Abstract
Purpose
To investigate the soluble Klotho (sKlotho) and fibroblast growth factor-23 (FGF-23) levels and echocardiographic findings in type 1 diabetic patients with no or early diabetic nephropathy.
Methods
A total of 147 subjects (mean age 34.1 ± 9.2 years, 55.8 % were females) including type 1 diabetic patients with glomerular filtration rate (GFR) >60 ml/min (n = 71, mean age 34.3 ± 9.5 years, 54.9 % were females) and healthy controls (n = 76, mean age 33.9 ± 9.1 years, 56.6 % were females) were included in this study. Data on demographic characteristics, blood biochemistry, urinalysis, diabetes-related complications and echocardiography were recorded. Serum levels for sKlotho and FGF-23 were determined by ELISA method.
Results
Patient and control groups were similar in terms of mean sKlotho (509.2 ± 183.5 and 547.6 ± 424.0 pg/ml, respectively) and FGF-23 (76.2 ± 15.6 and 77.2 ± 15.1 pg/ml, respectively) levels as well as echocardiographic findings. No significant correlation of sKlotho (pg/ml) and FGF-23 (pg/ml) levels with cardiac parameters was noted among diabetic patients. In subgroup analysis, the correlations between FGF-23 levels and isovolumic relaxation time (ms) and early diastolic velocity at medial/septal annulus (E’med) (m/s) were significant only in patients with early diabetic nephropathy (DN) but not in non-DN patients. No significant association of sKlotho levels with echocardiographic findings was noted.
Conclusions
Our findings in young adult type 1 diabetic patients with GFR >60 ml/min versus healthy controls revealed no difference between groups in terms of sKlotho and FGF-23 levels and echocardiographic findings, while a significant correlation of FGF-23 (pg/ml) levels and diastolic dysfunction was noted only in patients with DN.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite the 100 times greater risk of cardiovascular death in type 1 diabetic patients with diabetic nephropathy than in the general population [1], nephropathy is not present in the majority of type 1 diabetic patients who develop cardiovascular disease (CVD) [2].
While being frequent and characterized by severe clinical course, cardiovascular complications in type 1 diabetic patients are often underestimated and undertreated since they arise early in the disease [2]. Accordingly, consideration of cardiac disease as early as possible has been considered crucial for the appropriate control of established cardiovascular risk factors and to offer early cardiac follow-up in the affected patients [2].
However, cardiovascular complications have been more frequently discussed in patients with type 2 diabetes than in type 1 diabetes patients [3] with a serious lack of information concerning CVD in type 1 diabetes [2]. Scarce and inconclusive data are available on type 1 diabetes without diabetic nephropathy [4, 5].
Consistent with the recently identified critical role in the regulation of systemic calcium–phosphate homeostasis and metabolic network besides major well-established classical controllers [6, 7], the endocrine axis mediated by Klotho and FGF-23 has been the subject of intense research as the potential target for therapeutic interventions in multiple metabolic disorders including diabetes, obesity, hypercholesterolemia, chronic kidney disease (CKD) and bone disorders as well as human aging [6–8].
Named after the purported Greek goddess, Klotho, who spins the thread of life [9], Klotho was first identified as an aging-related gene and later demonstrated to be involved in the regulation of phosphate and vitamin D metabolism as an obligated cofactor for the binding of FGF-23 to its receptors, and sKlotho was shown to act as an endocrine factor with a multitude of renal and extra-renal effects [6, 10].
Among the functions of sKlotho, human longevity [6], regulation of insulin/insulin-like growth factor-1 signaling and suppression of oxidative stress, protection against endothelial dysfunction, regulation of the production of nitric oxide (NO) and participation in the maintenance of calcium homeostasis have been considered [9].
sKlotho seems to participate in important pathways of diabetic nephropathy in type 2 diabetes mellitus, like oxidative stress and endothelial dysfunction [4], and is suggested to protect the cardiovascular system by increasing nitric oxide production and inhibiting oxidative stress [9].
FGF-23 belongs to the so-called endocrine FGFs and is characterized for requiring sKlotho as a cofactor to exert its phosphaturic actions [6]. As a hormone secreted by osteocytes that plays a fundamental role in the regulation of phosphate and vitamin D metabolism [10, 11], high plasma concentrations were associated with more rapid kidney disease progression, [12, 13] CVD, heart failure and all-cause mortality in older adults [14] with much stronger associations evident in participants with CKD [14, 15].
High plasma FGF-23 [16, 17] and low plasma sKlotho [16] levels have been reported to contribute to ectopic calcification in soft tissues including the aorta and cardiac hypertrophy in CKD [10]. High FGF-23 concentrations were reported to be associated with left ventricular hypertrophy (LVH), cardiovascular events and death in patients with CKD [15, 18]. However, despite their putative role in CVD, the association between FGF-23/Klotho axis and vascular function, both in CKD and in subjects with normal renal function, remains uncertain [15, 18].
Likewise, scarce amount of data are available on the relation of FGF-23/Klotho axis in diabetic nephropathy [4], and to our knowledge, the role of FGF-23/Klotho axis in type 1 diabetic patients with no or early diabetic nephropathy has not been described previously.
Given that the clarification of the role of FGF-23/Klotho endocrine axis may provide new insight into mechanisms of cardiovascular risk in diabetes with or without early nephropathy, the present study was designed to evaluate sKlotho and FGF-23 levels in type 1 diabetic patients with glomerular filtration rate (GFR) >60 ml/min versus controls in relation to demographics, calcium and phosphate homeostasis, diabetes-related complications and cardiac function.
Methods
Study population
A total of 147 subjects (mean age 34.1 ± 9.2 years, 55.8 % were females) including type 1 diabetic patients with GFR >60 ml/min (n = 71, mean age 34.3 ± 9.5 years, 54.9 % were females) and healthy controls (n = 76, mean age 33.9 ± 9.1 years, 56.6 % were females) were included in this study conducted between February 2012 and March 2013 at Istanbul Medeniyet University Goztepe Training and Research Hospital, Istanbul. Type 1 diabetic patients with pregnancy, valvular heart disease, chronic inflammatory or infectious diseases, hypertension and GFR <60 ml/min, macroalbuminuria or a renal disease apart from diabetic nephropathy were excluded.
Written informed consent was obtained from each subject following a detailed explanation of the objectives and protocol of the study which was conducted in accordance with the ethical principles stated in the “Declaration of Helsinki” and approved by the institutional ethics committee (Protocol No. 14.02.2012/19/J-29/e).
Study parameters
Data on demographic characteristics (age and gender), anthropometrics [body mass index (BMI, kg/m2)], waist circumference (cm), blood biochemistry (fasting blood glucose, HbA1c, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglyceride, TSH, calcium, phosphate, parathyroid hormone (PTH), vitamin D, alkaline phosphatase (ALP), albumin, creatinine, urea, creatinine clearance and albuminuria) and eGFR (calculated using Cockcroft–Gault formula) were recorded in each subject. Echocardiographic findings including peak early diastolic myocardial tissue velocity (E), peak late (atrial contraction) diastolic myocardial tissue velocity (A), isovolumic relaxation time (IVRT), mitral E-wave deceleration time (Dec T), early diastolic velocity at lateral annulus (E’lat), early diastolic velocity at medial/septal annulus (E’med), systolic and diastolic blood pressure, aortic strain and distensibility of the ascending aorta (Ao Dis) were determined in each subject. Serum levels for sKlotho and FGF-23 were evaluated in relation to demographic characteristics, biochemical and echocardiographic findings in patient versus control groups, along with subgroup analysis with respect to the presence of early diabetic nephropathy in diabetic patients.
Biochemical assessments
In both patient and control groups, morning blood samples were taken after an overnight fasting period. Blood samples were left to clot for 2 h at room temperature and then centrifuged at 1500g for 10 min. Serum samples were stored at −80 °C until analysis. Due to its low stability even at −80 °C, sKlotho levels were analyzed within 2 months after serum samples were frozen. sKlotho levels were determined by ELISA method using the Klotho ELISA kit (Cusabio Biotech Co., Ltd., P.R. China), according to the manufacturer’s guidelines. The minimum detectable level of the test was 1.56 ng/ml. The intra- and inter-assay coefficients of variability of the test were <8 and <10 %, respectively. The normal mean values of serum Klotho levels were found to be 472 pg/ml (2.5–97.5 % reference limits: 204–741 pg/ml) with no difference between genders by using ELISA method [19]. To measure the levels of serum FGF-23, a commercial ELISA kit (EMD Millipore Corp., USA) was used. The limit of sensitivity of this assay was 3.5 pg/ml. The intra- and inter-assay coefficients of variability of the test at the concentrations of 75.7 and 78.5 pg/ml were 11.2 and 2.45 %, respectively. There is no comprehensive study regarding FGF-23 reference range for Millipore FGF-23 ELISA kit. Smith and colleagues reported mean plasma FGF-23 values in healthy individuals as 31.3 ± 8.4 pg/ml, using Millipore intact FGF-23 (iFGF- 23) assay [20]. Nevertheless, it is inapplicable to consider these values as a reference range due to small sample size.
Microalbuminuria was defined as a urine albumin/creatinine ratio (ACR) between 30 and 300 mg/g in the absence of urinary tract infection confirmed on at least two serial early morning samples. Glomerular filtration rate (GFR) was calculated using the Cockcroft–Gault formula: [(140 − age) × (Wt in kg) × (0.85 if female)/(72 × Cr)]. The presence of microalbuminuria and GFR >60 ml/min was defined as (early) diabetic nephropathy.
Echocardiographic assessment
All patients underwent transthoracic echocardiography in the left lateral lying position using a GE Vivid 7 (Horten, Norway) echocardiography machine. Aortic strain and distensibility were measured using M-mode echocardiography by calculating the systolic and diastolic diameters of the ascending aorta, approximately 3 cm above the aortic valve in the parasternal long-axis view. The systolic diameter of the aorta was measured at the point of highest forward motion of the aorta, whereas the diastolic diameter was measured at the area equivalent to the peak of the QRS complex on electrocardiography. Measurements were repeated at three cardiac beats and the mean value was obtained. The following formula was used for strain and distensibility measurements [21]:
The early diastolic peak flow velocity (E), late diastolic peak flow velocity (A) and E wave deceleration time (Dec. T.) were measured using transmitral Doppler imaging. The Doppler tissue-imaging (DTI) was obtained by using the pulsed-wave Doppler mode. Filters were set to exclude high-frequency signals, and the Nyquist limit was adjusted to a velocity range of –15 to –20 cm/s. Gains were reduced to achieve minimal background noise. All the DTI recordings were acquired during normal respiration. The velocities were noted by placing a 5-mm sample volume on the medial and lateral side of the mitral annulus, and they were recorded for 5–10 cardiac cycles at a sweep speed of 100 mm/s. Indexes of regional systolic function, namely the time velocity integral of the myocardial systolic (Sm) wave and diastolic function parameters, such as myocardial early (Em) and atrial peak (Am) velocities (m/s), were measured. The isovolumic relaxation time was measured as the time interval between the end of Sm and the onset of Em. All regional systolic and diastolic function parameters were measured in three consecutive cardiac cycles and averaged. The same blinded investigator performed the echocardiography.
Statistical analysis
Statistical analysis was made using computer software Number Cruncher Statistical System (NCSS) 2007 and Power Analysis and Sample Size (PASS) 2008 Statistical Software (Utah, USA). Numerical data were analyzed using Student’s t test and one-way ANOVA for variables with normal distribution, while Mann–Whitney U and Kruskal–Wallis tests were used for non-normally distributed variables. Yates Continuity Correction test (Yates corrected Chi-square test) was used for the comparison of categorical data. Pearson’s and Spearman’s rho correlation analyses were performed to evaluate the correlations between variables with normal distribution and non-normally distributed variables, respectively. Data were expressed as “mean [standard deviation (SD)],” median (minimum–maximum) and percent (%) where appropriate. p < 0.05 was considered statistically significant.
Results
Demographics, anthropometrics, laboratory and echocardiographic findings in patient versus control groups
Patient and control groups were similar in terms of age and gender distribution, while type 1 diabetic patients were determined to have significantly lower mean BMI (p = 0.033), GFR (p = 0.018) and PTH (p = 0.004), but higher values for fasting blood glucose (p < 0.001), HbA1c (p < 0.001), calcium (p = 0.015) and ALP (p = 0.004) when compared with controls (Table 1).
Patient and control groups were similar in terms of E, A, IVRT, Dec T, E’lat, E’med, systolic and diastolic blood pressure, aortic strain and distensibility of the ascending aorta (Table 2).
sKlotho (pg/ml) and FGF-23 (pg/ml) levels with respect to patient characteristics
Patient and control groups were similar in terms of mean sKlotho (509.2 ± 183.5 and 547.6 ± 424.0 pg/ml, respectively) and FGF-23 (76.2 ± 15.6 and 77.2 ± 15.1 pg/ml, respectively) levels. There was no significant change in sKlotho (pg/ml) and FGF-23 (pg/ml) levels with respect to age, gender, anthropometrics and duration of diabetes.
Correlation of Klotho (pg/ml) and FGF-23 (pg/ml) levels with cardiac parameters
Apart from negative correlation between FGF-23 (pg/ml) levels and A (r = −0.377, p = 0.040) in the control group, no significant correlation of sKlotho (pg/ml) and FGF-23 (pg/ml) levels with cardiac parameters was noted in patient and control groups (Table 3).
Subgroup analysis with respect to early diabetic nephropathy
Fourteen patients have stage 1 CKD (GFR >90 ml/min and microalbuminuria), and three have stage 2 CKD (GFR 60–90 ml/min and microalbuminuria). There were no differences in patients with diabetic nephropathy (DN group) compared with patients without DN (non-DN group) in terms of demographic data such as age, gender, BMI, waist circumference. Laboratory parameters were also similar in two groups. Only, albuminuria was significantly higher in DN group compared with non-DN counterparts (180.5 ± 17.3 mg/day vs. 12.1 ± 7.2 mg/day, p = 0.01). There were no differences in echocardiographic parameters between two groups. The sKlotho levels of patients with and without diabetic nephropathy were 487.7 ± 175.8 pg/ml and 516 ± 186.9 mg/ml, respectively (p = 0.582). There were no differences in plasma FGF-23 levels between the patients with and without diabetic nephropathy (76.9 ± 10.2 and 75.9 ± 17 pg/ml, respectively) (p = 0.810).
There were no correlations between serum Ca, P, PTH and sKlotho in patients without DN. Serum calcium and 25-hydroxy (OH) vitamin D levels were positively correlated with sKlotho levels only in DN group (r = 0.554, p = 0.021; r = 0.486, p = 0.048). We did not find any correlation between serum levels for Ca, P, PTH, 25-OH vitamin D and FGF-23 levels in patients with or without DN.
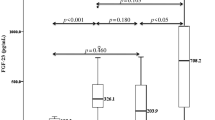
There were also positive correlation of FGF-23 levels with IVRT (r = 0.674, p = 0.023) and negative correlation with E’med (r = −0.681, p = 0.030) in DN group (Fig. 1). However, we did not observe any correlation between FGF-23 levels and echocardiographic parameters in non-DN group.
Correlations between FGF-23 (pg/ml) and isovolumic relaxation time (IVRT) (ms) and early diastolic velocity at medial/septal annulus (E’med) (m/s) in patients with early diabetic nephropathy (n = 17). a Positive correlation between FGF-23 levels with IVRT (r = 0.674, p = 0.023); b Negative correlation between FGF-23 levels with E’med (r = −0.681, p = 0.030)
Discussion
Our findings in a young adult population of type 1 diabetic patients with GFR >60 ml/min versus healthy controls revealed no difference between groups in terms of plasma levels for sKlotho and FGF-23 as well as echocardiographic findings, while a significant correlation of FGF-23 levels positively with IVRT (r = 0.674, p = 0.023) and negatively with E’med (r = −0.681, p = 0.030) was noted only in patients with early DN.
Given the inclusion of type 1 diabetic patients with GFR >60 ml/min, lack of a significant decrease in sKlotho levels in our patients compared with controls seems in line with the suggestion of advanced stage of CKD as a state of systemic Klotho deficiency and decline in Klotho levels parallel to CKD stage and GFR reduction [4, 6, 22]. Limited data are available concerning sKlotho levels in patients with diabetic nephropathy with lower levels reported in patients with HbA1c >6.5 % [23] along with experimental [24, 25] and clinical [4] evidence of reduced expression of diabetes indicating decline in Klotho levels parallel to CKD stage and GFR reduction in type 2 diabetic patients with nephropathy. In the literature, there is no study investigating sKlotho levels in type 1 diabetic patients with or without diabetic nephropathy, while lower sKlotho levels in diabetic CKD patients [4] and no change in serum levels for Klotho in diabetic patients without nephropathy compared with healthy controls [23] have been reported. In our study population besides in type 1 diabetic patients compared with controls, serum sKlotho levels were also similar between type 1 diabetics with and without DN.
Given the consideration of age-dependent decrease in Klotho levels in humans with implications of Klotho in a multitude of biological processes many of which implicated in human longevity [6, 26], it should be noted that our patients with type 1 diabetes with no or early diabetic nephropathy were young adults with mean age of 34.2 ± 9.6 years.
Consistent with lack of a significant increase in FGF-23 levels in our type 1 diabetic patients with GFR >60 ml/min as compared with controls, a progressive increase in FGF-23 as a compensatory response to maintain normal phosphatemia was associated with the decline in renal function [27, 28] where the raise can be up to 1000-fold higher in dialysis patients than in healthy individuals [28].
Lack of a significant increase in FGF-23 levels seems also consistent with the normal levels of sKlotho in our diabetic patients, since an end-organ resistance to the phosphaturic stimulus of FGF-23 because of a deficiency in Klotho has been suggested as an alternative hypothesis for the increase in FGF-23 levels [6, 29].
Considered as a biomarker for kidney function, sKlotho levels were reported to be higher in adolescence and lower in older adults and negatively correlate with creatinine and BUN, which are known to increase in an age-dependent fashion even in healthy humans [30]. Accordingly, lack of advanced diabetic nephropathy and younger age distribution might have a critical role in identification of no significant decrease in Klotho levels and no increase in FGF-23 in our type 1 diabetic patients as compared with controls.
In our study, no significant difference was noted between patients and controls in terms of echocardiographic parameters including E, A, IVRT, Dec T, E’lat, E’med, systolic and diastolic blood pressure, aortic strain and distensibility of the ascending aorta. However, while no significant correlation was determined between sKlotho (pg/ml) levels and cardiac parameters, FGF-23 (pg/ml) levels were shown to be correlated positively with IVRT (r = 0.674, p = 0.023) and negatively with E’med (r = −0.681, p = 0.030) in patients with early DN.
Cross-sectional data on the association of FGF-23 concentrations with echocardiographic evidence of cardiac hypertrophy such as LVM index (LVMI) and LVH were reported in subjects with CKD in several cohorts of general population with stronger associations noted in GFR of <60 ml/min/1.73 m2 [5, 15–17, 31, 32].
Notably, to be justified by further investigation related to the association between FGF-23 and future CVD in type 1 diabetes with early diabetic nephropathy, the correlation of FGF-23 levels positively with IVRT while negatively with E’med in our patients with GFR >60 ml/min seems to indicate the role of FGF-23 in prediction of diastolic dysfunction and therefore future cardiovascular risk in type 1 diabetic patients regardless of the severity of underlying diabetic nephropathy. Similarly, in the past study presenting novel information regarding potential vascular effects of FGF-23 in physiology, an independent association between higher FGF-23 and impaired vasoreactivity was reported in a community-based cohort, in the setting of normal renal function and without derangements in mineral metabolism [18].
While the exact mechanisms underlying association between FGF-23 and CVD remain unclear, strong correlation of increase in FGF-23 levels with decline in renal function [27] as one of the major risk factors for cardiovascular mortality [33], the suppression of 1,25-OH vitamin D by FGF-23 leading to cardiovascular events, [34] a phosphate burden that elevates circulating FGF-23, [35] the elevation in FGF-23 to compensate for the deficiency of Klotho in CKD [29] and finally the possible direct harmful influence of FGF-23 on the cardiovascular system have been suggested among the possible mechanisms [5].
The recent observational studies indicate relation of FGF-23 with endothelial dysfunction, atherogenic disease and cardiovascular events not only in populations with renal failure, but also in subjects without kidney impairment [6]. Furthermore, higher serum FGF-23 levels, even within the normal range, were reported to be independently associated with impaired vasoreactivity and increased arterial stiffness in the community [18]. High prevalence of preclinical diastolic dysfunction is a well-established fact among diabetic patients [36]. The evidence indicates that myocardial damage in diabetic subjects affects diastolic function before the systolic function [37]. Our findings are in line with the statement that the FGF-23–heart failure relationship may be principally driven by diastolic heart failure, which is associated with significant morbidity and mortality [38]. It is impressive that we observed a significant association between FGF-23 levels and diastolic dysfunction in only patients with microalbuminuria.
FGF-23 has been shown as a predictor of survival and cardiovascular morbidity independently of the serum phosphate levels, both in CKD and in subjects without kidney function impairment [6]. In fact, strongly suggesting that a component of cardiovascular risk in CKD patients could be directly attributable to FGF-23, [16] the cardiac hypertrophic effects of FGF-23 were reported to be FGF receptor-dependent but independent of Klotho [6]. A suggested pathological mechanism is the Klotho-independent low-affinity binding of FGF-23 even in the absence of Klotho in cardiomyocytes [39], which would occur under conditions with high FGF-23 concentrations, such as CKD [6].
Duration of the disease has been suggested as the predominant risk factor for CVD in patients with type 1 diabetes with limited data available on the occurrence of subclinical markers of CVD before the appearance of overt macrovascular disease (MVD) [3]. In this regard, since FGF-23 levels increase as renal function declines, we cannot predict the time course of FGF-23 by a single measurement in time [5], and therefore, a single FGF-23 value does not reflect the long-term outcome of patients which suggests measurement of serum FGF-23 levels repeatedly.
Hence, changes in Klotho and FGF-23 levels in diabetic patients without nephropathy need further investigation to clarify their involvement in the pathogenesis of diabetes mellitus in relation to age and kidney function as well as the impact of ongoing anti-diabetic treatments on the serum levels of these potential biomarkers [40].
This is a cross-sectional study, and this kind of study design is not adequate to reach a conclusion in causality, so it is difficult to state a causality between FGF-23 and echocardiographic findings. Another limitation is the small number of the patients with microalbuminuria (n = 17).
Currently, there are two types of commercial ELISA available for FGF-23 determination: iFGF-23 and C-terminal FGF-23 (cFGF-23). Although both types of FGF-23 have been found associated with disease severity in clinical conditions such as chronic renal failure, there is still debate on which assay is more helpful in clinical setting [41]. The kit which was used in the study was manufactured by Millipore Corporation to measure iFGF-23. There are also different commercial kits manufactured by different companies in the market for measuring both forms of FGF-23 [20]. To evaluate performances and to compare current ELISA kits, including Millipore Corp. FGF-23 kit, a study was conducted by Smith et al. [20]. The authors concluded that there was poor analytical agreement between ELISA for measuring both iFGF-23 and cFGF-23, largely due to the lack of standardization. Still it is not possible interchangeably to use the FGF-23 results which are determined by different ELISA. In our study, we measured plasma FGF-23 levels in all participants using same Millipore kit mentioned in Method section. In addition, the limit of sensitivity of the assay we used was 3.5 pg/ml, but the lowest plasma FGF-23 level measured was approximately 76 pg/ml in our study. Furthermore, there was no single patient who could have plasma FGF-23 level under the limit of sensitivity.
In conclusion, our findings in a young adult population of type 1 diabetic patients with GFR >60 ml/min versus healthy controls revealed no difference between groups in terms of plasma levels for sKlotho and FGF-23 as well as echocardiographic findings, while significant correlations of FGF-23 levels positively with IVRT and negatively with E’med were noted in diabetic patients with early DN. In this regard, our findings provide novel data supporting an association between circulating FGF-23 and diastolic dysfunction in type 1 diabetic patients even in the absence of clinically overt renal failure and related changes in sKlotho and FGF-23 levels. Future studies are required to validate the role of FGF-23 in CVD in type 1 diabetic patients without overt diabetic nephropathy to conclude FGF-23 as a modifiable cardiovascular risk factor.
References
Koivisto VA, Stevens LK, Mattock M, Ebeling P, Muggeo M, Stephenson J, Idzior-Walus B (1996) EURODIAB IDDM Complications Study Group. Cardiovascular disease and its risk factors in IDDM in Europe. Diabetes Care 19:689–697
Brindisi MC, Bouillet B, Vergès B, Halimi S (2010) Cardiovascular complications in type 1 diabetes mellitus. Diabetes Metab 36:341–344 (Review)
Shivalkar B, Dhondt D, Goovaerts I, Van Gaal L, Bartunek J, Van Crombrugge P, Vrints C (2006) Flow mediated dilatation and cardiac function in type 1 diabetes mellitus. Am J Cardiol 97:77–82
Kacso IM, Bondor CI, Kacso G (2012) Soluble serum Klotho in diabetic nephropathy: relationship to VEGF-A. Clin Biochem 45:1415–1420
Nakano C, Hamano T, Fujii N, Obi Y, Matsui I, Tomida K, Mikami S, Inoue K, Shimomura A, Nagasawa Y, Okada N, Tsubakihara Y, Rakugi H, Isaka Y (2012) Intact fibroblast growth factor 23 levels predict incident cardiovascular event before but not after the start of dialysis. Bone 50:1266–1274
Donate-Correa J, Muros-de-Fuentes M, Mora-Fernández C, Navarro-González JF (2012) FGF23/Klotho axis: phosphorus, mineral metabolism and beyond. Cytokine Growth Factor Rev 23:37–46
Long YC, Kharitonenkov A (2011) Hormone-like fibroblast growth factors and metabolic regulation. Biochim Biophys Acta 1812:791–795
Kurosu H, Kuro-O M (2009) The Klotho gene family as a regulator of endocrine fibroblast growth factors. Mol Cell Endocrinol 299:72–78
Wang Y, Sun Z (2009) Current understanding of klotho. Ageing Res Rev. 8:43–51 (Review)
Hu MC, Kuro-o M, Moe OW (2013) Renal and extrarenal actions of Klotho. Semin Nephrol. 33:118–129 (Review)
Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T (2004) FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res 19:429–435
Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, Wahl P, Gutiérrez OM, Steigerwalt S, He J, Schwartz S, Lo J, Ojo A, Sondheimer J, Hsu CY, Lash J, Leonard M, Kusek JW, Feldman HI, Wolf M (2011) Chronic Renal Insufficiency Cohort (CRIC) Study Group. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 305:2432–2439
Ix JH, Shlipak MG, Wassel CL, Whooley MA (2010) Fibroblast growth factor-23 and early decrements in kidney function: the Heart and Soul Study. Nephrol Dial Transplant 25:993–997
Ix JH, Katz R, Kestenbaum BR, de Boer IH, Chonchol M, Mukamal KJ, Rifkin D, Siscovick DS, Sarnak MJ, Shlipak MG (2012) Fibroblast growth factor-23 and death, heart failure, and cardiovascular events in community-living individuals: CHS (Cardiovascular Health Study). J Am Coll Cardiol 60:200–207
Jovanovich A, Ix JH, Gottdiener J, McFann K, Katz R, Kestenbaum B, de Boer IH, Sarnak M, Shlipak MG, Mukamal KJ, Siscovick D, Chonchol M (2013) Fibroblast growth factor 23, left ventricular mass, and left ventricular hypertrophy in community-dwelling older adults. Atherosclerosis 231:114–119
Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutiérrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro-O M, Kusek JW, Keane MG, Wolf M (2011) FGF23 induces left ventricular hypertrophy. J Clin Invest 121:4393–4408
Mirza MA, Larsson A, Melhus H, Lind L, Larsson TE (2009) Serum intact FGF23 associate with left ventricular mass, hypertrophy and geometry in an elderly population. Atherosclerosis 207:546–551
Mirza MA, Larsson A, Lind L, Larsson TE (2009) Circulating fibroblast growth factor- 23 is associated with vascular dysfunction in the community. Atherosclerosis 205:385–390
Pedersen L, Pedersen SM, Brasen CL, Rasmussen LM (2013) Soluble serum Klotho levels in healthy subjects. Comparison of two different immunoassays. Clin Biochem 46:1079–1083
Smith ER, McMahon LP, Holt SG (2013) Method-specific differences in plasma fibroblast growth factor 23 measurement using four commercial ELISAs. Clin Chem Lab Med 51:1971–1981
Lacombe F, Dart A, Dewar E, Jennings G, Cameron J, Laufer E (1992) Arterial elastic properties in man: a comparison of echo-Doppler indices of aortic stiffness. Eur Heart J 13:1040–1045
Hu MC, Shi M, Zhang J, Quiñones H, Griffith C, Kuro-o M, Moe OW (2011) Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol 22:124–136
Devaraj S, Syed B, Chien A, Jialal I (2012) Validation of an immunoassay for soluble Klotho protein: decreased levels in diabetes and increased levels in chronic kidney disease. Am J Clin Pathol 137:479–485
Zhao Y, Banerjee S, Dey N, LeJeune WS, Sarkar PS, Brobey R, Rosenblatt KP, Tilton RG, Choudhary S (2011) Klotho depletion contributes to increased inflammation in kidney of the db/db mouse model of diabetes via RelA (serine) 536 phosphorylation. Diabetes 60:1907–1916
Cheng MF, Chen LJ, Cheng JY (2010) Decrease of Klotho in the kidney of streptozotocin induced diabetic rats. J Biomed Biotechnol 2010:513853
Semba RD, Cappola AR, Sun K, Bandinelli S, Dalal M, Crasto C, Guralnik JM, Ferrucci L (2011) Plasma Klotho and cardiovascular disease in adults. J Am Geriatr Soc 59:1596–1601
Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, Jüppner H, Wolf M (2005) Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol 16:2205–2215
Larsson T, Nisbeth U, Ljunggren O, Juppner H, Jonsson KB (2003) Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int 64:2272–2279
Koh N, Fujimori T, Nishiguchi S, Tamori A, Shiomi S, Nakatani T, Sugimura K, Kishimoto T, Kinoshita S, Kuroki T, Nabeshima Y (2001) Severely reduced production of klotho in human chronic renal failure kidney. Biochem Biophys Res Commun 280:1015–1020
Yamazaki Y, Imura A, Urakawa I, Shimada T, Murakami J, Aono Y, Hasegawa H, Yamashita T, Nakatani K, Saito Y, Okamoto N, Kurumatani N, Namba N, Kitaoka T, Ozono K, Sakai T, Hataya H, Ichikawa S, Imel EA, Econs MJ, Nabeshima Y (2010) Establishment of sandwich ELISA for soluble alpha-Klotho measurement: age-dependent change of soluble alpha-Klotho levels in healthy subjects. Biochem Biophys Res Commun 398:513–518
Yilmaz MI, Sonmez A, Saglam M, Yaman H, Kilic S, Demirkaya E, Eyileten T, Caglar K, Oguz Y, Vural A, Yenicesu M, Zoccali C (2010) FGF-23 and vascular dysfunction in patients with stage 3 and 4 chronic kidney disease. Kidney Int 78:679–685
Gutierrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, Collerone G, Sarwar A, Hoffmann U, Coglianese E, Christenson R, Wang TJ, deFilippi C, Wolf M (2009) Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation 119:2545–2552
Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT (2010) Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative metaanalysis. Lancet 375:2073–2081
Wolf M, Shah A, Gutierrez O, Ankers E, Monroy M, Tamez H, Steele D, Chang Y, Camargo CA Jr, Tonelli M, Thadhani R (2007) Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int 72:1004–1013
Burnett SM, Gunawardene SC, Bringhurst FR, Juppner H, Lee H, Finkelstein JS (2006) Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J Bone Miner Res 21:1187–1196
Kazik A, Wilczek K, Polonski L (2010) Management of diastolic heart failure. Cardiol J 17:558–565
Patil VC, Patil HV, Shah KB, Vasani JD, Shetty P (2011) Diastolic dysfunction in asymptomatic type 2 diabetes mellitus with normal systolic function. J Cardiovasc Dis Res 2:213–222
Yancy CW, Lopatin M, Stevenson LW, DeMarco T, Fonarow GC (2006) ADHERE Scientific Advisory Committee and Investigators. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Hearth Failure National Registry (ADHERE) Database. J Am Coll Cardiol 47:76–84
Kardami E, Jiang ZS, Jimenez SK, Hirst CJ, Sheikh F, Zahradka P, Cattini PA (2004) Fibroblast growth factor 2 isoforms and cardiac hypertrophy. Cardiovasc Res 63:458–466
van Ark J, Hammes HP, van Dijk MC, Vervloet MG, Wolffenbuttel BH, van Goor H, Hillebrands JL (2013) Circulating alpha-klotho levels are not disturbed in patients with type 2 diabetes with and without macrovascular disease in the absence of nephropathy. Cardiovasc Diabetol 12:116
Smith ER, Cai MM, McMahon LP, Holt SG (2012) Biological variability of plasma intact and C-terminal FGF23 measurements. J Clin Endocrinol Metab 97:3357–3365
Acknowledgments
The study is granted by Sanofi-Aventis, Turkey. We thank Cagla Ayhan, MD, and Prof. Sule Oktay, MD, Ph.D., from KAPPA Consultancy Training Research Ltd., Istanbul, who provided editorial support funded by Sanofi-Aventis, Turkey.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Dogan, B., Arikan, I.H., Guler, D. et al. Fibroblast growth factor-23 but not sKlotho levels are related to diastolic dysfunction in type 1 diabetic patients with early diabetic nephropathy. Int Urol Nephrol 48, 399–407 (2016). https://doi.org/10.1007/s11255-015-1190-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-015-1190-y