Abstract
Many recent studies have suggested that cities are spatially heterogeneous. Only limited research has investigated whether urban heterogeneity influences the distribution of bats in a city. Between 2010 and 2012, we acoustically surveyed bats in Waco, Texas, a medium-sized city in the United States. Seven species were detected, five in adequate quantity for analyses. Three distinct distribution patterns were evident (Mexican free-tailed bats; red bats and evening bats; big brown bats and cave myotis), reflecting the distinct functional guilds of these species. Bayesian conditional autoregressive models indicated that tree-dwelling red bats and evening bats were influenced by variables describing heterogeneity of urban vegetation. Big brown bats and cave myotis were associated with variables related to water sources. Mexican free-tailed bat distribution could be explained well by variables related to urban buildings and other constructions. Our modeling also suggested that urban socioeconomic heterogeneity influenced bat distributions. Distributions of tree-dwelling bats corresponded to income level. Distributions of Mexican free-tailed bats, big brown bats, and cave myotis related to human density. These results support the idea that a city comprises a mosaic of habitats as perceived by various species of bats and likely by other species of wildlife in urban settings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patch in landscape ecology usually refers to surface area that differs from its surroundings in nature or appearance (Turner et al. 2001). At a broad scale, ecologists have tended to treat cities as homogeneous patches distinct from natural landscape elements, such as grassland and forest, because the urban environment has been modified by extensive impervious surfaces, anthropogenic constructions, and human activities (McKinney 2002; Cadenasso et al. 2007; McDonnell and Hahs 2008). However, researchers increasingly suggest that a city is a spatially heterogeneous complex mosaic at a fine scale. Socioeconomic processes thus interact with natural processes in cities to generate a heterogeneous urban landscape (Cadenasso et al. 2013).
Urban heterogeneity can be recognized from several perspectives. Prior to the founding of a city, the natural environment is heterogeneous in terms of topography, vegetation, open water sources, and other features (Marzluff et al. 2008), with humans modifying the natural environment (e.g., cutting of vegetation, adding artificial water bodies) as the city grows (Conzen 2001). The presence of many clusters of houses, office buildings and other man-made structures with different physical appearances and functions contributes to urban heterogeneity (Grimm et al. 2000; James and Bound 2009). A key mechanism promoting heterogeneity is that different economic functions drive districts within a city in different directions, thus affecting physical change of the landscape (Moses and Williamson 1967; Black and Henderson 2003). Historic and cultural factors also play an important role in determining the structure of a district through dynamic feedbacks (Miller and Hobbs 2002; Mommaas 2004; Irwin et al. 2009). Cities, therefore, are both physically and socioeconomically heterogeneous.
Many bat species are responsive to urbanization. When considered as patches and compared with natural settings at a broad scale, urbanized areas usually show lower bat species diversity than natural habitats, although bat activity level could be high and a few species could dominate these urban patches (Kurta and Teramino 1992; Johnson et al. 2008; Duchamp and Swihart 2008; Loeb et al. 2009; Jung and Kalko 2011). Urbanization adversely impacts some species, such as eastern red bats (Lasiurus borealis, Walters et al. 2007), white-striped free-tail bats (Tadarida australis, Rhodes et al. 2006; Rhodes and Catterall 2008), Indiana bats (Myotis sodalis, Sparks et al. 2005), common pipistrelle (Pipistrellus pipstrellus, Hale et al. 2012), and evening bats (Nycticeius humeralis, Duchamp et al. 2004). However other species, such as big brown bats (Eptesicus fuscus) and some species of genus Myotis, are better able to adapt to cities (Duchamp et al. 2004; Gehrt and Chelsvig 2004; Coleman and Barclay 2012).
Recent research suggested further that bats respond to spatial heterogeneity within cities at a fine scale. Bats recognized environmental heterogeneity related to vegetation conditions (Avila-Flores and Fenton 2005; Basham et al. 2011; Dixon 2011; Threlfall et al. 2012) and water source availability (Gaisler et al. 1998; Gehrt and Chelsvig 2003; Fabianek et al. 2011) in cities as they did in natural habitats. The distributions and activity levels of bats also respond to anthropogenic morphological heterogeneity related to building density (Gaisler et al. 1998; Threlfall et al. 2011), impervious surfaces (Dixon 2011), and artificial illumination (Avila-Flores and Fenton 2005). Many fine-scale studies suggested that bats might prefer to roost in tall and old buildings and forage on insects attracted by streetlights (Rydell 1991; Williams and Brittingham 1997; Neubaum et al. 2007), though that they might avoid streets with heavy traffic due to high collision potential (Zurcher et al. 2010).
Cities also demonstrate socioeconomic heterogeneity, but no published literature has specifically addressed whether bats might respond to socioeconomic variables portraying the human dimension of urban settings. The integration of socioeconomic factors into urban ecological study is still not mature, though land use type, human population density, and economic characteristics are theoretically suggested to capture variations in the pattern of urbanization (Rees 1997; Dow 2000; McIntyre et al. 2000; Hahs and McDonnell 2006). In practice, Troy et al. (2007) borrowed a system used for real estate marketing and advertising to categorize urban neighborhoods. They suggested several socioeconomic factors (e.g., age of dwelling, vacancy, average family size) as proxies for urban vegetation conditions. Gledhill and James (2012) used socioeconomic data (e.g., income, social class) to identify the conservation value of urban ponds. Farmer et al. (2013) demonstrated the correlation between house value and urban bird diversity. Urban bat research has revealed that bats tended to roost in old and accessible buildings (Davis et al. 1962; Williams and Brittingham 1997). Such building features might relate to income level and building maintenance effort (Troy et al. 2007; Lowry et al. 2012). Human’s general attitudes toward wildlife and pest control effort could also affect urban bat distribution by removal of bats from urban roosts (Adams and Lindsey 2010; Morzillo and Mertig 2011).
In this study, we sought to determine if bats would respond to natural environmental heterogeneity and man-made structural heterogeneity in urban settings as suggested in recent literature. Duchamp and Swihart (2008) and Luck et al. (2013) have suggested that the particular variables to which bats might respond would relate to each species’ life history or functional guild. Generally, we hypothesized that bats would respond to physical heterogeneity, whether natural or anthropogenic, in the city. More specifically, we expected that occurrences of tree-dwelling species, such as red bats, might relate to variation in vegetation conditions across the city. Similarly, we anticipated that species known to utilize buildings as roosts, such as Mexican free-tailed bats and big brown bats, might respond to the distribution of buildings in the city. We also tested the null hypothesis that bats would be indifferent to urban socioeconomic heterogeneity that normally could only be perceived by humans. We suspected that socioeconomic heterogeneity variables as recognized by humans might correspond with habitat suitability as perceived by bats.
Methods
Study area
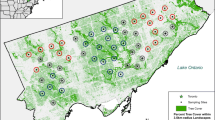
This study was conducted in the City of Waco (31°33′5″ N, 97°9′21″ W) and surrounding areas, McLennan County, Texas (Fig. 1). The city has a total area of 95.5 square miles (247.4 km2), including 84.2 square miles (218.1 km2) of land and 11.3 square miles (29.3 km2) of water. The Bosque River and Brazos River pass through the city limits. Lake Waco, a reservoir, is also contained within the city limits. Dense vegetation is present along the eastern bank of Lake Waco and the Bosque River. Waco is the 194th largest city in the United States and 21st in Texas in terms of population size, with an estimated total population of 120,465 (population density 1,350/sq. mi or 521.5/km2; U.S. Census, 2007). Most studies of urban bats have been conducted in major metropolitan areas, such as Sydney, Australia, Mexico City, and Chicago, United States. This study in Waco represents a more-commonly occurring situation, the medium-sized city, which has not been previously studied with regard to bat ecology.
Driving survey
We conducted mobile transect surveys in the city to identify locations where bats were active (Johnson et al. 2002; Roche et al. 2011). An AnaBat SD1 bat detector and PDA kit (Titley Scientific, Australia) was installed in the vehicle with the microphone mounted upward through the sunroof. The vehicle travelled at speeds of 30 – 50 km/h for most of the time, varying according to local speed limits and current traffic conditions. Variation in speed would have affected measures of activity levels or other measures of density of bats, but this study documented only presence or absence of bat activity along sampling transects. Possible effects of variation in speed were minimized by consistency of variation in speed within and across transects. We recorded the time and address when echolocation calls were detected. Calls were saved on the PDA for later species identification. The vehicle stopped for about 1 min at locations where bat calls were detected to observe bats and record more calls, except when traffic prohibited. As the detector could record bats within a 100 m radius (Rydell 1991; Russ et al. 2003), subsequent stops to sample for other calls detected were at least 100 m from the previous location, unless a different species was detected.
We divided the city into nine regions. Each region was sampled with one transect route. A total of nine survey routes, each 55 – 65 km in length, covered about 270 km2 of land area. Between fall 2010 and summer 2012, eight rounds of sampling were conducted, one each in the spring (March – April) and fall (September – October) of both years, and two rounds each summer (round 1 May – June, round 2 July – August). In each round, nine nights were selected for sampling of transects. To the extent possible, surveys were conducted on nights with similar weather conditions within each season (no severe weather conditions, similar night temperature, and maximum daytime temperature before the sampling night was at least 21 ° C). The sequence of nine transect routes among nine sampling nights was randomly generated. The time span for a sampling round was 7 weeks during the spring and fall, and 5 weeks in the summer. Driving surveys began approximately 10 min before sunset and lasted 90 – 120 min, depending on how many calls were recorded and the traffic conditions. We surveyed just after sunset because twilight and early evening is an interval of peak activity for bats as they leave their roosts for the first foraging and watering bouts of the evening (Schmidly 2004; Ammerman et al. 2012).
Species identification
Bat calls recorded during the driving survey were analyzed via Analook W 3.3f (C. Corben, IBM). Species identification was based on the frequency, shape, slope in octaves per second, and pulse interval of the calls within a sequence (O’Farrell et al. 1999). A known-species reference library was built based on published literature and online bat call libraries (O’Farrell et al. 1999; Kurta et al. 2007; Boland 2007; BatCall Library; BatSpecies List; Pacific northwest bat call library; Wyoming bat call library), and recordings from bats captured in other local research projects. Only sequences with at least three clear call pulses were compared with the library for species identification. McLennan County is within the geographic distribution of nine species of bats (Schmidly 2004): big brown bat (Eptesicus fuscus, EPFU), eastern red bat (Lasiurus borealis, LABO), hoary bat (Lasiurus cinereus, LACI), silver-haired bats (Lasionycteris noctivagans, LANO), Seminole bat (Lasiurus seminolus, LASE), cave myotis (Myotis velifer, MYVE), evening bats (Nycticeius humeralis, NYHU), eastern pipistrelles (Perimyotis subflavus, PESU), and Brazilian free-tailed bat (Tadarida brasiliensis, TABR).
GIS mapping
Based on the addresses recorded during the survey, we plotted locations with identified bat calls on a Bing base map via ArcMap (version 10.1, Esri, California, USA). A preliminary MRPP (multiresponse permutation procedure) analysis of locations’ coordinates showed no spatial variation between seasons (McCune et al. 2002). Therefore we pooled eight rounds of surveys as one map layer. Due to the limited availability of techniques for analyzing three-dimensional repeated-measures data (a temporal correlation among repeated measures over time and spatial correlations in geographic coordinates), we plotted a location only once even when multiple survey rounds included the same location with the same bat species multiple times (Gutzwiller and Riffell 2007). We defined locations with calls included for analyses as “bat active” locations. We also randomly established 300 locations on the transects, which were at least 100 m away from each other and from any locations with bat calls; we defined these locations as “no recording” locations and treated them as controls in further analyses. A 100 m radius buffer zone was generated at each location to collect data defining urban heterogeneity.
Defining urban heterogeneity
We selected three groups of variables to quantify urban heterogeneity, each group representing a different perspective. All variables were collected for both “bat active” and “no recording” locations. The first group described the heterogeneity of urban natural environment as reported in published literature (see Introduction). We measured the linear distance between a location and a water body (Lake Waco, Brazos River, Bosque River) or a forest patch (centroids of any forest patches larger than 50 ha) in km on a Bing base map as “distance to water body” and “distance to forest center”. Orthophotos from 2012 (http://www.tnris.org) were used to determine “vegetation coverage” (percentage of tree canopy within the 100 m buffer zone of each location) and “presence of a water body” (visually visible water body, either natural or artificial). Water sources were verified by field observations where feasible.
The second group of variables pertained to man-made structures in the city, including “presence of tall building”, “building density”, “street intersection density”, and “road level” (as suggested by literature cited in Introduction). Buildings with four or more floors were considered as “tall buildings” (Williams and Brittingham 1997; Mazurska and Ruczyński 2008). We used orthophotos to determine if a tall building was present in the 100 m radius buffer zone; presence of tall buildings was verified by field observation. Building density and street intersection density were based on counts of buildings and street intersections in the 100 m radius buffer zone. Road level was determined from a local road-level map (http://www.waco-texas.com/economic-development/map-disclaimer.asp). We recognized four levels of roads based on traffic, speed limit, and illumination condition (measured by EasyView 30 light meter, Extech Instruments Corp, MA): “highway” (high traffic volume, > 45 miles/h, > 200 lux), “major street” (medium to high traffic, 45 miles/h, > 100 lux), “local street” (low to medium traffic, 30 miles/h, > 50 lux), “private street” (low traffic, 30 miles/h or lower, < 50 lux).
The last group of variables sought to explore if bats might respond to socioeconomic heterogeneity in the city. We collected “median household income” and “human population density” from U.S. Census 2010 surveys, based on where locations fell into a census survey block. We re-classified land use categories based on the Anderson system (Anderson et al. 1976) and human behavior patterns, as suggested in literature (Cadenasso et al. 2007, 2013; James and Bound 2009). We recognized six categories of land use types: “low density residential” (mostly single family houses, either one or two floors, many people present at night), “medium or high density residential” (mostly two or three floor apartment complexes, many people present at night), “public” (mostly parks, few people present at night, poorly illuminated), “industrial” (extensive one or two floor factories or warehouse, extensive impervious parking area, few people present at night, medium level illumination), “commercial and office” (extensive impervious parking area, many people present early night, but few later, well illuminated), and “mixed use” (variety building structures, human activities and illumination levels). The variable “land use type” was determined by where each “bat active” or “no recording” location fell into the land use layer. “Land use diversity” was the count of total land use types within the buffer zone. These variables are summarized in Tables 1 and 2.
Mismatch of data scales (referred to as “the scale standardization problem”) is a common issue when using socioeconomic data as they are normally reported in areas rather than points (Turner et al. 2001). In our study, the finest scale for two census variables was census survey block. This scale, however, is still broader than the scale of 100 m buffer zone for bat active locations. Due to this limitation, a few bat active locations would have the same value for these variables when they were located in the same census survey block. Therefore, our models could not incorporate finer variations of median income or population density. It is possible that a species or species group of bats could have responded to either census variable at a finer scale not detectable by our analyses. However, the interpretation of patterns presented in Results should not be affected by this data scale mismatch.
Statistical analysis
To determine if “bat active” locations were distributed randomly within the survey range, we ran Complete Spatial Randomness (CSR) tests. Distance to the nearest event was used to compute CSR values (G values). A Monte Carlo test with 999 simulations was used to test the significance level of G values separately for each species recorded (Baddeley and Gill 1997; Davison and Hinkley 1997). We performed MRPP (multiresponse permutation procedure) tests to determine whether “bat active” locations (the coordinates) for different species shared the same distribution pattern (McCune et al. 2002).
Since bats are capable of flying over the entire city within a short time interval, it is possible that any bat recording might be related to other recordings nearby. Therefore, we performed Moran’s I test to determine if spatial autocorrelation might be occurring among “bat active” locations. Based on our preliminary analysis and research by Duchamp and Swihart (2008) and Kelejian and Prucha (2010), the neighbor scheme we used was based on the minimum distance band, the minimum distance needed to ensure that all the locations (both “bat active” and “no recording” locations) are linked to at least one neighbor. All points within the distance band were assigned as neighbors to the observation in the center. Binary weight style (all neighbors have the same influence) was used in distance-band based neighbor schemes (Bivand et al. 2008).
Before modeling any explanatory variable for bat presence/absence, we assessed whether multicollinearity existed in our data. We log-transformed these variables: median income, population density, distance to forest center, and distance to water body. Variance inflation factors (VIF) were calculated for continuous data. VIF values smaller than 3 indicate no correlation between continuous variables (Zuur et al. 2009); all VIF values in our data were smaller than 2. ANOVA tests were used to detect correlations between continuous data and categorical data and Chi-square tests for correlations among categorical data (Quinn and Keough 2002).
We constructed Bayesian logistic effect models to explore which urban heterogeneity variables might explain the distribution pattern of bat presence/absence in the city (Beale et al. 2010; McCarthy 2011). The model represents the logistic transform of the mean function for the set of binomial responses by a combination of covariates and a set of random effects. The latter incorporated spatial autocorrelation (http://cran.r-project.org/web/packages/CARBayes/CARBayes.pdf). Based on our preliminary analyses of different spatial models and suggestions in literature (Augustin et al. 1996; Lichstein et al. 2002; Bivand et al. 2008; Beale et al. 2010), we chose the CAR (conditional autoregressive) model to represent spatial correlation in the data. For each species recorded, we constructed binomial logistic random effect models with the location type (“bat active” vs. “no recording”) as response variable and urban heterogeneity variables as independent variables. The posterior quantiles (median and 95 % interval of explanatory variables) would indicate which variables significantly affect the distribution pattern of bats. We modeled three groups of urban heterogeneity variables separately instead of including all variables in one model to avoid unstable modeling results (Quinn and Keough 2002) and unexplainable variable interactions (Bivand et al. 2008). Preliminary analyses showed that land use type correlated with several variables. Thus we modeled this variable separately from others. Our modeling goal was to identify potential urban heterogeneity variables that might affect bat presence/absence. Therefore we conducted post hoc modeling for variables that were selected as significant explanatory variables by previous models (Burnham and Anderson 2002). Post hoc modeling explored whether variable interactions might further explain the bat activity distribution patterns. Each model included 5000 Markov chain Monte Carlo samples, with 500 of them discarded as the Burn-in period. The neighbor scheme used in these models was the same as used in Moran’s I test. All statistical analyses were conducted in R version 2.14.2 (2012-02-29).
Results
Sampling surveyed a total of more than 4,000 km of roadway in the Waco area. We recorded 3,622 separate bat calls with at least three pulses and were able to identify 3,215 calls representing seven species. These recordings generated 330 bat call locations among 8 rounds of surveys. We eliminated 46 of these locations (39 due to temporal correlation among survey rounds and 7 due to limited recordings). Thus, a total of 284 “bat active” locations were included for statistical analyses. All 284 locations had multiple call sequences and at least one bat call sequence with 10 pulses.
Mexican free-tailed bats were the dominant species with 216 active locations (76 % of all), followed by eastern red bats (26 locations), big brown bats (18 locations), evening bats (13 locations), and cave myotis (7 locations). Two species that migrate seasonally through Central Texas, the hoary bat (3 locations) and the silver hair bat (1 location), were recorded only rarely.
As is evident in the distribution maps (Fig. 1), most “bat active” locations occurred in the area comprising the central portion of the survey range. Within this central area, big brown bats and evening bats tended to occur in the southern sections, whereas Mexican free-tailed bats were widespread. Most red bat and evening bat calls were recorded near the eastern shore of Lake Waco. No bat presence was recorded near the Waco Regional Airport (northwest region of survey), though it is adjacent to the lake. The only silver-haired bat recording occurred near the lake. All three hoary bat locations were in the eastern side of the city.
We eliminated silver-haired bat and hoary bat from statistical analyses due to small sample sizes. The significance levels of the Complete Spatial Randomness (CSR) test calculated via the Monte Carlo test with 999 simulations were 0.004 for Mexican free-tailed bat (only 4 patterns similar to recorded TABR location pattern out of 1,000 random simulations), 0.002 for eastern red bat, big brown bat, and evening bat, <0.001 for cave myotis, indicating that the chances of these distribution patterns being random were very low. The CSR plots (Fig. 2) showed that cluster patterns occurred in these “bat active” locations, since there were more “bat active” locations with close neighbor than theoretical (Gobs(r) is above Gtheo (r) when r is at small values).
Complete Spatial Randomness (CSR) test plot for four bat species. G(r) estimates the nearest neighbor distance distribution function based on a point pattern (the cumulative distribution function of distance from a point to nearest other point, range 0 – 1), r is distance between points. Dashed red line indicates expected trend for a random point (Poisson) distribution. Shaded area shows the confidence range for 95 % significance. Solid black line, computed from actual observations, falls above 95 % confidence interval, suggesting there are more points having a close nearest neighbor than random, forming clusters in the point pattern (Bivand et al. 2008). Abbreviations for species defined in text
We conducted MRPP tests on the spatial coordinates of five species (Table 3, Fig. 3). Mexican free-tailed bats showed a distribution different from other four species (Fig. 3a). We found no significant distributional difference between red bat and evening bat (p = 0.172), or between big brown bat and cave myotis (p = 0.780). Other paired comparisons differed significantly from each other. Therefore, we pooled red bat and evening bat data (as the LANY group, Fig. 3b) as well as big brown bat and cave myotis data (as the EPMY group, Fig. 3c) for further analyses. An additional benefit of pooling these species’ data was to increase sample sizes and, thus, statistical robustness. Moran’s I tests showed that significant spatial autocorrelation existed in all three sets of bat locations (p < 0.0001 for Mexican free-tailed bat, LANY group, and EPMY group).
Bayesian CAR modeling suggested that three groups of bats responded to different types of urban heterogeneity (Table 4). Among variables describing urban natural environment, the presence of water source related positively to the probability of detecting EPMY group bats. The descriptive data indicated that 74.0 % of the locations with EPMY group detection had some water source within the buffer zone, compared to 33.3 % for “no recording” locations. LANY group species responded to both vegetation variables: It was more likely to detect LANY group bats at locations with higher canopy coverage (23.7 % canopy coverage at “no recording” locations vs. 56.0 % at LANY locations) and closer to a large forest patch (approx. 4 km at “no recording” vs. approx. 1 km at LANY locations). Mexican free-tailed bats did not respond to any variable related to the natural environment within urban settings.
In contrast to being sensitive to natural environment heterogeneity in urban settings, the EPMY and LANY groups showed no response to any variable describing the heterogeneity of man-made structures, except the probability of detecting the LANY group was negatively affected by the presence of highways. Modeling results suggested a similar avoidance of highways by Mexican free-tailed bats. The other three variables in this group significantly influenced Mexican free-tailed bats in the Bayesian CAR model: Mexican free-tailed bats were more likely to be detected in areas with tall buildings (61.1 % at TABR locations vs. 37.7 % at “no recording” locations), higher building density (approx. 25 buildings within the buffer zone at TABR locations vs. 14 at “no recording” locations), and high street intersection density (approx. 4 intersections within the buffer zone at TABR locations vs. 2 at “no recording” locations).
Our null hypothesis that bats were indifferent to socioeconomic heterogeneity was rejected. The socioeconomic variables selected by Bayesian CAR model for explaining the presence of bats vary among the bat groups. The LANY group was positively related to household median income. Average household median income at LANY locations was $57,470, compared to $44,431 at “no recording” locations. In contrast, EPMY and Mexican free-tailed bats were indifferent to median income but sensitive to human population density and land use diversity. Both Mexican free-tailed bats and EPMY species were more likely to be detected at locations with higher population density (3,830 people per square mile at TABR locations and 3,615 at EPMY locations vs. 1895 at “no recording” locations) and land use diversity (mean of 1.8 types of land use categories at TABR locations and 2.0 at EPMY locations vs. 1.6 at “no recording” locations). Modeling of land use types indicated that LANY bats were more likely to be detected at “low density residential” and “public” settings and less likely to be detected in “industrial” areas. EPMY bats seemed to prefer “low density residential” and avoid “medium/high density residential”, “mixed use” and “industrial” situations. “Mixed use” was the only land use type favored by Mexican free-tailed bats. Like the two other bat groups, Mexican free-tailed bats avoided “industrial” land. Other land use types showed no effect on bat distribution.
In post hoc modeling, we explored all possible combinations of significant variables in previous models after checking for multicollinearity between cross group variables. We excluded the variable road level, since only one category (highway) was significantly influential. Land use type was eliminated due to correlations with several other variables. For the LANY group, three variable combinations were considered. There was no interaction between median income and distance to forest center or between vegetation coverage and distance to forest center. However, the interaction between median income and vegetation coverage was positive, indicating that the positive effect of income would be more profound in areas with higher vegetation coverage, and vice versa. There was no interaction between the three significant variables for the EPMY group (water source, population density, and land use diversity). For Mexican free-tailed bats, we considered all 15 combinations among six significant variables (building density, presence of tall building, intersection density, population density, land use diversity, and structural change), and found only one significant interaction term in these models: population density negatively interacted with building density. Therefore, when population density increased, higher building density would become less important in the probability of detecting Mexican free-tailed bats. When building density was high, population density would have less influence on the probability of detecting Mexican free-tailed bats.
Discussion
The nonrandom distribution of bats in Waco clearly showed that bats did not view the city as a homogenous patch and that individual species, or subsets of species, view the city landscape differently. We found free-tailed bats having a distributional pattern distinct from other bat species in the city, yet overlapping the distributions of the other species. And, we found subsets of other bat species sharing similar distributional patterns. These findings support previous arguments that bats tend to follow their functional guilds in use of the urban landscape (Avila-Flores and Fenton 2005; Duchamp and Swihart 2008; Luck et al. 2013).
Both species in the LANY group, red bat and evening bat, are tree-dwelling bats (Schmidly 2004). Prior studies of urbanization have shown that these two species often occurred in well-vegetated habitats (Duchamp et al. 2004; Walters et al. 2007). As expected, the presence of LANY group species was positively affected by vegetation coverage and negatively by distance to forest center, which is consistent with published work (Furlonger et al. 1987; Gaisler et al. 1998; Avila-Flores and Fenton 2005; Dixon 2011). These bats’ life history requirements may also explain why locations in the LANY group were restricted to certain areas of the city and the number of locations was smaller than the most common species in the study area. We also found no interaction/correlation between these variables, which might suggest that local microenvironment created by dense trees might play a role as important as major forest patches in term of providing vegetation support for bats.
Water availability is important to bats for drinking and as foraging locations where insect prey are concentrated. High levels of bat activity near water sources have been demonstrated in many studies (Zimmerman and Glanz 2000; Everette et al. 2001; Perry et al. 2008). Roosting and foraging near water sources reduces energy costs associated with transit (Fenton 1997), likely a reason why the EMPY group responded to the presence of water sources at locations. Yet, the distance to a major water body (lakes, rivers) was not significant in our analyses: among 16 EPMY locations with water sources present, 14 had only anthropogenic water sources (12 with swimming pools, 2 with artificial ponds). It seems, therefore, that these widespread and easily accessible anthropogenic water sources were sufficient for bats in EPMY group. Observations of bats drinking from anthropogenic water sources are widely reported in published literature (Bowles et al. 1990; Rydell et al. 2002), and mammalogists know swimming pools to be excellent sites for productive bat-netting in situations where open water is otherwise scarce. Thus, certain features of the urban environment are clearly beneficial to wildlife (Adams and Lindsey 2010; Dearborn and Kark 2010).
The response of Mexican free-tailed bats to urban heterogeneity of man-made structures could be related to their roosting preferences and tolerances. Studies of roost selection by urban bat species showed that bats tended to select tall buildings (Williams and Brittingham 1997; Neubaum et al. 2007; Mazurska and Ruczyński 2008), consistent with our modeling results. In an intensive survey of Mexican free-tailed bats in South Texas during the 1950’s and 1960’s, Davis et al. (1962) found bats using a wide variety of types of buildings in 87 towns they visited. They suggested that Mexican free-tailed bats could roost in virtually any building as long as an opening leading to the building interior was available. A previous local study showed Mexican free-tailed bats used a wide array of anthropogenic structures in downtown Waco (Scales and Wilkins 2007). The large number of locations with Mexican free-tailed bat detections is consistent with this species’ being the most abundant and highly successful human commensal bat species in Texas (Wilkins 1989).
Higher building density should provide more roosting opportunities, thus explaining building density as a significant positive variable in our models on the probability of detecting Mexican free-tailed bats. However, this finding somewhat differs from some other published works, in which building densities (Avila-Flores and Fenton 2005; Neubaum et al. 2007) or housing density (Threlfall et al. 2012) negatively influenced urban bat presence. We believe the contradictory finding could relate to different species’ preferences. It could also relate to different urban architectural styles and the scale at which building density is assessed. The urban landscape varies across the U.S. and varies even more broadly when comparing cities globally. In medium-sized cities like Waco, high building density (about 40 buildings in the 100 m buffer zone) is found in areas with dense single-family housing, and residential districts comprise a greater proportion of the city than represented by downtown where most office buildings are located. Building density downtown is very similar to residential areas, and is very low in some industrial lands (less than 10 buildings in the buffer zone) where extensive parking infrastructure is present.
Co-occurrence of the Mexican free-tailed bats with big brown bats and cave bats (EPMY species group) is common in natural caves (Schmidly 2004; Ammerman et al. 2012), suggesting similar roosting requirements among these species. When present in cities, these same three species often utilize man-made structures (Schmidly 2004; Ammerman et al. 2012), with big brown bats particularly preferring tall buildings (Williams and Brittingham 1997; Neubaum et al. 2007). However, our modeling suggested that neither presence of tall buildings nor building density was recognized by species of the EPMY group in Waco. While relatively small sample size might be a factor (25 EPMY locations in restricted areas vs. 216 TABR locations widespread), we suggest that a competitive interaction might exist among those species where they are sympatric, with species in the EPMY group perhaps competitively inferior in ability to use buildings in comparison to Mexican free-tailed bats. The published studies for big brown bats (Williams and Brittingham 1997; Neubaum et al. 2007) are near the northern extent of distribution of Mexican free-tailed bats and beyond that of the cave bat (Wilson and Ruff 1999), and might reflect observations made without influence of the other two species.
Recent research on the relationship between bat activity and roadways showed that bats tended to avoid settings having roads with high traffic volumes (Gaisler et al. 1998; Russell et al. 2009; Zurcher et al. 2010; Berthinussen and Altringham 2012). This response may be due to disturbance caused by moving vehicles, or to the higher probability of bat-vehicle collisions, though most bats would not often be flying so low that they would collide with vehicles. Our models indicated that Mexican free-tailed bats and the LANY group species avoided areas with highways, and that all bat species in our study were indifferent to road levels other than highways. We found that Mexican free-tailed bats responded to street intersection density, such that their detection was more likely in areas with higher intersection density. This is consistent with what Neubaum et al. (2007) suggested in their studies of big brown bats.
In contrast to our null hypothesis, bats responded to the socioeconomic mosaic of urban settings in this study. Locations for LANY group species tended to occur in areas with high household median income. Median income, in turn, generally relates importantly to vegetation condition, house structure, and animal control effort (Troy et al. 2007; Morzillo and Mertig 2011; Lowry et al. 2012). Adams and Lindsey (2010) suggested that people’s attitude and opinion about wildlife could also be influenced by income. In our study, income did not correlate with natural environment variables or man-made structure variables (VIF smaller than 2). This suggested that bats’ selection of these high-income areas might not have been directly related to vegetation. Interestingly, we found a positive interaction term between income and vegetation coverage, meaning that the effect of income on suitability of areas to LANY group bats is stronger in well-vegetated areas than in less well-vegetated areas. This also indicated that income played a different role than vegetation when influencing bat distribution. We posit that income is an indirect indicator of suitable urban habitats for bats in the LANY group. More study is needed to explore which features in these high-income areas were potentially attractive to bats.
Modeling for both the Mexican free-tailed bat and the EPMY group selected human population density as a positive variable influencing bat distributions. Human population density has been used in various studies of urban environments to indirectly represent the composition of man-made structures (Alberti et al. 2008). In our study, the bat species responding to human population density are capable of roosting in man-made structures. Though we found no correlation of population density with building density or with presence of tall buildings, we still think that population density might indicate the availability of certain types of buildings, such as residential houses, which bats might find suitable. This may be why the interaction between population density and building density was negative. When the value of either variable was sufficiently high, enough bat-preferred roosting structures would be present and the effect of the other variable would reduce.
Compared to variables that describe only human demographics or features of the physical environment, land use type combines both sorts of information. A land use map is one of the most direct representations of urban mosaic. This likely explains correlations we found between land use type and many other variables (e.g., “industrial” strongly lacked vegetation, “medium/high density residential” had high population density). These modeling results also provided a different perspective on how bats might have perceived urban heterogeneity. All bat species in our study seemed to avoid “industrial” lands, since most natural elements such as water source and vegetation were generally absent. “Public” lands were favored by species in the LANY group probably because many parks with dense trees were located in this type of land use. Many tall buildings were present in the “mixed use” land type, which could explain the association between this land use type and Mexican free-tailed bat locations. “Low density residential” served as good habitat for many species. These conclusions are largely consistent with published work (Duchamp et al. 2004; Avila-Flores and Fenton 2005), since these neighborhoods usually provide features (e.g., houses for roosting, pools as water sources) that bats may favor. Cadenasso et al. (2007) suggested that within the same land use type there might still be differences due to the neighborhood age, income level, or other factors. Future work should explore what additional information could be considered with land use types to better explain how bats respond to urban heterogeneity.
Diversity of land use types is a variable not commonly considered in previous studies. Threlfall et al. (2011) considered a similar variable in an urban insectivorous bat study in Australia, but they did not find the diversity of land cover types to affect bat activity. Perry et al. (2008) found that red bats preferred forest habitats with less diverse patches. Our findings differed from both of those studies. In our models, land use diversity had a positive effect on the distribution patterns of species in the EPMY group and TABR. We speculated that a more diverse land use combination (a greater variety of physical structures) is more likely to provide more of the necessities that bats require. For example, bats likely can locate a roost in a tall building in mixed-use land type. A pool is probably present at a nearby house in the low-density residential land type. Meanwhile, the lighting in the nearby commercial and office land type might attract an abundance of insects for bats to consume. Another possible explanation for this result is that many unoccupied buildings tend to be present in the edge of each land use type. Most developments tend to utilize the center of each land use type first, leaving scattered unoccupied structures along the edge. Usually these structures are more favored by bats as roosts (Williams and Brittingham 1997; Mazurska and Ruczyński 2008).
Our research showed how heterogeneous urban environments affect bat activities near their roosts. It suggests that bats’ responses to urbanization are much as functional traits and life histories would predict. Bats in similar guilds may respond to cities in similar ways. However, when applying the guild concept, bat ecologists need to consider multiple dimensions, such as diet, roosting behavior, wing morphology, and other attributes. Bats in an urban environment are responsive not only to the heterogeneity of physical structure, but evidently also to socioeconomic heterogeneity. Interactions between physical heterogeneity and socioeconomic heterogeneity make the urban environment a very complex mosaic to bats. Many more factors remain to be considered as we strive to better understand the complexity of the urban ecosystem and how bats respond to the challenges and opportunities presented by this complexity.
References
Adams CE, Lindsey KJ (2010) Urban wildlife management. CRC Press/Taylor & Francis Group, Boca Raton
Alberti M, Marzluff JM, Shulenberger E et al (2008) Integrating humans into ecology: Opportunities and challenges for studying urban ecosystems. In: Marzluff JM, Shulenberger E, Endlicher W (eds) Urban ecology an international perspective on the interaction between humans and nature. Springer US, New York, pp 143–158
Ammerman LK, Hice CL, Schmidly DJ (2012) Bats of Texas. Texas A&M University Press, College Station
Anderson JR, Hardy EE, Roach JT, Witmer RE (1976) A land use and land cover classification system for use with remote sensor data. U.S. Government Printing Office
Augustin NH, Mugglestone MA, Buckland ST (1996) An autologistic model for the spatial distribution of wildlife. J Appl Ecol 33:339–347. doi:10.2307/2404755
Avila-Flores R, Fenton MB (2005) Use of spatial features by foraging insectivorous bats in a large urban landscape. J Mammal 86:1193–1204. doi:10.2307/4094543
Baddeley A, Gill RD (1997) Kaplan-Meier estimators of distance distributions for spatial point processes. Ann Stat 25:263–292. doi:10.2307/2242720
Basham R, Law B, Banks P (2011) Microbats in a “leafy” urban landscape: are they persisting, and what factors influence their presence? Aust Ecol 36:663–678. doi:10.1111/j.1442-9993.2010.02202.x
BatCall Library. http://www.msb.unm.edu/mammals/batcall/html/calllibrary.html. Accessed 6 May 2013
BatSpecies List. http://www.flybynightinc.org/bats.htm. Accessed 6 May 2013
Beale CM, Lennon JJ, Yearsley JM et al (2010) Regression analysis of spatial data. Ecol Lett 13:246–264. doi:10.1111/j.1461-0248.2009.01422.x
Berthinussen A, Altringham J (2012) The effect of a major road on bat activity and diversity. J Appl Ecol 49:82–89. doi:10.1111/j.1365-2664.2011.02068.x
Bivand RS, Pebesma EJ, Rubio VG (2008) Applied spatial data: analysis with R. Springer, New York
Black D, Henderson V (2003) Urban evolution in the USA. J Econ Geogr 3:343–372. doi:10.1093/jeg/lbg017
Boland JL (2007) Distribution of bats in southeast Alaska and selection of day-roosts in trees by Keen’s myotis on Prince of Wales Island, Southeast Alaska. Thesis
Bowles JB, Heideman PD, Erickson KR (1990) Observations on six species of free-tailed bats (Molossidae) from Yucatan, Mexico. Southwest Nat 35:151–157. doi:10.2307/3671536
Burnham KP, Anderson DR (2002) Model selection and multi-model inference: a practical information-theoretic approach. Springer, New York
Cadenasso ML, Pickett ST, Schwarz K (2007) Spatial heterogeneity in urban ecosystems: reconceptualizing land cover and a framework for classification. Front Ecol Environ 5:80–88
Cadenasso ML, Pickett STA, McGrath B, Marshall V (2013) Ecological heterogeneity in urban ecosystems: Reconceptualized land cover models as a bridge to urban design. In: Pickett STA, Cadenasso ML, McGrath B (eds) Resilience in ecology and urban design. Springer Netherlands, Houten, pp 107–129
Coleman JL, Barclay RMR (2012) Urbanization and the abundance and diversity of Prairie bats. Urban Ecosyst 15:87–102. doi:10.1007/s11252-011-0181-8
Conzen MP (2001) The study of urban form in the United States. Urban Morphol 5:3–14
Davis RB, Herreid CF, Short HL (1962) Mexican free-tailed bats in Texas. Ecol Monogr 32:311–346
Davison AC, Hinkley DV (1997) Bootstrap methods and their application. Cambridge University Press, Cambridge
Dearborn DC, Kark S (2010) Motivations for conserving urban biodiversity. Conserv Biol 24:432–440. doi:10.1111/j.1523-1739.2009.01328.x
Dixon MD (2011) Relationship between land cover and insectivorous bat activity in an urban landscape. Urban Ecosyst 15:683–695. doi:10.1007/s11252-011-0219-y
Dow K (2000) Social dimensions of gradients in urban ecosystems. Urban Ecosyst 4:255–275. doi:10.1023/A:1015767231137
Duchamp JE, Sparks DW, Whitaker JO Jr (2004) Foraging-habitat selection by bats at an urban–rural interface: comparison between a successful and a less successful species. Can J Zool 82:1157–1164
Duchamp JE, Swihart RK (2008) Shifts in bat community structure related to evolved traits and features of human-altered landscapes. Landsc Ecol 23:849–860. doi:10.1007/s10980-008-9241-8
Everette AL, O’Shea TJ, Ellison LE et al (2001) Bat use of a high-plains urban wildlife refuge. Wildl Soc Bull 29:967–973. doi:10.2307/3784424
Fabianek F, Gagnon D, Delorme M (2011) Bat distribution and activity in Montréal island green spaces: responses to multi-scale habitat effects in a densely urbanized area. Ecoscience 18:9–17. doi:10.2980/18-1-3373
Farmer MC, Wallace MC, Shiroya M (2013) Bird diversity indicates ecological value in urban home prices. Urban Ecosyst 16:131–144. doi:10.1007/s11252-011-0209-0
Fenton MB (1997) Science and the conservation of bats. J Mammal 78:1–14. doi:10.2307/1382633
Furlonger CL, Dewar HJ, Fenton MB (1987) Habitat use by foraging insectivorous bats. Can J Zool 65:284–288. doi:10.1139/z87-044
Gaisler J, Zukal J, Rehak Z, Homolka M (1998) Habitat preference and flight activity of bats in a city. J Zool 244:439–445. doi:10.1111/j.1469-7998.1998.tb00048.x
Gehrt SD, Chelsvig JE (2004) Species-specific patterns of bat activity in an urban landscape. Ecol Appl 14:625–635
Gehrt SD, Chelsvig JE (2003) Bat activity in an urban landscape: patterns at the landscape and microhabitat scale. Ecol Appl 13:939–950
Gledhill DG, James P (2012) Socio-economic variables as indicators of pond conservation value in an urban landscape. Urban Ecosyst 15:849–861. doi:10.1007/s11252-012-0242-7
Grimm NB, Morgan Grove J, Pickett STA, Redman CL (2000) Integrated approaches to long-term studies of urban ecological systems. Bioscience 50:571–584. doi:10.1641/0006-3568(2000)050[0571:IATLTO]2.0.CO;2
Gutzwiller KJ, Riffell SK (2007) Using statistical models to study temporal dynamics of animal—landscape relations. In: Bissonette JA, Storch I (eds) Temporal dimensions of landscape ecology. Springer, New York, pp 93–118
Hahs AK, McDonnell MJ (2006) Selecting independent measures to quantify Melbourne’s urban–rural gradient. Landsc Urban Plan 78:435–448. doi:10.1016/j.landurbplan.2005.12.005
Hale JD, Fairbrass AJ, Matthews TJ, Sadler JP (2012) Habitat composition and connectivity predicts bat presence and activity at foraging sites in a large UK conurbation. PLoS ONE 7:e33300. doi:10.1371/journal.pone.0033300
Irwin EG, Jayaprakash C, Munroe DK (2009) Towards a comprehensive framework for modeling urban spatial dynamics. Landsc Ecol 24:1223–1236. doi:10.1007/s10980-009-9353-9
James P, Bound D (2009) Urban morphology types and open space distribution in urban core areas. Urban Ecosyst 12:417–424. doi:10.1007/s11252-009-0083-1
Johnson JB, Gates JE, Ford WM (2008) Distribution and activity of bats at local and landscape scales within a rural–urban gradient. Urban Ecosyst 11:227–242. doi:10.1007/s11252-008-0055-x
Johnson JB, Menzel MA, Edwards JW, Ford WM (2002) A comparison of 2 acoustical bat survey techniques. Wildl Soc Bull 30:931–936
Jung K, Kalko EKV (2011) Adaptability and vulnerability of high flying Neotropical aerial insectivorous bats to urbanization. Divers Distrib 17:262–274. doi:10.1111/j.1472-4642.2010.00738.x
Kelejian HH, Prucha IR (2010) Specification and estimation of spatial autoregressive models with autoregressive and heteroskedastic disturbances. J Econ 157:53–67. doi:10.1016/j.jeconom.2009.10.025
Kurta A, Teramino JA (1992) Bat community structure in an urban park. Ecography 15:257–261. doi:10.1111/j.1600-0587.1992.tb00032.x
Kurta A, Winhold L, Whitaker JO, Foster R (2007) Range expansion and changing abundance of the eastern pipistrelle (Chiroptera: Vespertilionidae) in the central great lakes region. Am Midl Nat 157:404–411. doi:10.1674/0003-0031(2007)157[404:REACAO]2.0.CO;2
Lichstein JW, Simons TR, Shriner SA, Franzreb KE (2002) Spatial autocorrelation and autoregressive models in ecology. Ecol Monogr 72:445–463
Loeb SC, Post CJ, Hall ST (2009) Relationship between urbanization and bat community structure in national parks of the southeastern U.S. Urban Ecosyst 12:197–214. doi:10.1007/s11252-008-0075-6
Lowry JHL, Baker ME, Ramsey RD (2012) Determinants of urban tree canopy in residential neighborhoods: Household characteristics, urban form, and the geophysical landscape. Urban Ecosyst 15:247–266. doi:10.1007/s11252-011-0185-4
Luck GW, Smallbone L, Threlfall C, Law B (2013) Patterns in bat functional guilds across multiple urban centres in south-eastern Australia. Landsc Ecol 28:455–469. doi:10.1007/s10980-012-9842-0
Marzluff JM, Shulenberger E, Endlicher W (2008) Urban ecology: an international perspective on the interaction between humans and nature. Springer, New York
Mazurska K, Ruczyński I (2008) Bats select buildings in clearings in Bialowieza Primeval Forest. Acta Chiropterol 10:331–338. doi:10.3161/150811008X414908
McCarthy MA (2011) Bayesian methods for ecology. Cambridge University Press, Cambridge
McCune B, Grace JB, Urban DL (2002) Analysis of ecological communities. M G M Software Design, OR
McDonnell MJ, Hahs AK (2008) The use of gradient analysis studies in advancing our understanding of the ecology of urbanizing landscapes: current status and future directions. Landsc Ecol 23:1143–1155. doi:10.1007/s10980-008-9253-4
McIntyre NE, Knowles-Yánez K, Hope D (2000) Urban ecology as an interdisciplinary field: differences in the use of “urban” between the social and natural sciences. Urban Ecosyst 4:5–24. doi:10.1023/A:1009540018553
McKinney ML (2002) Urbanization, biodiversity, and conservation. Bioscience 52:883–890
Miller JR, Hobbs RJ (2002) Conservation where people live and work. Conserv Biol 16:330–337. doi:10.1046/j.1523-1739.2002.00420.x
Mommaas H (2004) Cultural clusters and the post-industrial city: towards the remapping of urban cultural policy. Urban Stud 41:507–532. doi:10.1080/0042098042000178663
Morzillo AT, Mertig AG (2011) Urban resident attitudes toward rodents, rodent control products, and environmental effects. Urban Ecosyst 14:243–260. doi:10.1007/s11252-010-0152-5
Moses L, Williamson HF (1967) The location of economic activity in cities. Am Econ Rev 57:211–222. doi:10.2307/1821622
Neubaum DJ, Wilson KR, O’shea TJ (2007) Urban maternity-roost selection by big brown bats in Colorado. J Wildl Manage 71:728–736. doi:10.2193/2005-684
O’Farrell MJ, Miller BW, Gannon WL (1999) Qualitative identification of free-flying bats using the Anabat detector. J Mammal 80:11–23
Pacific northwest bat call library. http://depts.washington.edu/sdwasm/pnwbat/batcall.html. Accessed 6 May 2013
Perry RW, Thill RE, Leslie DM (2008) Scale-dependent effects of landscape structure and composition on diurnal roost selection by forest bats. J Wildl Manage 72:913–925. doi:10.2193/2006-435
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, New York
Rees WE (1997) Urban ecosystems: the human dimension. Urban Ecosyst 1:63–75. doi:10.1023/A:1014380105620
Rhodes M, Catterall C (2008) Spatial foraging behavior and use of an urban landscape by a fast-flying bat, the molossid Tadarida australis. J Mammal 89:34–42
Rhodes M, Wardell-Johnson GW, Rhodes MP, Raymond B (2006) Applying network analysis to the conservation of habitat trees in urban environments: a case study from Brisbane, Australia. Conserv Biol 20:861–870. doi:10.1111/j.1523-1739.2006.00415.x
Roche N, Langton S, Aughney T et al (2011) A car-based monitoring method reveals new information on bat populations and distributions in Ireland. Anim Conserv 14:642–651. doi:10.1111/j.1469-1795.2011.00470.x
Russ JM, Briffa M, Montgomery WI (2003) Seasonal patterns in activity and habitat use by bats (Pipistrellus spp. and Nyctalus leisleri) in northern Ireland, determined using a driven transect. J Zool 259:289–299. doi:10.1017/S0952836902003254
Russell A, Butchkoski C, Saidak L, McCracken G (2009) Road-killed bats, highway design, and the commuting ecology of bats. Endang Species Res 8:49–60. doi:10.3354/esr00121
Rydell J (1991) Seasonal use of illuminated areas by foraging northern bats Eptesicus nilssoni. Ecography 14:203–207
Rydell J, Arita HT, Santos M, Granados J (2002) Acoustic identification of insectivorous bats (order Chiroptera) of Yucatan, Mexico. J Zool 257:27–36. doi:10.1017/S0952836902000626
Schmidly DJ (2004) The mammals of Texas. University of Texas Press, Austin
Scales JA, Wilkins KT (2007) Seasonality and fidelity in roost use of the Mexican free-tailed bat, Tadarida brasiliensis, in an urban setting. West N Am Naturalist 67:402–408
Sparks DW, Ritzi CM, Duchamp JE, Whitaker JO (2005) Foraging habitat of the Indiana bat (Myotis sodalis) at an urban–rural interface. J Mammal 86:713–718. doi:10.1644/1545-1542(2005)086[0713:FHOTIB]2.0.CO;2
Threlfall CG, Law B, Banks PB (2012) Sensitivity of insectivorous bats to urbanization: implications for suburban conservation planning. Biol Conserv 146:41–52. doi:10.1016/j.biocon.2011.11.026
Threlfall CG, Law B, Penman T, Banks PB (2011) Ecological processes in urban landscapes: mechanisms influencing the distribution and activity of insectivorous bats. Ecography 34:814–826. doi:10.1111/j.1600-0587.2010.06939.x
Troy AR, Grove JM, O’Neil-Dunne JPM et al (2007) Predicting opportunities for greening and patterns of vegetation on private urban lands. Environ Manage 40:394–412. doi:10.1007/s00267-006-0112-2
Turner M, Gardner R, O’Neill R (2001) Landscape ecology in theory and practice: pattern and process. Springer, New York
Walters BL, Ritzi CM, Sparks DW, Whitaker JO (2007) Foraging behavior of eastern red bats (Lasiurus borealis) at an urban–rural interface. Am Midl Nat 157:365–373. doi:10.1674/0003-0031(2007)157[365:FBOERB]2.0.CO;2
Williams LM, Brittingham MC (1997) Selection of maternity roosts by big brown bats. J Wildl Manage 61:359–368. doi:10.2307/3802592
Wilkins KT (1989) Tadarida brasiliensis. Mamm Species 331:1–10
Wilson DE, Ruff S (1999) The Smithsonian book of North American mammals. UBC Press, Vancouver
Wyoming bat call library. http://www.uwyo.edu/wyndd/data-dissemination/priority-data-comp/wyoming-bat-call-library/. Accessed 6 May 2013
Zimmerman GS, Glanz WE (2000) Habitat use by bats in eastern Maine. J Wildl Manage 64:1032. doi:10.2307/3803214
Zurcher AA, Sparks DW, Bennett VJ (2010) Why the bat did not cross the road? Acta Chiropterol 12:337–340. doi:10.3161/150811010X537918
Zuur A, Ieno EN, Walker N et al (2009) Mixed effects models and extensions in ecology with r. Springer, New York
Acknowledgments
We extend our thanks to colleagues and students at Baylor University who assisted in this research: Anica Debelica-Lee, Dr. Nick Green, and Dr. Thomas Pettit for constructing the bat echolocation library; Jonathan Cook for assistance with GIS techniques; Dr. Kevin Gutzwiller, Dr. David Kahle, and Tracy Pinney for discussion and advice regarding statistical analyses; Kevin Chen, Frany Dadhania, Therese Gavin, Vicky Ho, Robin Vander Pol, Kristine Williams, and Lucinda Yu for field assistance; Dr. Robert Doyle, chair of Baylor Biology Department; and the Graduate School and Glasscock Research Fund of Baylor University for financial support.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, H., Wilkins, K.T. Patch or mosaic: bat activity responds to fine-scale urban heterogeneity in a medium-sized city in the United States. Urban Ecosyst 17, 1013–1031 (2014). https://doi.org/10.1007/s11252-014-0369-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-014-0369-9