, dialyzer performance, and the whole blood citrate concentration in the extracorporeal circulation were found to be correlated, while no correlation was found between systemic iCa2+, ultrafiltration velocity, and blood flow velocity at the beginning of treatment (Table 3). Thus, the individualized RCA protocol can correct the influence of systemic iCa2+ and different blood flow velocities on coagulation in the extracorporeal circulation, and an individualized infusion dose of citrate can ensure that the whole blood citrate concentration in the extracorporeal circulation is in an appropriate range. Additionally, some non-anticoagulant factors can affect the occurrence of coagulation in the extracorporeal circulation. Compared with central venous catheter use, arteriovenous fistula use is less prone to coagulation, and the central venous catheter is more prone to poor blood flow and machine stop-pump alarms, increasing the probability of coagulation in the extracorporeal circulation [13]. The low-flux dialyzer had a lower scavenging capacity for citrate than the high-flux dialyzer, and the citrate concentrations in the dialyzer and venous bubble traps were relatively higher and the clotting rate was lower. Blood flow velocity is not a predictor because the calcium ions in the blood can better chelate with citrate to reduce the risk of clotting; however, the slow blood flow velocity itself is prone to clotting, and the two effects cancel each other, so the blood flow velocity cannot predict the occurrence of clotting.
Calatzis et al. [14] studied the influences of the whole blood citrate concentration on the iCa2+ level and coagulation function and found that the anticoagulation effect can be satisfactory when the whole blood citrate concentration is within the range of 2.26–3.39 mmol/L. The average whole blood citrate concentration in the extracorporeal circulation in this study is consistent with the results of Calatzis et al. (Table 1). Citrate cannot pass through the erythrocyte membrane [15]. Generally, the plasma citrate concentration is more valuable than the whole blood citrate concentration in RCA. The specific volume of hemoglobin was not included in the linear formula in the protocol used in this study because when the citrate infusion dose is constant, patients with a larger volume of hemoglobin have a lower plasma content and higher plasma citrate concentration. In these patients, the blood is more concentrated and prone to coagulation, which counteracts the effect of a high plasma citrate concentration to some extent. Hemodialysis requires less time than CRRT, and the requirement for the accuracy of the citrate infusion dose is also lower than that of CRRT. In addition, because the specific volume of hemoglobin is not tested, the operation is simplified, and the cost is reduced. No difference in hematocrit (HCT) changes was observed between the coagulation group and the non-coagulation group before and after treatment (Table 2), indicating that blood concentration under the individualized RCA protocol has little influence on coagulation. Regarding actual effectiveness, the deviation of the actual infusion rate of citrate from the theoretical rate was within ± 10% in nearly 80% of the treatments (Table 4), revealing the good applicability of this protocol. The deviation in this part is mainly because the rate of citrate infusion is set as an integer close to the theoretical value for convenience in clinical practice. In addition, the accuracy of infusion pumps and connecting pipes is not sufficient, and the problem of mismatching also has an impact and needs to be corrected regularly. An actual infusion rate deviation > ± 10% is usually associated with clinical factors that significantly affect coagulation status, such as high blood coagulation caused by HIT, severe hypoproteinemia, tumors, and long-term bed rest [16,17,18,19,20,21,22,23], which requires a full coagulation status evaluation in patients before treatment and timely adjustment of the anticoagulation program.
Conclusion
This study verified the safety, effectiveness, and applicability of an individualized RCA protocol for hemodialysis under real and complex clinical conditions. This protocol can meet the needs of individualized treatment, reduce the cost of treatment, and reduce restrictions on the use of RCA in hemodialysis to some extent. Limitations were also found in patients with significant hypercoagulability. As a retrospective study, this study also has certain limitations. First, this study did not enroll general hemodialysis patients, and no control group was included. The next step is to conduct a multicenter, prospective, parallel-controlled, and randomized clinical study with low-molecular-weight heparin as a control to confirm the differences in effectiveness and safety between an individualized RCA protocol and a conventional low-molecular-weight heparin anticoagulation protocol in general hemodialysis patients. Second, the sample size was not accurately calculated. Finally, we have multiple treatments for the same patient that may be biased.
Szamosfalvi et al. [24] proposed an automated RCA protocol more than 10 years ago, but it was not incorporated into practice. We hold that developing special automated RCA equipment and connecting catheters based on this protocol can simplify the operation and improve infusion accuracy, which is expected to be superior to anticoagulation using low-molecular-weight heparin in terms of effectiveness and adverse reactions while providing similar operational convenience and cost.
Data availability
Data are available from the corresponding author upon reasonable request.
References
Morita Y, Johnson RW, Dorn RE et al (1961) Regional anticoagulation during hemodialysis using citrate. Am J MedicalSci 242(1):32
Luo L, Fan M, Chen Q et al (2021) A simplified protocol for individualized regional citrate anticoagulation for hemodialysis: a single-center, randomized clinical study. Medicine 100(8):e24639
Buturovic-Ponikvar J, Cerne S, Gubensek J et al (2008) Regional citrate anticoagulation for hemodialysis: calcium-free Vs. calcium containing dialysate—a randomized trial. Int J Artif Organs 31(5):418–424
Tolwani A, Wille KM (2012) Advances in continuous renal replacement therapy: citrate anticoagulation update. Blood Purif 34(2):88–93
Oudemans-Van-Straaten H, Bosman RM, Van-Der-Voort P et al (2009) Citrate anticoagulation for continuous venovenous hemofiltration. Crit Care Med 37(2):545
Khwaja A (2012) KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 120(4):c179–c184
Kramer L, Bauer E, Joukhadar C et al (2003) Citrate pharmacokinetics and metabolism in cirrhotic and noncirrhotic critically ill patients. Crit Care Med 31(10):2450–2455
Hartmann J, Strobl K, Fichtinger U et al (2012) In vitro investigations of citrate clearance with different dialysis filters. Int J Artif Organs 35(5):352–359
Song L (2017) Calcium and bone metabolism indices. Adv Clin Chem 82:1–46
Umerah CO, Momodu II. Anticoagulation. 2021 Dec 27. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan–. PMID: 32809486.
Lin T, Song L, Huang R et al (2019) Modified regional citrate anticoagulation is optimal for hemodialysis in patients at high risk of bleeding: a prospective randomized study of three anticoagulation strategies. BMC Nephrol 20(1):472
Al Saran K, Sabry A, Abdulghafour M, Yehia A (2010) Online conductivity monitoring of dialysis adequacy versus Kt/V derived from urea reduction ratio: a prospective study from a Saudi Center. Ren Fail 32(1):36–40
Masud A, Costanzo EJ, Zuckerman R, Asif A (2018) The complications of vascular access in hemodialysis. Semin Thromb Hemost 44(1):57–59
Calatzis A, Toepfer M, Schramm W et al (2001) Citrate anticoagulation for extracorporeal circuits: effects on whole blood coagulation activation and clot formation. Nephron 89(2):233–236
Kozik-Jaromin J (2005) Citrate kinetics during regional citrate anticoagulation in extracorporeal organ replacement therapy. universität freiburg
Arepally, Gowthami, M (2017) Heparin-induced thrombocytopenia. Blood: J Am Soc Hematol 129(21):2864–2872
Debaugnies F, Azerad MA, Noubouossié D et al (2010) Evaluation of the procoagulant activity in the plasma of cancer patients using a thrombin generation assay. Thromb Res 126(6):531–535
LinnemannB L-L (2012) Risk factors, management and primary prevention of thrombotic complications related to the use of central venous catheters. Vasa 41(5):319–332
De Pietri L, Montalti R, Beqliomini B et al (2010) Thromboelastographic changes in liver and pancreatic cancer surgery: hypercoagulability, hypocoagulability or normocoagulability? Eur J Anaesthesiol 27(7):608–616
Saccullo G, Malato A, Raso S et al (2012) Cancer patients requiring interruption of long-term warfarin because of surgery or chemotherapy induced thrombocytopenia: the use of fixed sub-therapeutic doses of low-molecular weight heparin. Am J Hematol 87(4):388–391
Huang MJ, Wei RB, Li QP et al (2016) Hypercoagulable state evaluated by thromboelastography in patients with idiopathic membranous nephropathy. J Thrombosis Thrombolysis 41(2):321–327
Levi M, Tom V (2017) Coagulation and sepsis. Thrombosis Res 149:38–44
Liew N, Alemany G, Angchaisuksiri P et al (2017) Asian venous thromboembolism guidelines: updated recommendations for the prevention of venous thromboembolism. Int Angiol 36(1):1
Szamosfalvi B, Frinak S, Yee J (2010) Automated regional citrate anticoagulation: technological barriers and possible solutions. Blood Purif 29(2):204–209
Funding
This study was supported by Nanchang Medical and health science and technology Support Plan (2022-KJZC-027).
Author information
Authors and Affiliations
Contributions
LL designed the study, organized the research, conducted the statistical analysis, and revised the paper; YF, FW, and MZ collected the data, conducted the statistical analysis, and wrote the paper. YG, ZH, TJ, and CG implemented the individualized protocol of regional citrate anticoagulation in hemodialysis, collected blood samples, and scored blood coagulation in the extracorporeal circulation.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fan, Y., Wu, F., Zou, M. et al. An individualized regional citrate anticoagulation protocol for hemodialysis: a real-world retrospective study. Int Urol Nephrol 56, 295–302 (2024). https://doi.org/10.1007/s11255-023-03677-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-023-03677-z