Abstract
Purpose
Epithelioid angiomyolipoma (EAML) is a rare entity of the kidney. The guideline for grossing and reporting of renal EAML has not been established for Chinese patients. We planned this study to provide some preliminary indicators for draft guidelines of pathological diagnosis among Chinese people.
Methods
The histopathological characteristics of 11 EAML cases from Cancer Hospital, Chinese Academy of Medical Sciences, were reviewed, and a pooled analysis based on our cases and cases from published articles was performed on the histopathological characteristics and prognosis of 56 Chinese patients with EAML. All the cases met the criteria of the 2004 World Health Organization classification of renal tumors.
Results
The ratio of female to male was 1.2:1 with the mean age of 43.4 in the 11 cases. All the 11 cases were sampled following the guideline of renal cell carcinoma. The mean tumor size was 6.5 cm. Four (36.4 %) cases showed necrosis. Six (54.5 %) cases showed invasive borders. Only one case showed metastases. In pooled analysis of the total 56 cases with EAML, 10 cases (17.9 %) showed adverse prognosis. Tumor size, necrosis and invasive edge showed significant difference between favorite and adverse prognostic groups (P < 0.05).
Conclusion
The majority of EAML is benign, and true malignant EAML is rare. The sample of EAML should follow the sample guidelines of renal cell carcinoma with some modifications, emphasizing the presence of necrosis and invading edge. The information of tumor size, necrosis and invasive edge should be included in the diagnostic report of each EAML case.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Renal angiomyolipoma (AML), a mesenchymal neoplasm, is composed of various proportions of dysmorphic blood vessels, smooth muscle and adipose tissue [1]. Since many studies indicated that AML is related to perivascular epithelioid cell (PEC) differentiation, it belongs to the PEComa tumor family [2]. Immunohistochemically, PEC expresses melanocytic and myogenic markers such as HMB45, HMSA-1, MelanA/Mart1, microphthalmia transcription factor (Mitf), actin and less commonly desmin [3]. Epithelioid angiomyolipoma (EAML) of the kidney is a rare subtype of angiomyolipoma (AML), which was first reported by Mai [4]. EAML is characterized by polygonal cells with clear to eosinophilic cytoplasm, round to oval nuclei that may show various degrees of nuclear atypia, and the same immunoreactivity as AML [1, 5–8]. AMLs, including EAML, are strongly associated with tuberous sclerosis (TSC) [1, 5–8]. In contrast to the classic AML which is benign, local recurrence and distant metastases have been reported in EAML [5–8]. Approximately one-third of EAMLs have been reported to have metastasis to lymph nodes, liver, lungs or spine [9]. However, no prognostic histopathological features have been established.
Faraji et al. [10] studied the pathologic features of six EAML cases and conducted a meta-analytic study. They indicated that male patient and larger tumor size may be two potential predictive factors of adverse clinical course in EAML. Brimo et al. [11] purposed the clinicopathologic prognostic indicators of malignant EAML. Their study focused on adult patients (age ≥ 17) with EAML, which have no less than moderate atypia and the proportion of the epithelioid cells was no less than 5 %. In their study, the proportion of atypical epithelioid cells (≥70 %), the number of mitotic figures (≥2/10), atypical mitotic figures and necrosis were the four prognostic factors. Nese et al. [12] also proposed the risk stratification for pure renal EAML. They mainly focused patients (age ≥ 14) with pure EAML of different atypia. Their study indicated that TSC and/or concurrent AML, tumor size (>7 cm), carcinoma-like growth pattern, extrarenal extension and/or involvement of renal vein and necrosis were the five prognostic factors in the risk stratification. Yang et al. [13] indicated that atypical mitotic figures, blood vessel invasion and tumor embolus may be predicting malignant behavior of EAML focused on 27 Chinese patients with EAML from kidney and liver. Overall, these studies revealed: (1) EAML may have uncertain malignant potential; (2) the clinic courses of EAMLs were partially different based on the clinicopathologic factors; (3) the application of clinicopathologic features of EAML to predict its clinical course may be feasible. Today, a lot of genitourinary pathologists agreed that the EAMLs should be divided into low, intermediate and high risk of malignant behavior based on published criteria [14].
In China, some case reports and small series of EAML were published since 2000 [15]. Although the diagnosis of EAML is not an issue when following the criteria of the 2004 World Health Organization classification of renal tumors [9], there are no guidelines on how to sample and what standard information should be reported in the pathological report in China. The purpose of this retrospective study was to: (1) investigate the clinicopathologic features of EAML cases in Cancer Hospital, Chinese Academy of Medical Sciences, to find some histopathological features, which may be related to prognosis and (2) perform pooled analysis of histopathological features and prognosis in Chinese patients with renal sporadic EAML. The results may provide useful information for the establishing guideline of properly sampling and reporting EAML.
Materials and methods
The summary of clinicopathologic features in the 11 cases with renal EAML in Cancer Hospital, Chinese Academy of Medical Sciences, was a part of a series of researches that was approved by NCC Ethics Committee/IRB. In this part, patient consent was not required as there were no risks anticipated to the participants. All the information of patient’s identification was de-identified.
Clinical data and postoperative pathological evaluation on EAML cases from Cancer Hospital, Chinese Academy of Medical Sciences, in China
The EAML cases from 2005 to 2011 at our hospital were reviewed. All cases were sampled following the guideline of handling renal cell carcinoma, which was according to the tumor size as well as the relationship between tumor and perinephric fat or renal sinus fat.
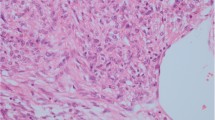
The inclusion criteria were listed as follows: (1) Chinese adult (age ≥ 19); (2) radical nephrectomy or partial nephrectomy; (3) located in kidney only; (4) a minimum of 5 % of epithelioid component in tumor [11]; and (5) diagnosed according to the histological and immunohistochemical criteria of EAML in the current World Health Organization classification of renal tumors (2004) [9], including: (1) polygonal larger cells with abundant granular cytoplasm; (2) enlarged vesicular nuclei, prominent nucleoli, multinucleated or enlarged ganglion-like cells; (3) nuclear anaplasia or atypical mitotic figure; (4) expression of one or more melanocytic markers (HMB-45, Mart-1/MelanA, etc); (5) positive or negative for myoid markers (actin, smooth muscle actin, etc); and (6) a negative immunoreaction for one or more epithelial markers, pan-cytokeratin, CK8, CK18 and/or epithelial membrane antigen (Fig. 1).
Representative histopathological features of epithelioid angiomyolipoma. a Invasive edge (red arrow) and tumor embolus (black arrow). b Polygonal larger cells with abundant granular cytoplasm (red arrow) and multinucleated cells (black arrow). c Abundant granular cytoplasm (red arrow) and nuclear anaplasia with prominent nucleoli (black arrow). d Extensive necrosis (black arrow). e Immunohistochemistry for HMB45 showing diffuse strong reactivity. f Immunohistochemistry of SMA showing partly strong reactivity
Clinical data including sex, age and mode of surgery were collected from medical records. Follow-up information was obtained from medical records or telephone, including local recurrence (Rec), metastasis (Mets), death of disease (DOD) or no evidence of disease (NED) during the follow-up period. An adverse clinical course was defined as local Rec, Mets and DOD at different intervals after surgical excision [16, 17]. The pathological features including tumor size, degree of atypia, growth pattern, mitotic figures, necrosis, multinucleate giant cells, extrarenal extension, invasive edge and/or involvement of renal vein are evaluated. Tumor size was obtained according to the gross description of the specimen after formalin fixation. All slides were reviewed by three pathologists (S. Z, Z.Y. and H. T. Z.).
Pooled analysis of histopathological characteristics and prognosis of renal EAML in published Chinese cases
We reviewed the literature to retrieve reported Chinese patients with EAML. Literatures of EAML were obtained from databases of PubMed (http://www.ncbi.nlm.nih.gov/pubmed/) and Wanfang (http://www.wanfangdata.com.cn/) from January 1, 2000, to December 31, 2014. Search terms were listed as follows: “renal or kidney” and “epithelioid AML or PEComa” and “pathology or pathological” and “prognosis” and “Language = English or Chinese”. As very few reports of Chinese patients with EAML were associated with TSC [18, 19], only EAML without TSC was selected in this study. Papers published in English and Chinese core journals [20] were selected in this study.
Inclusion criteria
The inclusion criteria were the same as the cases in our cases (see in “Clinical data and postoperative pathological evaluation on EAML cases from Cancer Hospital, Chinese Academy of Medical Sciences, in China” section). Only case reports with available prognostic information were collected in this pooled analysis.
Approaches in assessing the methodological quality of the studies
Each study was evaluated independently by three investigators (S.Z., X.G. B. and Q. K. S.). We used the Oxman and Guyatt index to evaluate the research quality [21]. H. T. Z. and J. H. M. may be consulted when necessary. The data quality from each paper was valued by the integrity of information: age, sex, mode of surgery, tumor size, necrosis and invasive edge. According the previous researches mainly [10–13], we gave each factor different scores: (1) age and type of surgery scored 1; (2) sex and invasive edge scored 2; and (3) tumor size and necrosis scored 3. After scoring them separately, we met and compared the scores of each case report or article. When necessary, we read the contents of the study to identify the sources of disagreement and discussed them until an agreed score for each study. We add each score as the total score.
Data extraction and strategy
After this assessment was completed, the following information was extracted by S. Z.: name of first author; journal; date of publication; language; age; sex; type of surgery; tumor size; necrosis; invasive edge; and follow-up. Necrosis and invasive edge were divided into two groups of “+” and “−.” All the patients were divided into two groups based on the information of follow-up—favorable group (patients of NED) or adverse group (patients of Rec, Mets and DOD) [16, 17].
Statistical analysis
Analyses of two parts were performed in this study. In the first part, a descriptive statistical analysis was provided for the cases from our hospital. All target variables were described using the descriptive statistics. Counting data frequency and proportion were reported. For continuous data, mean, standard deviation (SD), minimum and maximum were reported. The second part is a pooled summary in which data from the eligible studies and our cases were pooled. For age, sex, mode of surgery and tumor size, only the data from our patients and the individual data from the selected papers were used. The aggregated data in the selected papers were not included in the analyses. For showing necrosis and invasive edge, the data from aggregated data can be used as the number of “+” (or “−”) cases was provided in these papers. Therefore, the sample size for each variable might be different. Final sample size for each variable was included in the summary tables. Wilcoxon test was provided for continuous variables. Similarly, for categorical variables, Fisher’s exact test was used. All of the tests were two-tailed with the p value to indicate the strength of the evidence of the difference between two classified groups. Data were analyzed by SAS 9.2 version (SAS institute Inc., Cary, NC).
Results
Clinicopathologic features of EAML cases from Cancer Hospital, Chinese Academy of Medical Sciences, in China
According to the inclusion criteria, 11 patients with EAML from our hospital were selected. No case was associated with TSC. There were 5 (45.5 %) males and 6 (54.5 %) females. The mean age of the patients was 43.4 years old (23–58). Radical nephrectomy was performed in 8 (72.7 %) patients, while partial nephrectomy was performed in the other 3 (27.3 %) patients. The mean tumor size was 6.5 cm (2.0–17.5 cm). Four (36.4 %) cases showed necrosis. Six (54.5 %) cases showed invasive edge (Fig. 1). Other pathologic characters, including degree of atypia, growth pattern, mitotic figures, multinucleate giant cells, extrarenal extension and/or involvement of renal vein, will be described in detail in a separate paper (unpublished data). Information on prognosis was retrieved for 10 patients, while 1 patient was lost during follow-up (Table 1, case number 47–57).
Pooled analysis of histopathological characteristics and prognosis of renal EAML in Chinese cases
There were 104 reports of Chinese patients with EAML during 2000–2014. Thirteen papers were published in Scientific Citation Index in English language, and 91 papers were published in Chinese journals. Eighteen papers were selected according to the inclusion criteria [15, 22–38] (Fig. 2). The Oxman and Guyatt index of each study was no less than three. The data quality score was no less than six.
Twelve papers are case series report, while others six papers are case reports [15, 22–38] (Table 1). All the eligible studies had intact information of age and sex, while the information of mode of surgery, tumor size, necrosis and invasive edge was not reported in 3, 1, 5 and 6 cases individually [23, 33, 34, 38]. The intact rate of each information was from 87.0 to 100.0 % (40/46–46/46), while the rate of intact information case/total case was 84.8 % (39/46) [15, 22–38]. All the pathologic diagnoses were confirmed by immunohistochemistry. The range of number of cases in case series articles was 2–10.
There were 56 cases included in the pooled analysis of histopathological features and prognosis of renal EAML in Chinese population (Table 2). Tumor size, necrosis and invasive edge showed statistically significant difference between favorable and adverse clinical course groups (P < 0.05).
Discussion
As EAML is one of the malignant potential tumors [9], possible predictive pathologic features should be identified and included in the pathologic report by the pathologist. As we known, some important features can only be recorded correctly when performing the grossing at the appropriate level. In the practice, grossing relevant features may be neglected due to no standard grossing and reporting protocol. Currently, there is no guideline on grossing and reporting of EAML in China. We believe that data from Chinese population should be more helpful in clinical practice in China. In this study, we reviewed the clinicopathologic characters of our own hospital’s EAML patients to get some potential pathologic characters related to malignancy, which were gotten from grossing. Based on the results from our patients and published articles, we explored the possibility of using histopathological features for prognosis of renal EAML malignancy from pooled Chinese patients without TSC. Our findings may be useful for establishing the guideline of grossing and reporting EAML in China and may also help the clinical practices in this disease in other countries.
The mean age of EAML in our 11 cases was 43.4 years, with slightly female predominance. All the 11 cases were sporadic. Only 10 % patients with EAML showed advanced clinical course, which is much lower than reported in the literature [9]. The possible reasons were as follows: (1) all of our cases were sporadic and none of them was associated with TSC; (2) the follow-up time in some cases was short (only 7 months), while it may need more time to confirm the clinical course in the corresponding case; (3) there may be some selection bias in the literatures as benign EAML may not be reported. Based on our reviews of 11 cases with EAML in our hospital, large tumor size, tumor necrosis and invasive tumor edge may be three grossing relevant pathologic characters related to malignant potential.
In the past decade, a lot of published cases indicated that the potential pathologic features (such as male patient, larger tumor size and tumor necrosis) may be associated with malignant EAML, although definitive pathologic indictors were not affirmed [10–13]. Patients with tumors showing one or more such features may have a worse outcome. In order to have a relative large sample size, data from the eligible studies and our cases were pooled. Based on the indication from our patients and the findings from published articles, we selected tumor size, tumor necrosis and invasive tumor edge, as the possible pathologic features relevant to grossing in the pooled data analysis. The age, sex and mode of surgery were investigated as well.
In our study, we did not find that sex was related to the outcome of EAML. Compared with other studies, we only chose adult patients with sporadic EAML, while others included all patients with EAML in all age groups irrespective of sporadic or associated with TSC [10–12]. The difference in case selection may lead to the different results. In this study, we found for the first time that invasive tumor edge may also have potential for predicting malignant EAML. As shown in our series, invasive tumor edge was a relatively frequent finding, compared with extrarenal extension and involvement of renal vein (unpublished data). In our series, all cases were sampled according to the guideline of renal cell carcinoma grossing, i.e., sampling 1 block/cm of tumor. All the tumors were sampled no less than 4 blocks and tumors no more than 2 cm were entirely submitted, since all cases were suspicious of renal cell carcinoma before operation. Based on the findings in this study, we suggested that when renal EAML is confirmed, the tumor interface with adjacent structures and areas of tumors suspicious for necrosis should be carefully re-sampled to offer enough information if necessary. For the reporting, the information of tumor size, the presence of tumor necrosis and invasive tumor edge should be reported as well.
As far, there were no guidelines concerning follow-up management, adjuvant treatment, treatment types or response. The length of follow-up will affect the grouping. To overcome those limits in maximum degree, we used strict inclusion criteria, methodological quality control to guarantee the maximum of clinical and methodological homogeneity in each selected study. This is the first paper that focused on the potential pathological features for malignant sporadic EAML in Chinese. As EAML is a rare disease, we believe that it is necessary to initiate a multicenter clinical research of EAML in China in the future. Not only the grossing protocol but also the microscopic features will be included in that clinical research. A larger sample size study makes the results more reliable.
In conclusion, the majority of EAML is benign, and true malignant EAML is rare. Detailed clinical information, sufficient sampling and necessary test of immunophenotype were three key points for accurate diagnosis. For grossing, we suggested that the sampling of EAML should follow the guidelines of renal cell carcinoma with some modifications [39], emphasizing the presence of necrosis and invading tumor edge. Larger tumor size, the presence of tumor necrosis and invasive tumor edge may be the potential indicators for malignant EAML and should be reported in each case. When such characters are present, we should emphasize them in the pathologic diagnosis and communicate with clinicians for attention and provide better clinical management for these patients.
References
Aydin H, Magi-Galluzzi C, Lane BR, Sercia L, Lopez JI, Rini BI, Zhou M (2009) Renal angiomyolipoma: clinicopathologic study of 194 cases with emphasis on the epithelioid histology and tuberous sclerosis association. Am J Surg Pathol 33:289–297. doi:10.1097/PAS.0b013e31817ed7a6
Pan CC, Jong YJ, Chai CY, Huang SH, Chen YJ (2006) Comparative genomic hybridization study of perivascular epithelioid cell tumor: molecular genetic evidence of perivascular epithelioid cell tumor as a distinctive neoplasm. Hum Pathol 37:606–612
Martignoni G, Pea M, Reghellin D, Zamboni G, Bonetti F (2008) PEComas: the past, the present and the future. Virchows Arch 452:119–132
Mai KT, Perkins DG, Collins JP (1996) Epithelioid cell variant of renal angiomyolipoma. Histopathology 28:277–280
Pea M, Bonetti F, Martignoni G, Henske EP, Manfrin E, Colato C, Bernstein J (1998) Apparent renal cell carcinomas in tuberous sclerosis are heterogeneous: the identification of malignant epithelioid angiomyolipoma. Am J Surg Pathol 22:180–187
Eble JN, Amin MB, Young RH (1997) Epithelioid angiomyolipoma of the kidney: a report of five cases with a prominent and diagnostically confusing epithelioid smooth muscle component. Am J Surg Pathol 21:1123–1130
Mete O, van der Kwast TH (2011) Epithelioid angiomyolipoma: a morphologically distinct variant that mimics a variety of intra-abdominal neoplasms. Arch Pathol Lab Med 135:665–670. doi:10.1043/2009-0637-RSR.1
Varma S, Gupta S, Talwar J, Forte F, Dhar M (2011) Renal epithelioid angiomyolipoma: a malignant disease. J Nephrol 24:18–22
Eble JN, Organization WH, Cancer IAfRo (2004) Pathology and genetics of tumours of the urinary system and male genital organs. IARC Press, Lyon
Faraji H, Nguyen BN, Mai KT (2009) Renal epithelioid angiomyolipoma: a study of six cases and a meta-analytic study. Development of criteria for screening the entity with prognostic significance. Histopathology 55:525–534. doi:10.1111/j.1365-2559.2009.03420.x
Brimo F, Robinson B, Guo C, Zhou M, Latour M, Epstein JI (2010) Renal epithelioid angiomyolipoma with atypia: a series of 40 cases with emphasis on clinicopathologic prognostic indicators of malignancy. Am J Surg Pathol 34:715–722. doi:10.1097/PAS.0b013e3181d90370
Nese N, Martignoni G, Fletcher CD, Gupta R, Pan CC, Kim H, Ro JY, Hwang IS, Sato K, Bonetti F, Pea M, Amin MB, Hes O, Svec A, Kida M, Vankalakunti M, Berel D, Rogatko A, Gown AM, Amin MB (2011) Pure epithelioid PEComas (so-called epithelioid angiomyolipoma) of the kidney: a clinicopathologic study of 41 cases: detailed assessment of morphology and risk stratification. Am J Surg Pathol 35:161–176. doi:10.1097/PAS.0b013e318206f2a9
Yang L, Feng XL, Shen S, Shan L, Zhang HF, Liu XY, Lv N (2012) Clinicopathological analysis of 156 patients with angiomyolipoma originating from different organs. Oncol Lett 3:586–590
Srigley JR, Delahunt B, Eble JN, Egevad L, Epstein JI, Grignon D, Hes O, Moch H, Montironi R, Tickoo SK, Zhou M, Argani P; ISUP Renal Tumor Panel. (2013) The International Society of Urological Pathology (ISUP) Vancouver Classification of Renal Neoplasia. Am J Surg Pathol 37:1469–1489. doi:10.1097/PAS.0b013e318299f2d1
Wu XL, Wang QJ, Zhu QJ, Fang ZX (2000) Atypical angiomyolipoma of the kidney: report of four cases and review for literature. J Clin Exp Pathol 16:367–370. doi:10.3969/j.issn.1001-7399.2000.05.005
Martignoni G, Pea M, Bonetti F, Zamboni G, Carbonara C, Longa L, Zancanaro C, Maran M, Brisigotti M, Mariuzzi GM (1998) Carcinomalike monotypic epithelioid angiomyolipoma in patients without evidence of tuberous sclerosis: a clinicopathologic and genetic study. Am J Surg Pathol 22:663–672
Serrano Frago P, Del Agua Arias Camisón C, Gil Sanz MJ, Allué López M, Gonzalvo Ibarra A, Plaza Mas L, Rioja Sanz LA (2006) Controversies related to epithelioid variant of renal angiomyolipoma: a review of the literature. Urology 67(846):e3–e5
Xu C, Jiang XZ, Zhao HF, Zhang NZ, Ma L, Xu ZS (2013) The applicability of Ki-67 marker for renal epithelioid angiomyolipoma: experience of ten cases from a single center. Neoplasma 60:209–214
Liu H, Wang HQ, Li X, Tang LO, Sun XL, Ji XR (2007) One case of malignant epithelioid amgiolipoma. Chin J Pathol 36:640–641. doi:10.3760/j.issn:0529-5807.2007.09.017
Zhu Q, Cai RH, He J (2011) A main list of the Chinese Core journals, 6th edn. Beijing University Press, Beijing
Jadad AR, McQuay HJ (1996) Meta-analyses to evaluate analgesic interventions: a systematic qualitative review of their methodology. J Clin Epidemiol 49:235–243
Li J, Zhu M, Wang YL (2012) Malignant epithelioid angiomyolipoma of the kidney with pulmonary metastases and p53 gene mutation. World J Surg Oncol 10:213. doi:10.1186/1477-7819-10-213
Qiao LD, Yan W, Liu D, Liu YX, Zhang GY, Chen S (2012) Clinical features of renal epithelioid angiomyolipoma. Chin J Urol 33:495–498. doi:10.3760/cma.j.issn.1000-6702.2012.07.004
Li DC, Yue Y, Ren L (2012) Malignant renal angiomyolipoma: a case and review of literature. Chin Med 7:598–600. doi:10.3760/cma.j.issn.1673-4777.2012.05.029
Cui L, Zhang JG, Hu XY, Fang XM, Lerner A, Yao XJ, Zhu ZM (2012) CT imaging and histopathological features of renal epithelioid angiomyolipomas. Clin Radiol 67:e77–e82. doi:10.1016/j.crad.2012.08.006
Zhang GX, Fang SL, Qi F, Ding K, Yao ZP, Zhan YF (2011) The clinic-pathological and immunohistopathologic characters of renal epithelioid angiomyolipoma: 2 cases report. Prac Med 27:86–87. doi:10.3969/j.issn.1006-5725.2011.01.036
Wen J, Li HZ, Ji ZG, Mao QZ, Shi BB, Yan WG (2011) Renal epithelioid angiomyolipoma without obvious local progress in 10 years: a case report and literature review. Ir J Med Sci 180:557–560. doi:10.1007/s11845-010-0616-x
Luo D, Gou J, Yang L, Xu Y, Dong Q (2011) Epithelioid angiomyolipoma with involvement of inferior vena cava as a tumor thrombus: a case report. Kaohsiung J Med Sci 27:72–75. doi:10.1016/j.kjms.2010.05.003
Zheng XG, Meng K, Wu B, Zhou XJ (2004) Clinicopathological and immunohistochemical characteristics of renal epithelioid angiomyolipoma: case report. J Med Postgraduates 17:151–154. doi:10.3969/j.issn.1008-8199.2004.02.020
Dai JY, Jue X, Hong L, Li Y, Zhou H, Wang CY, Liu N (2010) Epithelioid angiomyolipoma of the kidney. Chin J Urol 31:595–597. doi:10.3760/cma.j.issn.1000-6702.2010.09.007
Yan LM, Song WJ, Liu ZH, Bai X (2009) Clinicopathologic analysis of 3 cases of renal epithelioid angiomyolipoma. Chin J Clin Oncol 36:1152–1155. doi:10.3969/j.issn.1000-8179.2009.20.004
Wang ZH, Weng HY, Xing XW, Li CY, Wu HB, Wang XQ (2009) Epithelioid angiomyolipoma of kidney: a report of three cases and review of literature. J Clin Exp Pathol 25:367–370. doi:10.3969/j.issn.1001-7399.2009.04.007
Li J, Teng XD, Yan LP, Xiao WB, You QH, Li YY (2008) Malignant renal angiomyolipoma with metastases (report of 3 cases). Chin J Urol 29:744–747. doi:10.3321/j.issn:1000-6702.2008.11.009
Pan CC, Chung MY, Ng KF, Liu CY, Wang JS, Chai CY, Huang SH, Chen PC, Ho DM (2008) Constant allelic alteration on chromosome 16p (TSC2 gene) in perivascular epithelioid cell tumour (PEComa): genetic evidence for the relationship of PEComa with angiomyolipoma. J Pathol 214:387–393
Yang XP, Pan HX, Guo T, He J, Weng MX (2008) Renal epithelioid angiomyolipoma: a clinicopahtological analysis of 3cases. J Clin Urol 23:687–689. doi:10.3969/j.issn.1001-1420.2008.09.013
Huang KH, Huang CY, Chung SD, Pu YS, Shun CT, Chen J (2007) Malignant epithelioid angiomyolipoma of the kidney. J Formos Med Assoc 106:S51–S54
Meng YH, Pei F, Lu P, Yu JY, Zheng J (2007) Epithelioid angiomyolipoma of kidney: clinicopathologic study of two cases and review of literature. Chin J Pathol 36:19–23. doi:10.3760/j.issn:0529-5807.2007.01.007
Zhou L (2006) Renal epithelioid angiomyolipoma (One case). ShanXi Med J 35:470–471. doi:10.3969/j.issn.0253-9926.2006.05.069
Trpkov K, Grignon DJ, Bonsib SM, Amin MB, Billis A, Lopez-Beltran A, Samaratunga H, Tamboli P, Delahunt B, Egevad L, Montironi R, Srigley JR; members of the ISUP Renal Tumor Panel (2013) Handling and staging of renal cell carcinoma: the International Society of Urological Pathology Consensus (ISUP) conference recommendations. Am J Surg Pathol 37:1505–1517. doi:10.1097/PAS.0b013e31829a85d0
Acknowledgments
The authors thanked Professor Ximing J. Yang from Northwestern Memorial Hospital, Northwestern University, Feinberg School of Medicine for his kind advises to this manuscript. The authors also thanked Dr. Guangyuan Zheng from HuaZhong University of Science and Technology and Dr. Zhiyong Ren from Department of Pathology, Huntsman Cancer Institute, University of Utah for their kind advises to this manuscript.
Funding
This project was supported by Beijing Hope Run Special Fund (LC2011B35).
Author contributions
S.Z. and X.G.B. helped to interpret the data and drafted the initial manuscript. Q.K.S. helped to analyze the data. H.T.Z. and J.H.M. helped to design the study. S.Z., Z.Y. and H.T.Z. helped to review the slides of all the 11 cases from the participating Cancer Hospital, Chinese Academy of Medical Sciences, in China. X.G.B. and J.H.M. helped to review all the clinical information and follow up all the 11 cases from the participating Cancer Hospital, Chinese Academy of Medical Sciences, in China. G.L. helped the data management. H.T.Z. and J.H.M. both did critical revisions of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Additional information
Shan Zheng and Xin-gang Bi have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zheng, S., Bi, Xg., Song, Qk. et al. A suggestion for pathological grossing and reporting based on prognostic indicators of malignancies from a pooled analysis of renal epithelioid angiomyolipoma. Int Urol Nephrol 47, 1643–1651 (2015). https://doi.org/10.1007/s11255-015-1079-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-015-1079-9