Abstract
Urban areas consist of wide expanses of impervious surfaces which are known to negatively affect insect biodiversity in general, but green spaces within cities have the potential to provide necessary habitat and foraging resources. Although, communal gardens were primarily intended to provide fresh, regional food to denizens, these green islands also host a surprisingly high number of wild bee species.
The gardens were characterized based on structural elements such as flower frequency, the relative percentage of lawn, trees, shrubs, planted crops and infrastructure (e.g. seating possibilities or garden houses). Further, the effects of different landscape structures surrounding the gardens and distance to the city center were analyzed on the total wild bee species richness and functional traits. Focusing on these putative influencing factors, statistical analyses calculating random decision forests along with generalized linear mixed models were applied. With 113 observed wild bee species, communal gardens provide habitat for a quarter of all known species in Vienna. In conclusion, results revealed that only elements within the gardens had an effect on species richness, with flower frequency as the major positive driver. The examined communal gardens promote and conserve wild bees independent from the location within the city or garden size. Furthermore, these green patches are important sanctuaries, hosting rare and threatened species as well as remarkably special wild bee communities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cities have grown steadily since 1950 and more than half of the world’s population along with 74% of all Europeans live in urban areas (United Nations 2018). Increasing urbanization and associated changes in the surrounding environment lead to habitat loss or degradation, which is an actual threat for the local wild bee biodiversity (Biesmeijer 2006). Besides fewer foraging and nesting possibilities for wild bees resulting from soil-sealing, expanding urban areas bring with them increased anthropogenic pressures. Additionally, exotic plants often planted as ornamental flowers in parks and flowerbeds influence the native insect community in cities. For these reasons urbanization is negatively associated with wild bee species richness (Hernandez et al. 2009; Ahrné et al. 2009; Banaszak-Cibicka and Żmihorski 2012).
Conversely, urban areas also provide special and diverse microclimates for several species. Green spaces in cities have the potential to provide habitats for insects and maintain corridors between green patches for movement (Smith et al. 2006). Moreover, a long-term study evaluating intrinsic and extrinsic extinction traits of local bee communities has shown that bees, which occur in urban areas, have a lower extinction risk in contrast to wild bees preferring other habitats, like forests or wastelands (Hofmann et al. 2019). In general, cities are warmer and drier due to infrastructure and heat generated by human activity. Some species have adapted to these conditions and can be considered urbanophilic. This is why, wild bee communities within these urban heat islands (UHIs) differ in comparison to surrounding landscapes. Urban habitats enable wild bees to emerge earlier and thermophilic species are able to settle within cities (Banaszak-Cibicka 2014; Fortel et al. 2014). But also, plants can benefit from the microclimatic conditions found in urban habitats. UHIs lead to an earlier ripeness of crops, an affect which is utilized by the increasingly more popular urban agriculture movement. Urban agriculture has the potential to provide environmental, economic and social values, through its ability to reduce the ecological footprint of a city, while increasing quality of life and allowing inhabitants to experience nature (Potter and LeBuhn 2015). Green urban areas have important ecological impacts such as cooling effects or absorbing water from rainfalls (Bowler et al. 2010; Norton et al. 2015). Additionally, in rapidly growing cities, urban gardening is one possible answer to the growing demand for regional, sustainable and safe food production (Gunnarsson and Federsel 2014). Whereas, the most common motivating factors are being in contact with nature and the ability to consume fresh food (Matteson and Langellotto 2010; Guitart et al. 2012). Urban gardens also have the potential to conserve the local biodiversity as some crops act as important foraging resources for flower-visiting wild bees, as a variety of plants provide pollen, nectar or both (Matteson and Langellotto 2010). Intensification of agriculture is one of the major drivers causing insects decline globally (Sánchez-Bayo and Wyckhuys 2019). On the contrary, a recently published study revealed that communal gardens in four UK cities have the potential to counteract this trend as these gardens were shown to be diversity hotspots for wild bees (Baldock et al. 2019).
Communal gardens are defined as open space designed gardens, where the local community cultivates flowers and crops (Holland 2004; Kingsley et al. 2009). The production of crops like apples, cherries and pears, which are important crops in Austria, is highly dependent on insect pollination (Leonhardt et al. 2013). City beekeeping is gaining more and more popularity (Lorenz and Stark 2015; Stange et al. 2017). In Vienna 5000 honey bee hives are situated in the city center (Magistrat der Stadt Wien 2019). But within this study, the focus is on wild bees as one of the most important pollinator group for crops planted in urban areas. These important ecosystem service providers pollinate multiple plant species enhancing the fruit yield as well as the seed set of entomophilous plants (Lowenstein et al. 2015). Wild bees can be considered key species in urban areas. They are not just simply pollinators but are also members of trophic webs and represent important prey for different organisms, such as birds or wasps. Additionally, wild bees have the potential to raise awareness of urban biodiversity to city inhabitants and sensitize to conservation actions (Banaszak-Cibicka and Żmihorski 2012; Fortel et al. 2014).
Wild bees in urban habitats have been a research topic in several projects investigating cities like Gothenburg, Poznan, Lyon, Vancouver and San Francisco (Tommasi et al. 2004; Banaszak-Cibicka and Żmihorski 2012; Fortel et al. 2014; Gunnarsson and Federsel 2014; Potter and LeBuhn 2015). Furthermore, communal gardens have been well studied by social sciences, but not so much by the natural sciences (Alaimo et al. 2010; Firth et al. 2011; Guitart et al. 2012). Most biological research of community gardens has been performed in the UK, New York City and Sydney, but information about Central European communal gardens is lacking completely (Matteson and Langellotto 2010; Makinson et al. 2017; Baldock et al. 2019). The capital city of Austria is an exceptionally species-rich city due to its special climate conditions and extensive natural sites surrounding the city. With 465 reported Apiformes, Vienna has the highest number of species among cities in Central Europe (Zettel et al. 2015, 2016).
Many ecological studies have been carried out along urban-to-rural gradients, although these gradients do not often reflect the importance of (semi-) natural structures within urban areas. Green patches within cities are of great significance for projects concerning urban biodiversity (Ramalho and Hobbs 2012). To gain knowledge of the local wild bee biodiversity in urban agriculture, twelve communal gardens in Vienna were investigated. As flight distance is a function of body length, especially for small bee species it is crucial that nesting and foraging resources are only a few hundred meters apart (Gathmann and Tscharntke 2002; Zurbuchen et al. 2010). In close proximity to the city center, the proportion of impervious surface increases, while the amount of green areas decreases. This is why, the effect of the distance to the city center on species richness was tested. However, species diversity data alone is not always sufficient evaluating the habitat quality for bees (Sheffield et al. 2013), this is why we determined species-specific life history traits (LHTs) such as nesting and foraging types for the gardens’ bee communities. The aim of this study was to answer the following research questions: (1) Which wild bee communities are attracted to communal gardens? (2) How does the flower frequency and certain bee-attracting plants within communal gardens influence the bee community? (3) How are different garden structures and surrounding landscape structures affecting wild bee species richness?
Material and methods
Study location
Vienna, the capital city of Austria, counts 1,888,776 inhabitants in 2018, which is an increase of 13% in comparison to the year 2008 (Himpele 2018). With a unique geographical location underlined by influences of the Atlantic and the continental climates, the average annual temperature was 12.7 °C with a maximum of 38.9 °C and a minimum of −10.4 °C in the year 2017, whereas the average annual precipitation was approximately 563 mm (ZAMG 2017). Vienna is characterized by spacious natural habitats surrounding the city, such as the Lobau as part of the Danube Floodplains National Park and the biosphere reserve Wienerwald. Therefore, the city includes 49.6% green areas at 41,487 ha and presents an exceptional interesting region for investigations into wild bee biodiversity (Himpele 2018).
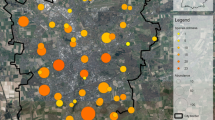
Wild bees were studied in twelve communal gardens (Fig. 1) during seven periods between the end of June through the end of August 2016 and from early March until June 2017 in three weeks intervals. All community gardens were part of the “Gartenpolylog” club, which manages 69 gardens in Vienna (Madlener 2007). The twelve gardens are located throughout Vienna and were selected according to the amount of interest from the gardeners in supporting the present project. Nevertheless, as every examined garden cultivated crop in Vienna, in this study community gardens are synonymously exchangeable as urban agriculture.
Species sampling
Wild bees were collected by hand-netting specimens. The average sampling duration was 64 min per garden, but was adjusted to the respective garden size. The study focused on species richness and did not consider species abundance, thus only a few individuals per species were collected.
Sampling took place between 10 am and 4 pm in dry weather conditions, moderate wind and an average temperature of 24 °C (minimum of 16 °C in March and a maximum of 30 °C in June). Wild bees were stored in a snap cap with ethyl acetate and later prepared and identified at species level using appropriate literature (ScheuchI 1995; Amiet 1996, 2001; Scheuchl 1996; Schmid-Egger and Scheuchl 1997; Amiet et al. 2004; Gokcezade et al. 2010). Sampled specimens are stored in the wild bee collection at the Institute for Integrative Nature Conservation Research (University of Natural Resources and Life Sciences, Vienna). The majority of individuals of the genus Bombus were identified in the field alive and released afterwards. Counts of Apis mellifera L., 1758 were not included, as it is a domesticated species and two gardens hosted bee hives which would likely lead to bias in the results. Additionally, each garden was examined for nesting activities of wild bees. Species-specific ecological information (LHTs) the nesting type, sociality and floral specificity were compiled from primary literature (Westrich 1989, 2018; Michener 2007; Amiet and Krebs 2014; Scheuchl and Willner 2016) and described in Table 1.
Garden and landscape characters
The garden sizes ranged from 120 m2 to 4000 m2. On each sampling date, the communal gardens were characterized by the flower frequency as a proxy for foraging resources of wild bees. Studies have shown that a visual estimate of the floral cover is an acceptable and widely used method in ecology projects (Gallegos Torell and Glimskär 2009). Therefore, the relative flower frequency was estimated according to a previous project (Gunnarsson and Federsel 2014). All blooming plants were estimated with regard to each garden in four categories: “none” (<11%), “low” (11–25%), “moderate” (26–50%) to “high” (>51%). Additionally, the cover of the four major garden structures (i.e. crop plants; trees & shrubs; lawn; infrastructure) in each communal garden was estimated. On one hand, crops, trees and shrubs constitute an important foraging resource for these pollinators. On the other hand, a well-tended lawn and garden infrastructure, such as garden houses or seating accommodations can hardly be utilized by wild bees (Grimm et al. 2008; Wastian et al. 2016; Fischer et al. 2016; Zhao et al. 2019). Plants, where wild bees were observed feeding on pollen and/or nectar, were identified immediately or photos were taken for a later identification at least at the genus level. A detailed list including site coordinates, size and structure is attached in appendix Table 5.
The urban structures were mapped in ArcGis 10.4 (Environmental Systems Research Institute 2016) within a 500 m radius around each community garden. The data was extracted from the geodata viewer of the city of Vienna (Stadt Wien 2015). The urban structure data sets were summarized into 8 categories (Table 2). The percentages of urban structures within each circle were used for statistical analyses. Further entities (landscape types) such as agricultural lands, vineyards, wood and water areas were not included in the analyses as they occurred infrequently and therefore, would have led to biased statistical results.
Data analysis
Data analyses were carried out using the program R (R Core Development Team 2016). A detailed data exploration was carried out prior to the analyses (Zuur et al. 2010), whereas models including garden size and sampling time were calculated to evaluate possible biases in further calculations.
We tested the effect of a set of variables on wild bee species richness, calculating generalized linear mixed models (GLMM) with a Poisson error distribution and sampling interval as a random structure to account for temporal dependence of sampling with the R package “lme4” (Bates et al. 2014). Therefore, a model set of 21 GLMMs were calculated. For these model sets either one predictor (i.e. flower frequency, garden structures, landscape structures) or a combination were included. The entity “distance to the city center” and species richness of important crops were modeled without the flower frequency as they appeared to be collinear. In regard to GLMMs including the flower frequency, the category “none” was defined as baseline for parameter estimation. Model selection was performed using the second order Akaike Information Criterion (AICc), which is suitable for small sample sizes (Motulsky and Christopoulos) with the R package “AlCcmodavg” (Mazerolle 2017). A cut-off ∆AICc ≥2 was defined previously and all models with an AICc difference > 2 from the most parsimonious model were not considered further to determine the reliability of the data.
To analyze the effect of the predictors on the species richness of LHTs a two-step approach was applied. First, for each trait a Random Forest (RF) with 500 trees was calculated using the R package “party” (Hothorn et al. 2018). The “Variable Importance” (VI) for each RF was based on a conditional permutation scheme (Strobl et al. 2008, 2009) and applied to assess which predictors were most important for the LHTs. In the second step GLMMs were formulated to assess the effect of these most important predictors on the species richness of the LHTs. Again, the sampling event was set as random structure, a Poisson error distribution was applied, and model selection was based on AICc.
Plants and crops found in communal gardens were compared with the primary literature (Pawelek et al. 2009; Zurbuchen and Müller 2012; Westrich 2018) and examined according to their attractiveness as pollen and / or nectar resources for wild bees. A detailed list of the crops can be found in appendix Table 6.
Results
In total, 113 wild bee species belonging to 22 genera were collected in twelve communal gardens in Vienna. A detailed list of all collected species including their LHTs “lecty”, “sociality” and “nesting type” is listed in appendix Table 7. The genus containing the highest number of species counted was Lasioglossum Curtis, 1833 with 23 species followed by Andrena Fabricius, 1775 and Hylaeus Fabricius, 1793 with 12 species. The species richest garden (CG8) hosted 48 wild bee species in comparison to a garden with only 17 (CG9).
Wild bee communities
When considering foraging preferences, the majority of all species were generalists (95 species), as opposed to oligolectic bees (18 sp.). The wild bee community was composed of mainly solitary species (76 sp.). Nesting types were separated into parasitic bees and two main groups of above- (34 sp.) and below-ground (54 sp.) nesting bees (Fig. 2, Tab. 2). In addition to the 59% (54 sp.), which were below-ground nesting species, 22% (25 sp.) cavity nesting wild bees were found in Vienna’s communal gardens. Nesting-activity of wild bees within the gardens were observed for Osmia cornuta (Latreille, 1805) at an artificial bee home as well as for Hylaeus moricei (Friese, 1898) in a reed fence. Honey bees were observed in every communal garden and two gardens hosted bee hives (CG4 & CG5) (Table 3).
Flower frequency and wild bee attracting crops
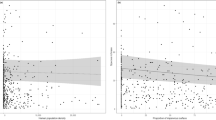
The effect of flower frequency was strongly positive from none to moderate level, but wild bee species richness decreased from the moderate to high level of flower frequency (Fig. 3).
Thirty-seven wild bee-attracting crops and wild flowers were identified in communal gardens. These plants were used by wild bees as resources for nectar, pollen or both. Furthermore, these plant species are known to attract many wild bee generalists and also some foraging specialists. Among these wild bee attracting plants were 12 Asteraceae, 10 Lamiaceae, 3 Rosaceae and Boraginaceae, 2 Malvaceae, one species each of Ranunculaceae, Campanulaceae, Cucurbitaceae, Fabaceae, Apiaceae, Araliaceae and Amaryllidaceae.
In 6 out of 12 communal gardens most wild bee species were found from the end of June to the beginning of July, followed by the sampling period at the end of July (4 gardens). The lowest number of species was found in March (4 gardens) and in April (3 gardens). On average, wild bee species richness in all communal gardens was lowest in March, increasing until the end of June and dropping again until August. Species richness of wild bee-attracting crops and plants showed a similar result, although numbers dropped at the end of June and increased again in July (Fig. 4).
Garden structures and surrounding entities
Another three models including flower frequency and all four observed garden structures “crop”, “trees & shrubs”, “infrastructure” and “lawn” were equaly parsimonious (Fig. 5). The effect of flower frequency did not change (Appendix Table 8). The percentage of “crops”, infrastructure and “trees & shrubs” in the gardens increased wild bee species richness (Fig. 5a-c), while an increasing proportion of lawn had a negative effect on wild bee species richness. Models including flower frequency and landscape structures around the gardens (e.g. buildings, traffic, green areas surrounding the garden, artificial elements) had ∆AIC values higher than 2 and were therefore not considered as influencing variables on wild bee species richness (Table 4).
When considering LHTs and structural elements, the calculated RFs of VI for the traits: “sociality” and “nesting type” presented a clear outcome for flower frequency as the most important predictor. The VI values differed from graph to graph, but it is possible to compare them only for the same response variable. Flower frequency was the most important predictor of species richness for each trait and its effect was the same as in fig. 3 with the largest influence at moderate scales. Other elements within and outside of the communal gardens were of minor importance in contrast to flower frequency (Fig. 6). The proportion of crops as well as trees and shrubs within the gardens were of importance to eusocial bee populations and had a positive effect on the diversity of solitary wild bees. For solitary bee communities the proportion of crops was of greatest significance (Appendix Fig. 7). When examining nesting types, again flower frequency affected both above- and below-ground nesters, but also crops and buildings influenced the species richness of below-ground nesters (Appendix Fig.8). For ground-nesting Apiformes, crops and flower frequency had a positive effect and the increasing proportion of buildings surrounding the communal garden had a negative effect. When analyzing above-ground nesting species only flower frequency had a conclusive VI.
Calculated Random Forests (RF) for structure elements influence on species richness of A: eusocial and B: solitary; C: below-ground nesters, D: above-ground nesting wild bee communities. On the y-axis the structure elements were presented, while on the x-axis the Variable Importance (VI) was applied. In all four graphs A to D flower frequency had an informative VI
Discussion
Within this study, 113 wild bee species were found in twelve communal gardens, which constitutes 25% of all known species in Vienna. Therefore, communal gardens host similar species numbers as Vienna’s roof tops (90 sp.), cemeteries (96 sp.) or the extensive area of the Danube Island (144 sp.) (Pachinger and Hölzler 2006; Pachinger et al. 2014; Kratschmer et al. 2018). Green islands within urban areas have the potential to conserve the local wild bee biodiversity by acting as sanctuaries (Sirohi et al. 2015), as very uncommon or threatened species were found in the investigated gardens. In particular Halictus tectus Radoszkoswki 1876, a species assumed to be locally extinct in Austria was documented in garden CG1. This very uncommon furrow bee as well as the flower bee Anthophora aestivalis (Panzer, 1801) are typically residents of dry habitats such as sand or gravel pits (Wiesbauer 2017), which communal gardens in Vienna can provide through their diverse microhabitats.
Comparison of different studies with previously generated results from literature focusing on species richness may be problematic due to differences in sampling methods and primary study focus. Additionally, every city has different developmental histories and geographic settings resulting in different species assemblages (Sirohi et al. 2015). Thus, studies focusing on communities or guilds specified by their LHTs instead of taxonomy-based groups have the potential to solve these issues and make conclusive comparisons between the same landscape type possible (Sheffield et al. 2013).
Wild bee communities in communal gardens
The majority of all wild bees found were pollen generalists (84%), soil nesting (48%) and solitary (67%), which is comparable to another study in Poland investigating urban public gardens (Banaszak-Cibicka and Żmihorski 2012). In Austria more than 30% are oligolectic species (Zurbuchen and Müller 2012; Amiet and Krebs 2014) in comparison to the low numbers of pollen specialists (16%) found in communal gardens. Outside of this study, other projects also reported a lack of oligolectic species in urban habitats (Matteson and Langellotto 2010; Banaszak-Cibicka et al. 2016; Makinson et al. 2017). In Vienna’s communal gardens mostly crops and a few wild flowers were cultivated. Here, specialized wild bees obtain few if any foraging resources, thus explaining the low species richness of oligolectic species found. Gardens designed with diverse flower compositions, support high species numbers and special communities such as oligolectic bees (Biesmeijer 2006; Goulson et al. 2008; Pawelek et al. 2009). Nevertheless, rare oligolectic representatives observed in communal gardens, were discovered: e.g. the uncommon sand bee Andrena agilissima (Scopoli, 1770), which prefers different cabbage species as foraging resources or Rophites quinquespinosus Spinola, 1808, which forages on Lamiaceae (Falk 2015; Westrich 2018). Species of the families Lamiaceae and Fabaceae are considered especially attractive for bumble and other wild bees (Ahrné et al. 2009). Labiates were often found in every communal garden due to the popularity of herbs like mint, sage or oregano.
When discussing nesting sites, the results revealed that above-ground nesters find appropriate habitat in communal gardens. The proportion of above-ground nesting wild bees is comparable to other studies focusing on green patches in urban areas (Banaszak-Cibicka and Żmihorski 2012; Sirohi et al. 2015). Such an example of an above-ground nesting bee, which benefits from communal gardens, is Ceratina chalybea Chevrier, 1872, which prefers blackberry or rose stems as nesting sites (Wiesbauer 2017).
An unusual discovery was Hylaeus moricei, as it is assumed that it depends on reed galls in wetlands for nesting (Amiet and Krebs 2014; Scheuchl and Willner 2016). Here, H. moricei was captured exiting a reed fence. Therefore, this provides another example where artificial garden structures are able to enhance species richness. Besides this uncommon mask bee, the gardens generally hosted a relatively high proportion of Colletidae with 12% of all species collected. On the contrary, from all known wild bee species in Vienna Colletidae accounted for 41 species (9%) (Gusenleitner et al. 2012; Zettel et al. 2015). The two collected species of the genus Colletes Latreille, 1802 are oligolectic specialists for Asteraceae (Müller and Kuhlmann 2008). Colletes daviesanus Smith, 1846 prefers foraging on Asteraceae like chamomile species (Falk 2015), which are a popular plant in communal gardens. Furthermore, the 12 sampled mask bee species were with the exception of Hylaeus signatus (Panzer, 1789), which feeds exclusively on Reseda (Michener 2007), all generalists. Hylaeus species prefer carrots and diverse allium plants as pollen resources (Gosek et al. 1995; Müller et al. 2006). These tendencies might have led to the high occurrence of Colletidae in communal gardens.
As cavity nesters are not reliant on sparsely vegetated ground patches, they are considered “moderately urbanophilic” wild bees (Fortel et al. 2014). Beside cracks in walls as nesting possibilities, appropriate artificial bee homes are able to host cavity nesting species, such as O. cornuta and Osmia bicornis (Linnaeus, 1758) (Wilkaniec and Giejdasz 2003; Sedivy and Dorn 2014). Therefore, the sampling sites hosted a remarkably high species richness of mason bees (7 species) or leafcutter bees (8 species). Beside the artificial bee home inhabitants, another mason bee, Osmia niveata (Fabricius, 1804), is worth mentioning along with its cleptoparasitic counterpart Stelis punctulatissima (Kirby, 1802), both found in Vienna’s communal gardens.
Within this study, parasitic wild bees composed 11% (12 sp.) of all species found. In comparison, a study on the Danube Island revealed that 19% of the sampled bees were parasitic (Pachinger and Hölzler 2006). Studies have shown that cleptoparasites can act as indicator taxa for the stability of wild bee communities (Sheffield et al. 2013), because cuckoo bees respond quickly to disturbances and thus reflect the high quality and diversity of habitats (Fortel et al. 2014). Therefore, cities can be considered as a disturbing factor resulting in low numbers of parasitic species, as their species richness and abundancy depends on their hosts.
Foraging resources within communal gardens
With the communal garden season starting in March, most flower beds were empty and hardly any crop or wild flower was blooming in early spring. Therefore, foraging resources were probably the major limiting factor in low species numbers found in March and April. Additionally, with the main phenology in June or July, early spring species are generally rare in urban areas (Banaszak-Cibicka and Żmihorski 2012). This is why, not only in Vienna but also other cities (Tommasi et al. 2004), most wild bee species were reported from late spring and early summer.
When considering foraging resources, flower frequency had clearly the strongest effect on wild bee species richness. This means that the availability of pollen or nectar resources was the main factor enhancing wild bee richness in communal gardens. This result conforms with previous studies, which demonstrated that increased flower frequencies enhanced the species richness and abundance of wild bees in parks, ornamental flower beds, green strips along roads and roof tops (Werrell et al. 2009; Hennig and Ghazoul 2012; Gunnarsson and Federsel 2014; Makinson et al. 2017; Kratschmer et al. 2018). The effect of flower frequency on species richness peaked at the moderate level and dropped off at the level “high”. This may be an artifact, due to high flower frequencies being measured only four times in the gardens. Another possible explanation might be that the mass-flowering of a few plant species provided foraging resources to a restricted number of wild bee species. This pattern was also observed in other studies, where the mass-flowering of single crops influenced the abundance of some wild bee taxa and certain functional traits like cavity-nesters positively (Holzschuh et al. 2013; Kovács-Hostyánszki et al. 2013).
However, gardeners have the power to design the green area to attract wild bees and in return yield more harvest (Werrell et al. 2009). By beginning the garden season with early blooming crops (e.g. fruit trees or Allium species like onions or garlic) and extending the flowering period in autumn, a wide range of wild bees will be attracted (Gunnarsson and Federsel 2014). Consequently, special wild bee communities can be enhanced, such as some species of the genera Anthophora Latreille, 1803 and Andrena, these are considered spring species which emerge in late March and April, whereas bumble bees are active until September (Amiet and Krebs 2014; Westrich 2018). Further, wild flowers attract wild bees and with higher abundancies and species numbers, the pollination service increases resulting in a rich harvest (Potter and LeBuhn 2015; Lowenstein et al. 2015). This positive feedback loop can also be exploited by deliberately planting wild bee attracting plants (Appendix Table 6). Referring to high species numbers and observed structure elements, two gardens (CG1 and CG8) were in accordance with this scheme.
Garden elements and landscape structures
Several landscape structures were modeled in statistical calculations to test the effect on species richness. Neither buildings, traffic nor artificial elements like courtyards surrounding the studied communal gardens influenced the wild bee species richness. As the distance to the city center had no effect on species richness, it appeared that urban areas of Vienna include adequate green spaces to act as corridors or habitats for wild bees. Also, garden size and sampling duration did not influence the number of wild bees found.
Furthermore, in contrast to projects in Sydney and Stockholm (Andersson et al. 2007; Makinson et al. 2017), green areas nearby did not influence the total number of observed species. Although, it can not be concluded that green areas nearby were not affecting the existing wild bee diversity at all, the areas included into the statistical models might have been too small for an observable effect. Only buildings surrounding the communal gardens had a negative effect on soil nesting bee communities as extensive areas of impervious surfaces in building developments within cities are the primary reason for a limitation of suitable habitats for below-ground nesters (Matteson et al. 2008).
Nevertheless, several studies have shown that landscape heterogeneity and green corridors promote wild bee species and abundance (Tommasi et al. 2004; Jauker et al. 2009). Conservation corridors can shelter diverse insect groups by providing feeding resources and nesting possibilities, but are also very important for migration (Samways 2018). By connecting habitats, individuals have the opportunity to move from one population to another group (Forman 1995; Hong et al. 2017), thus geneflow can be maintained between isolated populations minimizing the risk of an inbreeding depression. In an urban setting, patches and green corridors that increase habitat connectivity are likewise flower beds, parks, botanical gardens, green roofs, backyards (Kadas 2006; Pinheiro et al. 2006; Smith et al. 2006; Hunter and Hunter 2008; Braaker et al. 2014) or designated wild corners like the “Stadtwäldchen” opposite the communal garden CG8.
Our results show that elements in the communal gardens were affecting wild bees. As garden size was no influencing factor, which has also been supported by previous studies (Gunnarsson and Federsel 2014; Potter and LeBuhn 2015), even small green islands like communal gardens within an urban landscape have the potential to support the local wild bee community. With regard to structural elements within the gardens, species numbers are significantly higher when the garden contained more crops, trees and shrubs. The positive effect of crops was also observed for soil nesting and solitary wild bee communities.
On the other hand, a higher percentage of lawn areas negatively affected species richness. Therefore, tidy gardens with a perfect lawn may be aesthetically pleasing for some humans but cannot be considered bee friendly. Whereas, wild or unmanaged corners have a huge positive effect on insects (Matteson and Langellotto 2010). One of the communal gardens (CG8) with the highest number of observed species offered several unmanaged areas creating different microhabitats. These wild areas contained wild flowers or a mound of loose soil. Furthermore, a brick wall with countless small holes hosted cavity nesters.
Conclusion
The examined communal gardens host a quarter of all described species in Vienna. These green islands within the city are able to provide feeding and nesting resources for diverse wild bee communities as well as for rare specimens. Although, the gardens host a surprisingly high number of species, there was lack of oligolectic wild bees. Also, the city is a disturbing factor resulting in fewer cuckoo bee species. The more diverse the garden was designed, the more attractive it was for wild bees. As the flowering frequency on moderate scales proved itself a major driver for species richness, diverse crops mixed with wild flowers extending the flowering season from March to October are recommended to promote these pollinators. Also, microhabitats such as sparsely vegetated ground patches, walls, a reed fence or unmanaged areas within communal gardens provide nesting sites for rare species. Therefore, diverse communal gardens within urban areas are important spaces for conserving the local wild bee biodiversity. In cooperation with architects, city planners and biologists environmental damage can be minimized by taking green areas, such as communal gardens, into account when planning landscapes that enhance biodiversity.
According to the presented results, it is simple to create a communal garden, which is wild bee friendly by following these suggestions:
The design of structurally diverse communal gardens should be a primary aim during the planning process.
Woody plants like shrubs and trees as well as wild corner with extensive maintenance should be prioritized to increase the structural diversity of communal gardens.
Microhabitats such as sparsely vegetated ground patches, walls, dead-wood elements or a bamboo fence provide nesting sites for a variety of species.
Establishing a mix of different insect pollinated crops and native wild flowers attract both, oligo- and polylectic species.
In order to promote species richness, extending the flowering season from March to October is recommended.
The extend of frequently mown lawn should be reduced to a minimum.
References
Ahrné K, Bengtsson J, Elmqvist T (2009) Bumble Bees (Bombus spp) along a Gradient of Increasing Urbanization. PLoS One 4:e5574. https://doi.org/10.1371/journal.pone.0005574
Alaimo K, Reischl TM, Allen JO (2010) Community gardening, neighborhood meetings, and social capital. J Commun Psychol 38:497–514. https://doi.org/10.1002/jcop.20378
Amiet F (1996) Apidae, 1. Teil. Allgemeiner Teil, Gattungsschlüssel, die Gattung Apis, Bombus und Psithyrus. Centre suisse de cartographie de la faune: Schweizerische Entomologische Gesellschaft, Neuchâtel
Amiet F (ed) (2001) Apidae. 3: Halictus, Lasioglossum. Centre suisse de cartographie de la faune: Schweizerische Entomologische Gesellschaft, Neuchâtel
Amiet F, Krebs A (2014) Bienen Mitteleuropas. Gattungen, Lebensweise, Beobachtungen, 2nd edn. Haupt Natur Verlag, Bern
Amiet F, Herrmann M, Müller A, Neumeyer R (2004) Apidae. 4: Anthidium, Chelostoma, Coelioxys, Dioxys, Heriades, Lithurgus, Megachile, Osmia, Stelis. Neuchâtel
Andersson E, Barthel S, Ahrné K (2007) Measuring social-ecological dynamics behind the generation of ecosystem services. Ecol Appl 17:1267–1278. https://doi.org/10.1890/06-1116.1
Baldock KCR, Goddard MA, Hicks DM et al (2019) A systems approach reveals urban pollinator hotspots and conservation opportunities. Nat Ecol Evol 3:363–373. https://doi.org/10.1038/s41559-018-0769-y
Banaszak-Cibicka W (2014) Are urban areas suitable for thermophilic and xerothermic bee species (Hymenoptera: Apoidea: Apiformes)? Apidologie 45:145–155. https://doi.org/10.1007/s13592-013-0232-7
Banaszak-Cibicka W, Żmihorski M (2012) Wild bees along an urban gradient: winners and losers. J Insect Conserv 16:331–343. https://doi.org/10.1007/s10841-011-9419-2
Banaszak-Cibicka W, Ratyńska H, Dylewski Ł (2016) Features of urban green space favourable for large and diverse bee populations (Hymenoptera: Apoidea: Apiformes). Urban For Urban Green 20:448–452. https://doi.org/10.1016/j.ufug.2016.10.015
Bates D, Mächler M, Bolker B, Walker S (2014) Fitting Linear Mixed-Effects Models using lme4. J Stat Softw 67:1–48
Biesmeijer JC (2006) Parallel Declines in Pollinators and Insect-Pollinated Plants in Britain and the Netherlands. Science 313:351–354. https://doi.org/10.1126/science.1127863
Bogusch P, Straka J (2012) Review and identification of the cuckoo bees of central Europe (Hymenoptera: Halictidae: Sphecodes). Zootaxa:1–41
Bogusch P, Kratochvil L, Jakub S (2006) Generalist cuckoo bees (Hymenoptera: Apidae: Sphecodes) are species-specialist at the individual level. Behav Ecol Sociobiol 60:422–429
Bowler DE, Buyung-Ali L, Knight TM, Pullin AS (2010) Urban greening to cool towns and cities: A systematic review of the empirical evidence. Landsc Urban Plan 97:147–155. https://doi.org/10.1016/j.landurbplan.2010.05.006
Braaker S, Ghazoul J, Obrist MK, Moretti M (2014) Habitat connectivity shapes urban arthropod communities: the key role of green roofs. Ecology 95:1010–1021. https://doi.org/10.1890/13-0705.1
Environmental Systems Research Institute (2016) Geodatenviewer der Stadtvermessung Wien. Magistrat der Stadt Wien (MA41), 2015
Falk S (2015) Field Guide to the Bees of Great Britain and Ireland. British Wildlife Field Guides, Bloomsbury
Firth C, Maye D, Pearson D (2011) Developing “community” in community gardens. Local Environ 16:555–568. https://doi.org/10.1080/13549839.2011.586025
Fischer LK, Eichfeld J, Kowarik I, Buchholz S (2016) Disentangling urban habitat and matrix effects on wild bee species. PeerJ 4:e2729. https://doi.org/10.7717/peerj.2729
Forman RTT (1995) Some general principles of landscape and regional ecology. Landsc Ecol 10:133–142. https://doi.org/10.1007/BF00133027
Fortel L, Henry M, Guilbaud L et al (2014) Decreasing Abundance, Increasing Diversity and Changing Structure of the Wild Bee Community (Hymenoptera: Anthophila) along an Urbanization Gradient. PLoS One 9:e104679. https://doi.org/10.1371/journal.pone.0104679
Gallegos Torell Å, Glimskär A (2009) Computer-aided calibration for visual estimation of vegetation cover. J Veg Sci 20:973–983. https://doi.org/10.1111/j.1654-1103.2009.01111.x
Gathmann A, Tscharntke T (2002) Foraging ranges of solitary bees. J Anim Ecol 7:757–764
Gokcezade J, Gereben-Krenn B-A, Neumayer J, Krenn H (2010) Feldbestimmungsschlüssel für die Hummeln Österreichs, Deutschlands und der Schweiz (Hymenoptera, Apidae). Linzer biologischer Beitrag 42:5–42
Gosek J, Ruszkowski A, Kaczmarska K (1995) Food plants and an economic importance of Hylaeus species of subgenera Spatulariella Popov, Abrupta Popov and Koptogaster Alfken (Hymenoptera, Colletidae). Pszczelnicze Zeszyty Naukowe 39:265–272
Goulson D, Lye GC, Darvill B, Words K (2008) Decline and Conservation of Bumble Bees. Annu Rev Entomol 53:191–208
Grimm NB, Faeth SH, Golubiewski NE et al (2008) Global Change and the Ecology of Cities. Science 319:756–760. https://doi.org/10.1126/science.1150195
Guitart D, Pickering C, Byrne J (2012) Past results and future directions in urban community gardens research. Urban For Urban Green 11:364–373. https://doi.org/10.1016/j.ufug.2012.06.007
Gunnarsson B, Federsel LM (2014) Bumblebees in the city: abundance, species richness and diversity in two urban habitats. J Insect Conserv 18:1185–1191. https://doi.org/10.1007/s10841-014-9729-2
Gusenleitner F, Schwarz M, Mazzucco K (2012) Apidae (Insecta: Hymenoptera). In: Schuster R (ed) Schuster R (ed) Biosystematices and Ecology Series No. 29: Checklisten der Fauna Österreichs, No. 6. Verlag der Österreichsichen Akademie der Wissenschaften, Wien
Hennig EI, Ghazoul J (2012) Pollinating animals in the urban environment. Urban Ecosyst 15:149–166. https://doi.org/10.1007/s11252-011-0202-7
Hernandez JL, Frankie GW, Thorp RW (2009) Ecology of Urban Bees: A Review of Current Knowledge and Directions for Future Study. Cities and the Environment 2:1–15. doi: https://doi.org/10.15365/cate.2132009
Himpele K (2018) Wien in Zahlen 2018. MA 23 Wirtschaft, Arbeit und Statistik, Wien.
Hofmann MM, Zohner CM, Renner SS (2019) Narrow habitat breadth and late-summer emergence increases extinction vulnerability in Central European bees. Proc R Soc B Biol Sci 286:20190316. https://doi.org/10.1098/rspb.2019.0316
Holland L (2004) Diversity and connections in community gardens: a contribution to local sustainability. Local Environ 9:285–305. https://doi.org/10.1080/1354983042000219388
Holzschuh A, Dormann CF, Tscharntke T, Steffan-Dewenter I (2013) Mass-flowering crops enhance wild bee abundance. Oecologia 172:477–484. https://doi.org/10.1007/s00442-012-2515-5
Hong W, Guo R, Su M et al (2017) Sensitivity evaluation and land-use control of urban ecological corridors: A case study of Shenzhen, China. Land Use Policy 62:316–325. https://doi.org/10.1016/j.landusepol.2017.01.010
Hothorn T, Hornik K, Strobl C, Zeileis A (2018) Package “party” A Laboratory for Recursive Partytioning 1.3–1. http://party.R-forge.R-project.org
Hunter MR, Hunter MD (2008) Designing for conservation of insects in the built environment. Insect Conserv Divers 1:189–196. https://doi.org/10.1111/j.1752-4598.2008.00024.x
Jauker F, Diekötter T, Schwarzbach F, Wolters V (2009) Pollinator dispersal in an agricultural matrix: opposing responses of wild bees and hoverflies to landscape structure and distance from main habitat. Landsc Ecol 24:547–555. https://doi.org/10.1007/s10980-009-9331-2
Kadas G (2006) Rare Invertebrates Colonizing Green Roofs in London. Urban Habitats 4:66–86
Kingsley JY, Townsend M, Henderson-Wilson C (2009) Cultivating health and wellbeing: members’ perceptions of the health benefits of a Port Melbourne community garden. Leis Stud 28:207–219. https://doi.org/10.1080/02614360902769894
Kovács-Hostyánszki A, Haenke S, Batáry P et al (2013) Contrasting effects of mass-flowering crops on bee pollination of hedge plants at different spatial and temporal scales. Ecol Appl 23:1938–1946. https://doi.org/10.1890/12-2012.1
Kratschmer S, Kriechbaum M, Pachinger B (2018) Buzzing on top: Linking wild bee diversity, abundance and traits with green roof qualities. Urban Ecosyst 21:429–446. https://doi.org/10.1007/s11252-017-0726-6
Leonhardt SD, Gallai N, Garibaldi LA et al (2013) Economic gain, stability of pollination and bee diversity decrease from southern to northern Europe. Basic Appl Ecol 14:461–471. https://doi.org/10.1016/j.baae.2013.06.003
Lorenz S, Stark K (2015) Saving the honeybees in Berlin? A case study of the urban beekeeping boom. Environ Sociol 1:116–126. https://doi.org/10.1080/23251042.2015.1008383
Lowenstein DM, Matteson KC, Minor ES (2015) Diversity of wild bees supports pollination services in an urbanized landscape. Oecologia 179:811–821. https://doi.org/10.1007/s00442-015-3389-0
Madlener N (2007) Verein Gartenpolylog - Gaertnerinnen der Welt kooperieren - Gemeinschaftsgaerten. https://gartenpolylog.org/de/home . .
Magistrat der Stadt Wien (2019) Bienenvölker in Wien. In: Stadt Wien. https://www.wien.gv.at/umwelt-klimaschutz/bienen-wien.html . .
Makinson JC, Threlfall CG, Latty T (2017) Bee-friendly community gardens: Impact of environmental variables on the richness and abundance of exotic and native bees. Urban Ecosyst 20:463–476. https://doi.org/10.1007/s11252-016-0607-4
Matteson KC, Langellotto GA (2010) Determinates of inner city butterfly and bee species richness. Urban Ecosyst 13:333–347. https://doi.org/10.1007/s11252-010-0122-y
Matteson KC, Ascher JS, Langellotto GA (2008) Bee Richness and Abundance in New York City Urban Gardens. Ann Entomol Soc Am 101:140–150. https://doi.org/10.1603/0013-8746(2008)101[140:BRAAIN]2.0.CO;2
Mazerolle M (2017) AICcmodavg: Model selection and multimodel inference based on (Q)AIC(c). R package version 2.1–1. https://cran.r-project.org/package=AICcmodavg . Accessed 3 Mar 2019
Michener C (2007) The Bees of the World, 2nd edn. The Johns Hopkins University Press, Baltimore
Müller A, Kuhlmann M (2008) Pollen hosts of western palaearctic bees of the genus Colletes (Hymenoptera: Colletidae): the Asteraceae paradox: Pollen hosts of Colletes bees. Biol J Linn Soc 95:719–733. https://doi.org/10.1111/j.1095-8312.2008.01113.x
Müller A, Diener S, Schnyder S et al (2006) Quantitative pollen requirements of solitary bees: Implications for bee conservation and the evolution of bee–flower relationships. Biol Conserv 130:604–615. https://doi.org/10.1016/j.biocon.2006.01.023
Norton BA, Coutts AM, Livesley SJ et al (2015) Planning for cooler cities: A framework to prioritise green infrastructure to mitigate high temperatures in urban landscapes. Landsc Urban Plan 134:127–138. https://doi.org/10.1016/j.landurbplan.2014.10.018
Pachinger B, Hölzler G (2006) Die Wildbienen (Hymenoptera, Apidae) der Wiener Donauinsel. Beiträge zur Entomofaunistik 7:119–148
Pachinger B, Neumüller U, Eckl L-M et al (2014) Friedhöfe als Rückzugsraum für Wildbienen (Hymenoptera: Apidae) in der Grossstadt Wien. Beiträge zur Entomofaunistik 15:81–93
Pawelek JC, Frankie GW, Thorp RW, Przybylski M (2009) Modification of a Community Garden to Attract Native Bee Pollinators in Urban San Luis Obispo, California. Cities Environ 2:1–20
Pinheiro MHO, De Neto LCA, Monteiro R (2006) Urban Areas and Isolated Remnants of Natural Habitats: An Action Proposal for Botanical Gardens. Biodivers Conserv 15:2747–2764. https://doi.org/10.1007/s10531-005-1133-5
Potter A, LeBuhn G (2015) Pollination service to urban agriculture in San Francisco, CA. Urban Ecosyst 18:885–893. https://doi.org/10.1007/s11252-015-0435-y
R Core Development Team (2016) A Language and Environment for Statistical Computing. V 3.3.2 R Foundation for Statistical Computing. Vienna
Ramalho CE, Hobbs RJ (2012) Time for a change: dynamic urban ecology. Trends Ecol Evol 27:179–188. https://doi.org/10.1016/j.tree.2011.10.008
Samways MJ (2018) Insect Conservation for the Twenty-First Century. In: Shah MM, Sharif U (eds) Insect Science-Diversity. Conservation and Nutrition, InTechOpen, pp 19–41
Sánchez-Bayo F, Wyckhuys KAG (2019) Worldwide decline of the entomofauna: A review of its drivers. Biol Conserv 232:8–27. https://doi.org/10.1016/j.biocon.2019.01.020
ScheuchI E (1995) Illustrierte Bestimmungstabellen der Wildbienen Deutschlands und Österreichs. Band 1: Anthophoridae. Eigenverlag, Velden.
Scheuchl E (1996) Illustrierte Bestimmungstabellen der Wildbienen Deutschlands und Österreichs. Bd. 2: Megachilidae - Melittidae. Eigenverlag, Velden
Scheuchl E, Willner W (2016) Taschenlexikon der Wildbienen Mitteleuropas. Alle Arten im Porträt, Quelle & Meyer Verlag, Wiebelsheim
Schmid-Egger C, Scheuchl E (1997) Illustrierte Bestimmungstabellen der Wildbienen Deutschlands und Österreichs. Bd. 3: Andrenidae. Eigenverlag, Velden
Sedivy C, Dorn S (2014) Towards a sustainable management of bees of the subgenus Osmia (Megachilidae; Osmia) as fruit tree pollinators. Apidologie 45:88–105. https://doi.org/10.1007/s13592-013-0231-8
Sheffield C, Pindar A, Packer L, Kevan P (2013) The potential of cleptoparasitic bees as indicator taxa for assessing bee communities. Apidologie 44:501–510. https://doi.org/10.1007/s13592-013-0200-2
Sirohi MH, Jackson J, Edwards M, Ollerton J (2015) Diversity and abundance of solitary and primitively eusocial bees in an urban centre: a case study from Northampton (England). J Insect Conserv 19:487–500. https://doi.org/10.1007/s10841-015-9769-2
Smith RM, Warren PH, Thompson K, Gaston KJ (2006) Urban domestic gardens (VI): environmental correlates of invertebrate species richness. Biodivers Conserv 15:2415–2438. https://doi.org/10.1007/s10531-004-5014-0
Stadt Wien - ViennaGIS (2015) Geodatenviewer der Stadtvermessung Wien. Magistrat der Stadt Wien (MA41), 2015, ESRI, Vienna, https://www.wien.gv.at/ma41datenviewer/public/
Stange E, Zulian G, Rusch G et al (2017) Ecosystem services mapping for municipal policy: ESTIMAP and zoning for urban beekeeping. One Ecosyst 2:e14014. https://doi.org/10.3897/oneeco.2.e14014
Strobl C., Boulesteix A.-L., Kneib T., Augustin T., Zeileis A. (2008) Conditional variable importance for random forests, BMC Bioinformatics 9:1471–2105
Strobl C., Hothorn T., Zeileis A. (2009) Party on! Contributied Research Articles 1:14–17
Tommasi D, Miro A, Higo HA, Winston ML (2004) Bee diversity and abundance in an urban setting. Can Entomol 136:851–869. https://doi.org/10.4039/n04-010
United Nations (2018) World Urbanization Prospects: The 2018 Revision. Department of Economic and Social Affairs/ Population Division
Wastian L, Unterweger PA, Betz O (2016) Influence of the reduction of urban lawn mowing on wild bee diversity (Hymenoptera, Apoidea). J Hymenopt Res 49:51–63. https://doi.org/10.3897/JHR.49.7929
Werrell PA, Langellotto GA, Morath SU, Matteson KC (2009) The Influence of Garden Size and Floral Cover on Pollen Deposition in Urban Community Gardens. Cities Environ 2:1–16
Westrich P (1989) Die Wildbienen Baden-Württembergs: Allgemeiner Teil. Lebensräume, Verhalten, Ökologie und Schutz. Eugen Ulmer GmbH & Co., Stuttgart
Westrich P (2018) Die Wildbienen Deutschlands. Eugen Ulmer Verlag, Stuttgart
Wiesbauer H (2017) Wilde Bienen. Biologie - Lebensraumdynamik am Beispiel Österreich - Artenporträts, 1. Auflage. Eugen Ulmer KG, Stuttgart
Wilkaniec Z, Giejdasz K (2003) Suitability of nesting substrates for the cavity-nesting bee Osmia rufa. J Apic Res 42:29–31. https://doi.org/10.1080/00218839.2003.11101084
ZAMG (2017) Klimaspiegel Wien Innere Stadt für 2017. ZAMG - Zentralanstalt für Meterologie und Geodynamik, In https://www.zamg.ac.at/cms/de/klima/klimauebersichten/jahrbuch .
Zettel H, Ockermüller E, Wiesbauer H et al (2015) Kommentierte Liste der aus Wien (Österreich) nachgewiesenen Bienenarten (Hymenoptera: Apidae). Zeitschrift der Arbeitsgemeinschaft Österreichischer Entomologen 67:137–194
Zettel H, Zimmermann D, Wiesbauer H (2016) Ergänzungen zur Bienenfauna (Hymenoptera: Apidae) von Wien, Österreich. Beiträge zur Entomofaunistik 17:85–107
Zhao C, Sander HA, Hendrix SD (2019) Wild bees and urban agriculture: assessing pollinator supply and demand across urban landscapes. Urban Ecosyst 22:455–470. https://doi.org/10.1007/s11252-019-0826-6
Zurbuchen A, Müller A (2012) Wildbienenschutz- von der Wissenschaft zur Praxis. Zürich, Bristol-Stiftung, Bern, Stuttgart, Wien
Zurbuchen A, Landert L, Klaiber J et al (2010) Maximum foraging ranges in solitary bees: only few individuals have the capability to cover long foraging distances. Biol Conserv 143:669–676. https://doi.org/10.1016/j.biocon.2009.12.003
Zuur AF, Ieno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems: Data exploration. Methods Ecol Evol 1:3–14. https://doi.org/10.1111/j.2041-210X.2009.00001.x
Acknowledgements
First of all, we want to thank the gardeners for their support and interest in this project. Furthermore, we wish to thank Andreas Werner Ebmer for the confirmation of the determination of Halictus tectus, Sabine Schoder for assisting with the identification of difficult Hylaeus species, Monika Kriechbaum with the determination of certain plant species and David Horner for proof reading.
Author information
Authors and Affiliations
Contributions
JL wrote the manuscript and along with BäPa conceived the original idea and identified the specimens on species level. Field work was conducted by JL and FG in the context of FG’s Bachelor thesis. SK performed the statistical computations with R. BoPe contributed the GIS results. All authors discussed the results and contributed to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Appendices
Appendix 1
Appendix 2
Appendix 3
Appendix 4
Appendix 5
Appendix 6
Rights and permissions
About this article
Cite this article
Lanner, J., Kratschmer, S., Petrović, B. et al. City dwelling wild bees: how communal gardens promote species richness. Urban Ecosyst 23, 271–288 (2020). https://doi.org/10.1007/s11252-019-00902-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-019-00902-5