Abstract
Purpose
The age-related decline of the testosterone-to-estrogen (T-to-E2) ratio in serum is associated with the increased prevalence of prostatic inflammation. The goal of the study was to induce prostatic inflammation with E2 and androgen treatment and to explore the inflammatory markers and apoptosis on prostatitis.
Methods
Castrated SD rats were treated with E2 and different doses of androgens to achieve an elevated concentration of E2 and a wide range of the androgen-to-E2 ratio in serum. Inflammatory markers TNF-α, COX-2 and MIP-1α were immunohistochemically stained. Apoptosis detection was evaluated by TUNEL staining. E2, T and DHT concentrations in serum were measured, and the relative weight of the prostate and seminal vesicles were determined.
Results
T was anti-inflammatory at the doses which normalized or over stimulated the growth of the prostate and seminal vesicles. Experimentally, prostatitis induced by E2 alone increased the prostatic levels of the inflammatory markers TNF-a, COX-2 and MIP-1a. As signs of anti-estrogenic actions, androgens dose-dependently decreased the expression of TNF-α, COX-2 and MIP-1α. Prostatitis induced by E2 alone caused extensive apoptosis in the castrate-resistant cells and E2-induced apoptosis occurred dependently of T manipulation.
Conclusions
Estrogen-alone-induced inflammatory response could promote the expression of inflammatory markers; however, T supplementation reduces the expression of inflammatory markers and E2-induced apoptosis occurs dependently on T manipulation in prostatitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostatitis is the common urological diagnosis in men which is poorly defined and difficult to diagnose and treat. Initial studies have estimated that up to 50 % of men may suffer from prostate inflammation at some point in their life [1]. According to the National Institutes of Health (NIH) classification of prostatitis in 1999, chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) is the most prevalent type, accounting for at least 90 % of all the prostatitis cases. It is ascribed to unknown (nonbacterial) origins, and the symptoms, both acute and chronic, are common, bothersome, and burdensome in terms of health-related quality of life [2, 3]. The etiology and pathogenesis of CP/CPPS are unknown.
One possible initiator of the inflammation is the hormone. Environmental pollutants and industrial chemicals disrupt and have the potential to alter the action of gonadal steroid hormones by virtue of their anti-androgenic or estrogenic properties and, in so doing, affect the hormonal balance [4–6]. Besides, the age-related increased prevalence of CP/CPPS is associated with the decrease of the testosterone (T) concentration and T-to-estrogen (E2) ratio (T-to-E2 ratio) in serum [7–9]. The association can be explained by the weakening anti-inflammatory action of T and the intensifying proinflammatory influence of E2 [10]. The causal role of the decreased ratio of T-to-E2 is primarily supported by the findings in preclinical models of human prostatic diseases.
In theory, when androgen concentrations decline and estrogen concentrations remain unaltered or elevate with the age and the hormonal balance is disrupted by environment [11, 12], the unbalanced estrogen may induce inflammation. Androgen substitution would be then expected to balance estrogen actions and have preventive actions. In this study, the impact of the estrogen and androgen was studied on the development of nonbacterial inflammation, the expression of inflammatory biomarkers and the apoptosis of the prostate of the adult SD rat.
Methods
Animals
Adult male Sprague–Dawley (SD) rats, weighing 320–370 g, were purchased from SIPPR-B&K laboratory animal Corp. [License Number: SCXK (Shanghai) 2008-0016]. Rats were housed four per cage in an animal room maintained at 20–26 °C and 40–70 % relative humidity with an alternating 12-h light/dark cycle(the lights came on automatically at 6:00 a.m.). They were given free access to tap water and food. All protocols were approved by Shanghai Institute of Planned Parenthood Research Animal Care. Care was taken to minimize discomfort, distress and pain to the animals.
Surgery and experimental groups
SD rats were castrated via the scrotal route by removing the epididymal fat pads with the testes under general anesthesia. Rats were divided into 6 groups of 8 rats each: (1) the normal control group, (2) the castrated control group, (3) the E2-treated (0.25 mg/kg/day) group, (4) the E2 + T(0.25 mg)-treated group, (5) the E2 + T(0.50 mg)-treated group and (6) the E2 + T(1.0 mg)- treated group. The experimental protocol according to the method of Bernoulli J et al. [9] is shown in Table 1. E2 and T were dissolved in olive oil. E2 was injected subcutaneously at a dose of 0.25 mg/kg/day; however, T was injected subcutaneously at the dose of 0, 0.25, 0.50 and 1.0 mg/kg/day, respectively. In the normal control group and castrated group, rats were treated only with olive oil by subcutaneous injection, but without drugs. Hormone/vehicle was injected subcutaneously for 30 days (Table 1).
Relative of prostate and seminal vesicle weights and histopathology
Rats were weighed twice a week and killed on the 31st day, and the prostate and seminal vesicles were extirpated. After the prostate gland weight was measured without bladder or seminal vesicles, both lateral lobes of prostates were dissected and used for histopathologic evaluation. Relative weights were obtained by dividing the organ weight by body weight and multiplying by 100. Thereafter, prostate samples were fixed in neutral 10 % formalin solution for 24 h at room temperature, dehydrated in ethanol, cleared in xylene and embedded in paraffin. For routine histological analysis with hematoxylin and eosin (H&E) or immunohistochemical methods, the prostate lateral lobe sections of paraffin-embedded tissues were cut at 4–6 µm thickness. Tissues were considered positive for prostatitis if one area of inflammatory cell infiltration was observed in a microscopic section. Prostatic lesions were observed by two pathologists (Dr Yan and Dr Liu) who had no knowledge of the animal treatment group.
Serum hormone measurements
On the 31st day, rats were anesthetized with 3 %pentobarbital sodium. Blood samples were collected by abdominal aorta. Blood was centrifuged for 15 min (3,000 rpm, 4 °C), and the supernatant was immediately transferred to a tube. Serum samples were stored at −80 °C until the measurement of hormone concentrations. Thereafter, E2, DHT and T concentration was measured by E2, DHT and T immunoassay kit according to the instructions given by the manufacturer (E2 ELISA for rat serum, DHT ELISA for rat serum and T ELISA for rat serum, Hufeng Chemical Co., Ltd, China), using spectrophotometer at 450 nm.
Immunohistochemical staining of TNF-α, COX-2 and MIP-1α
Tissue sections were deparaffinized and rehydrated, and antigens were retrieved by incubating sections in a microwave oven by using 10 MM sodium citrate buffer, pH 6.0, for 15 min. Sections were allowed to cool and were rinsed with PBS buffer. They were incubated with primary antibodies overnight at +4 °C. TNF-α, COX-2 and MIP-1α antibodies were diluted 1:150, respectively, in PBS. On the following day, the sections were washed with PBS and incubated for 30 min with secondary antibody (HRP-conjugated anti-mouse or anti-rabbit secondary antibody) at room temperature according to the manufacturer’s instructions. Color was developed with diaminobenzidine substrate. Sections were then slightly counterstained with Mayer’s hematoxylin, dehydrated and mounted.
Apoptosis detection in tissue sections
TUNEL staining was performed using the DeadEnd™ Colorimetric Apoptosis Detection System (Promega, USA) with the following changes to the recommended protocol.
-
1.
The sections were deparaffinized and hydrated.
-
2.
Nuclei of the sections were stripped of proteins by incubation with 20 µg/ml proteinase K for 15 min at room temperature.
-
3.
The sections were equilibrated by equilibration buffer at room temperature for 5 min.
-
4.
TdT reaction mix was added to the tissue sections on the slides. Slides were covered with plastic coverslips to ensure even distribution of the mix. Slides were incubated for 60 min at 37 °C in a humidified chamber.
-
5.
The reaction was terminated by transferring the slides to 2X SSC for 15 min.
-
6.
Endogenous peroxidase was inactivated by 0.3 % hydrogen peroxide for 5 min at room temperature.
-
7.
The sections were covered with Streptavidin HRP (diluted 1:500 in PBS), incubated for 30 min at room temperature and stained with DAB and developed until a light brown background appears.
-
8.
Finally, counterstaining was done using Mayer’s hematoxylin.
Statistical analysis
All data were presented as mean ± SD. Analysis by one-way ANOVA was used to assess statistical differences of data obtained in the normal control and treated groups. SPSS ver. 16.0 for windows was used. P values lower than 0.05 were considered significant.
Result
Body weight and relative weights of the organs
There was a significant decrease in body weight of rats that were castrated and subsequently administrated with estrogen or androgen hormones (Fig. 1a). T had no effect on the body weight of estrogen-treated, castrated rats.
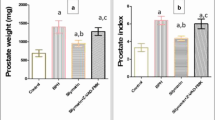
Body weight and relative weights of the organs. a Effect of E2 +T and oil on body weight. b Relative weight of prostate. Values are mean ± SD. c Relative weight of the seminal vesicles. Values are mean ± SD. *Significantly different from the normal control group at P < 0.05. **Significantly different from the normal control group at P < 0.01. ##Significantly different from the castrated treated at P < 0.01
The castrated control and E2-treated groups showed significant reduction in the relative weights of prostate compared with the normal control group; however, no significant difference in the relative weights of prostate was noted between the two groups (Fig. 1b). Administration of T and estrogen rats increased the prostate weights significantly. T increased the prostate weight in a dose-dependent manner.
E2 administration to castrated rats slightly increased the relative weights of the seminal vesicles compared to the castrated control group, (Fig. 1c). Seminal vesicle weights increased further when estrogen treatment was combined with T (Fig. 1c). The weight-increasing effect of T was dose dependent.
Histopathology
In the normal control group, there was a normal appearance of the glandular epithelium and stroma with no obvious leukocyte infiltration into the lumina and stroma in all rats. On the other hand, extensive infiltration of inflammatory cells in the lumina, mononuclear cells in the stroma of the gland and epithelial degeneration were observed in the hormone-treated group, suggesting CP (Fig. 2).
Histopathologic findings of the prostate in 6 groups (hematoxylin and eosin stain, 400×). a The normal control group showed normal appearance of the glandular epithelium without leukocyte infiltration. b In castrated control group, there was no inflammatory cell found in the prostate. c In the E2-treated group, extensive infiltration of inflammatory cells in the glandular lumens was found suggesting chronic prostatitis. d In the E2 + T(0.25 mg)-treated group, less inflammatory cells were noted in the stroma between and around the glands. e In the E2 + T(0.50 mg)-treated group, less inflammatory cells were noted in the stroma between and around the glands. f In the E2 + T(1.0 mg)-treated group, less inflammatory cells were noted in the stroma between and around the glands
Serum hormone concentrations (E2, T and DHT)
Total concentrations of the unconjugated E2, T and DHT in serum are shown in Fig. 5a, b, c, respectively. E2 administration significantly increased the E2 concentrations in E2-treated group. T had no significant effects on serum E2 concentration in any group (Fig. 3a). T concentrations significantly decreased in E2-treated group and E2 + T(0.25 mg)-treated group, compared with normal control group (Fig. 3b). T doses of 0.5 mg⁄kg and 1.0 mg⁄kg resulted in total concentration of T in serum (116.07 nmol/L and 120.47 nmol/L), which did not differ significantly from the concentration of untreated, non-castrated rats (135.92 nmol/L). Serum DHT concentrations did not differ significantly compared to untreated, non-castrated rats (Fig. 3c).
Serum estrogen (a) testosterone (b) and dihydrotestosterone (c) concentrations in serum. Values are mean ± SD.*Significantly different from the normal control group at P < 0.05. **Significantly different from the normal control group at P < 0.01. ##Significantly different from the castrated treated at P < 0.01
Determination of inflammatory markers TNF-α, COX-2 and MIP-1α
The expression of TNF-α, COX-2 and MIP-1α in normal control group is negative or not significant. However, the levels of the proinflammatory cytokines TNF-a, COX-2 and MIP-1α in the prostate of the male SD rat nonbacterial model were significantly increased in the E2-treated group compared to the sham-operated group and castrated control group. The expressions of TNF-α, COX-2 and MIP-1α in the co-administration of estrogen and androgen group were significantly decreased (Fig. 4).
TNF-α (A–F), COX-2 (G–L) and MIP-1α (M–R) immunohistochemical staining of the prostate. Weakly positive TNF-α, COX-2 and MIP-1α were detected in normal control group. E2 treatment induced TNF-α, COX-2 and MIP-1α-expression (C, I, O), whereas androgen treatment blocked their expression in the prostate
Apoptosis detection in tissue sections
Contemporary morphometric analysis shows that castration significantly increased epithelial but not stromal apoptosis, whereas E2 alone significantly increased epithelial and/or stromal apoptosis compared with normal control group and castrated control group in SD rats (Figs. 5, 6). These data showed that E2 uniquely caused apoptosis in the castrate-resistant cells, reducing cell proliferation and increasing apoptosis in the epithelial and stromal cells of prostatitis.
Apoptosis cells (%) on prostatitis induced by E2 and T. Values are mean ± SD. *Significantly different from the normal control group at P < 0.05. **Significantly different from the normal control group at P < 0.01. #Significantly different from the castrated treated at P < 0.05. ##Significantly different from the castrated treated at P < 0.01
To determine whether the mechanism of E2 action was androgen independent, we compared the effect of doses of androgen supplementation on apoptosis in prostatitis. Morphometric analyses showed that apoptosis in E2 + T (0.25 mg)-treated group is largely consistent with the castrated control group. Quantification of apoptosis (%) in the dose (0.50 mg and 1.0 mg) of T significantly decreased compared with the castrated control group (P < 0.05 and P < 0.01). T supplementation altered the apoptotic response to E2 in cellular compartment of the epithelium or the stroma in a dose-dependent manner (Figs. 5d, e, f, 6).
Discussion
Although an appropriate animal model that mimics human prostatitis has not been established yet, some hormone-induced models produced by co-administration with estrogen and T or by administration with estrogen alone in castrated rats have been proposed to elucidate the mechanisms of the molecular pathology of nonbacterial prostatitis (NBP) [13]. In the present study, we studied the effects of the estrogen to T ratio on the development of prostatitis. Co-administration with estrogen and T results in a significant increase in the relative weight of the prostate and seminal vesicles, although some reports show that co-administration of exogenous T, with its anti-inflammatory effect, attenuates the estrogen-induced increase in the incidence and severity of prostatitis [14]. The prostate gland and seminal vesicles are androgen-dependent organs in males. Therefore, androgens play a key role in the regulation of prostatic growth, function and disease. When the androgen level drops below a threshold, as is the case after surgical or chemical castration, the secretory cells undergo apoptosis, causing glandular involution. They will regrow to original size upon androgen replacement.
The ratio of estrogen and T retains a significant and negative association with the majority of inflammatory markers. Although association does not mean causality, it is possible to speculate that a decline in T and/or a rise in estrogen might be responsible for prostatic inflammation [15]. In the present study, the lower dose (0.25 mg) of T normalized the weight of the prostate and seminal vesicles while the highest dose (1.0 mg) promoted overgrowth of the prostate and seminal vesicles, which support the fact that androgen is a crucial hormone for prostate and seminal vesicles development. T was anti-inflammatory, but even the highest dose (1.0 mg) did not eliminate the inflammatory response completely. This implies that T substitution in doses may yield useful preventive effects on the prostatic inflammation and subject the sex accessory glands to more intense androgenic stimulation than is normal for the male. Thus, T may not be optimal for the prevention and treatment of estrogen-related prostatic inflammation [16]. All these features are markedly counteracted by T supplementation. Hence, T prevents but not induces prostatic diseases.
Cytokines, including TNF-α, are regulatory proteins that are released by various types of cells and that promote intercellular communication and immune responses. Chemokines, such as MIP-1a, are chemotactic cytokines that recruit and activate immune cells at sites of inflammation. In the present study, the proinflammatory cytokines TNF-α and COX-2 and the chemokine MIP-1α were significantly increased in the E2-treated group compared with the normal control group and castrated control group, which is consistent with the previous studies [17, 18]. T supplementation reduces the expression of proinflammatory cytokines and the chemokines, as reported by other investigators [19, 20]. However, the precise mechanisms of T-mediated immunomodulation are still unknown.
There is a close relationship between hormone and cell apoptosis. Androgens promote cell proliferation and differentiation [21]. Castration is effective because it reduces cell proliferation, but as the rate of proliferation in the human prostate gland is relatively low [22–24], its most important effect is to promote apoptosis and cell death. Our study showed that E2 uniquely caused apoptosis in the castrate-resistant cells, reducing cell proliferation and increasing apoptosis in the epithelial and stromal cells of prostatitis [25]. E2-induced apoptosis occurs dependently of testosterone manipulation. Therefore, our study provides insight into its mechanism of action and cellular targets.
The present results suggest that the androgen concentrations required to antagonize the inflammatory action of increased estrogen might over stimulate the prostate growth. Hence, the effects of estrogen and testosterone on the mechanism of prostatitis would require further studies in the future.
References
Kim DS, Lee EJ, Cho KS, Yoon SJ, Lee YH, Hong SJ (2009) Preventive effects of oligomerized polyphenol on estradiol-induced prostatitis in rats. Yonsei Med J 50(3):391–398
McNaughton Collins M, Pontari MA, O’Leary MP, Calhoun EA, Santanna J, Landis JR, Kusek JW, Litwin MS (2001) Quality of Life Is Impaired in Men with Chronic Prostatitis the Chronic Prostatitis Collaborative Research Network*. J Gen Intern Med 16(10):656–662
Turner JA, Ciol MA, Von Korff M, Berger R (2005) Health concerns of patients with nonbacterial prostatitis/pelvic pain. Arch Intern Med 165(9):1054–1059
Foster P (2006) Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. Int J Androl 29(1):140–147
Foster PM, Mcintyre BS (2002) Endocrine active agents: implications of adverse and non-adverse changes. Toxicol Pathol 30(1):59–65
Kelce WR, Wilson EM (1997) Environmental antiandrogens: developmental effects, molecular mechanisms, and clinical implications. J Mol Med 75(3):198–207
Vermeulen A, Kaufman J, Goemaere S, Van Pottelberg I (2002) Estradiol in elderly men. Aging Male 5(2):98–102
Kaufman JM, Vermeulen A (2005) The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev 26(6):833–876
Bernoulli J, Yatkin E, Konkol Y, Talvitie EM, Santti R, Streng T (2008) Prostatic inflammation and obstructive voiding in the adult Noble rat: impact of the testosterone to estradiol ratio in serum. Prostate 68(12):1296–1306
Straub RH (2007) The complex role of estrogens in inflammation. Endocr Rev 28(5):521–574
Schneider G, Kirschner MA, Berkowitz R, Ertel NH (1979) Increased Estrogen Production in Obese Men*. J Clin Endocrinol Metab 48(4):633–638
Cowin PA, Foster P, Pedersen J, Hedwards S, McPherson SJ, Risbridger GP (2008) Early-onset endocrine disruptor induced prostatitis in the rat. Environ Health Perspect 116(7):923–929
Vykhovanets E, Resnick M, MacLennan G, Gupta S (2007) Experimental rodent models of prostatitis: limitations and potential. Prostate Cancer Prostatic Dis 10(1):15–29
Naslund MJ, Strandberg J, Coffey D (1988) The role of androgens and estrogens in the pathogenesis of experimental nonbacterial prostatitis. J Urol 140(5):1049–1053
Vignozzi L, Morelli A, Sarchielli E, Comeglio P, Filippi S, Cellai I, Maneschi E, Serni S, Gacci M, Carini M (2012) Testosterone protects from metabolic syndrome-associated prostate inflammation: an experimental study in rabbit. J Endocrinol 212(1):71–84
Yatkin E, Bernoulli J, Lammintausta R, Santti R (2008) Fispemifene [Z-2-{2-[4-(4-chloro-1, 2-diphenylbut-1-enyl)-phenoxy] ethoxy}-ethanol], a novel selective estrogen receptor modulator, attenuates glandular inflammation in an animal model of chronic nonbacterial prostatitis. J Pharmacol Exp Ther 327(1):58–67
Sugimoto M, Oka M, Tsunemori H, Yamashita M, Kakehi Y (2011) Effect of a phytotherapeutic agent, Eviprostat®, on prostatic and urinary cytokines/chemokines in a rat model of nonbacterial prostatitis. Prostate 71(4):438–444
Seiji MYK, Michiko O et al (2013) Bladder function in 17 β-estradiol- induced nonbacterial prostatitis model in Wister rats. Int Urol Nephrol 45:749–754
Bebo BF, Schuster JC, Vandenbark AA, Offner H (1999) Androgens alter the cytokine profile and reduce encephalitogenicity of myelin-reactive T cells. J Immunol 162(1):35–40
Fijak M, Schneider E, Klug J, Bhushan S, Hackstein H, Schuler G, Wygrecka M, Gromoll J, Meinhardt A (2011) Testosterone replacement effectively inhibits the development of experimental autoimmune orchitis in rats: evidence for a direct role of testosterone on regulatory T cell expansion. J Immunol 186(9):5162–5172
Isaacs JT (1984) Antagonistic effect of androgen on prostatic cell death. Prostate 5(5):545–557
Schleipen B, Hertrampf T, Fritzemeier K, Kluxen F, Lorenz A, Molzberger A, Velders M, Diel P (2011) ERβ-specific agonists and genistein inhibit proliferation and induce apoptosis in the large and small intestine. Carcinogenesis 32(11):1675–1683
English HF, Santen RJ, Lsaacs JT (1987) Response of glandular versus basal rat ventral prostatic epithelial cells to androgen withdrawal and replacement. Prostate 11(3):229–242
Hussain S, Lawrence MG, Taylor RA, Lo CY-W, BioResource A, Frydenberg M, Ellem SJ, Furic L, Risbridger GP (2012) Estrogen receptor β activation impairs prostatic regeneration by inducing apoptosis in murine and human stem/progenitor enriched cell populations. PLoS One 7(7):e40732
McPherson SJ, Hussain S, Balanathan P, Hedwards SL, Niranjan B, Grant M, Chandrasiri UP, Toivanen R, Wang Y, Taylor RA (2010) Estrogen receptor–β activated apoptosis in benign hyperplasia and cancer of the prostate is androgen independent and TNFα mediated. Proc Natl Acad Sci 107(7):3123–3128
Acknowledgments
This work was supported by National Science and Technology Major Project of Original New Drug Research of China (Grant No.2011ZX09301-005) and Shanghai “Innovative Action Plan” Experimental Animal Study (Grant No.11140901300 and 11140901303).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Jia, Yl., Liu, X., Yan, Jy. et al. The alteration of inflammatory markers and apoptosis on chronic prostatitis induced by estrogen and androgen. Int Urol Nephrol 47, 39–46 (2015). https://doi.org/10.1007/s11255-014-0845-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-014-0845-4