Abstract
The present study evaluated the metabolic and functional effects of adding garra meal to a broiler chicken diet. Three hundred twenty Sasso-breed day-old chicks were randomly assigned to four dietary treatments with either 0, 10, 20 or 30% garra meal added on top of formulated starter and grower basal diets. The experiment lasted for 42 days. Feed intake and body weight gain increased at the starter and grower phases of broilers with garra meal addition (P < 0.05). Broiler chickens fed 30% garra meal were more efficient in converting feed to body weight and yielded the highest carcass weight (P < 0.05). Crude protein ileal digestibility coefficient was higher with 20% (76.2%), and crude fat with 20 (92.1) and 30% (92.6%) garra meal receiving groups (P < 0.05). The increase in individual and total esterified carnitine concentrations in dried blood spots demonstrated the elevated metabolic rate with garra meal addition (P < 0.05). A better supply of glucogenic substrate to the citric acid cycle was seen with garra meal addition due to the increase of propionylcarnitine to acetylcarnitine ratio (P < 0.05) without any apparent effect on ketogenesis in terms of serum 3-hydroxybutyrylcarnitine to acetylcarnitine ratio. Yet, it likely showed that part of the amino acids from garra meal were used as glucogenic substrate (P < 0.05). Histomorphometry data showed 20% garra meal addition elevated villus height, crypt depth and their ratio in the proximal parts of the small intestine (duodenum and jejunum) with the opposite results observed in the more distal part (ileum) with the highest for the control group (P < 0.05). It can be concluded that garra meal improved broiler performance when added to a plant-based diet and only few parameters warranted for caution when using more up to 30% garra meal addition. Beyond growth performance, garra meal generated a shift to a more efficient digestion, absorption and nutrient metabolism.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fish meal has been a successful ingredient in poultry diets throughout the world. In some regions such as the European Union, its use has been banned together with meat and bone meals after the bovine spongiform encephalopathy crisis in 1996 (Eliaszewicz 2004; Thiry et al. 2004). In Ethiopia, Garra spp., an indigenous fish, are commonly considered a waste from fisheries despite its importance as a valuable source of nutrients (Bayissa et al. 2021a). Despite their presence in some Asian and African countries (Getahun and Stiassny 1998), about 60% of the African garra fish species are found in Ethiopia (Dejenie et al. 2008; Getahun 2000).
The present poultry diets in Ethiopia are usually imported and based on cereals (Ebsa et al. 2019). The latter contain anti-nutritional factors such as difficult-to-digest fibre fractions and may have imbalanced amino acid and mineral profiles if not properly formulated (Fickler 2002; Blair 2018). Using local garra “waste” to produce fish meal could improve the ecological sustainability of poultry diets in Ethiopia and improve their nutritive value.
Fish meal is indeed known as a highly digestible nutrient source, in particular protein, with a well-balanced amino acid pattern that can support growth of the chickens (Karimi 2006). Despite these interesting nutritional characteristics of fish meal, poor processing and preservation methods may lead to the formation of putrefactive compounds such as biogenic amines (Auerswald et al. 2006; Feng et al. 2016). In most studied cases, these products are negatively associated with intestinal health (Apajalahti and Vienola 2016). In particular, the addition of garra may exert biogenic amines (e.g. histamine) that can increase intestinal permeability in chickens. Higher levels of addition can be related with increased histidine decarboxylation effects by L-histidine decarboxylase, hence histamine levels (Holecek 2020). In general, chickens have been reported to have low histamine levels and its concentration can considered an indicator of food quality to ensure consumer safety (Mejrhit et al. 2018).
Furthermore, the lipid fraction may trigger opposite effects; on the one hand, the high proportion on long-chain n-3 fatty acids may benefit the health of the chickens and their consumers, but these lipids are also highly susceptible to peroxidation, leading to increased dietary content of oxidative agents (Amaral et al. 2018; Alagawany et al. 2019). As a third example of nutrients that may determine the optimal addition level of garra meal in poultry diets, the concentration and bioavailability of important minerals may need consideration. A large proportion of Ethiopian infants suffer from several micromineral deficiencies, such as iron and selenium (Mekonnen et al. 2005). Preliminary data demonstrated that those minerals are quite high in garra, and one may assume that their bioavailability will be high because they are mainly chelated (e.g. iron in haemoglobin) or covalently bound (e.g. selenium in selenomethionine). Recent works on the Ethiopian garra nutrient composition have reported a higher zinc and iron concentration than in other fish in the same aquatic environment (Bayissa et al. 2021a).
Because small garra fish by-catch is still discarded as a waste in several locations, the use of its fish meal in poultry nutrition could be a sustainable solution to reduce the need for dietary protein source in the sector. We here investigate how fish meal made of garra, a small fish species endemic to Ethiopia, may affect nutrient metabolism and health parameters beyond overall performance in broiler chickens. Knowledge on the metabolic effects of garra meal can identify additional benefits or disadvantages beyond growth performance that are often neglected in similar poultry trials and for which case we intended to add garra meal on top of a finished basal diet. The choice for adding the garra meal on top of the diet instead of substituting with another ingredient evidently led to different dietary nutrient and energy concentrations, but allowed for a net identification of the garra meal effects, independent of a substituted ingredient. Therefore, this project is aimed at investigating the effects of graded addition of garra meal on production performance, apparent nutrient digestibility, intestinal microarchitecture, serum profile of acylcarnitine, blood concentrations of malondialdehyde, histamine, selected free amino acids, intestinal permeability and nutrient profiles in broilers.
Materials and methods
Garra-based poultry feed formulation
The experimental feed formulation and feeding trials were carried out at Mekelle University, Mekelle, Ethiopia. Garra (G. dembecha) fish were caught from several water bodies and reservoirs of the Tigray region, Northern Ethiopia. Whole fish were uniformly distributed for drying on a triangular shaped entirely mesh-enclosed drier stand (2 m × 12 m × 2.5 m) established on the roof of a 15 m high building and sun-dried for a fixed time period of 5 ± 1 day. The drying environment was with maximum average temperature of 28 ± 1.4 °C and wind speed at 2.72 m/s across the drying periods, December, January and February 2019/20. During the drying period, whole fish were uniformly turned over using a brush-ended clean stick to facilitate drying. Dried garra meal was collected and milled to acceptable smaller size using a grinder machine and made ready as feed substituent. Commercially available formulated broiler starter (days 0–21) and finisher (days 22–42) feeds were purchased from EthioChicken Plc, Mekelle, Ethiopia. For both growth phases, a 10, 20 and 30% garra meal addition to the formulated commercial diets were prepared. We deliberately chose for addition of the garra meal instead of substitution with other ingredients to obtain the same nutrient profile. Both scenarios inevitably lead to confounding factors; since we wanted to know the pure effect of garra meal, we opted for addition, in the awareness that this implied different dietary nutrient concentrations. The macronutrient compositions of the garra meal, broiler starter and finisher basal diets were determined by proximate analysis (AOAC 1990) (Table 1).
Similarly, as a starting protocol for the experiment, garra meal, broiler starter and finisher basal diets were further evaluated for amino acid, minerals and fatty acid contents. Amino acids assay was done using reversed-phase high-performance liquid chromatography analysis at feed and food quality laboratory, Belgium (Table 2). The total ash content was determined following the ISO method (ISO 936, 1998) and the mineral (Ca, P, Mg, K, Na, Br, Cu, Fe, Mn and Zn) concentrations of the garra meal and feed samples were evaluated after dry ashing mineralization according to ISO method (ICP-AES: ISO 11885) as described by Gorsuch (1970) at the Chemistry laboratory, Ghent University (Table 3) and the fatty acid composition at Lanupro, Ghent University (Table 4).
Experimental animal set up and feeding trial
The experiment involved 320 day-old broiler chickens, randomly divided over 20 indoor floor pens with 16 chickens each (at a density of 0.28 m2/chicken). Each pen was randomly attributed to one of four dietary treatments, i.e. 0, 10, 20 and 30% garra meal additions on top of the broiler starter and finisher diets. Broiler chickens (Sasso-C44 breed) were purchased from hatchery supply of EthioChicken Plc, Mekelle, Ethiopia.
Pens were considered experimental units. All pens were designed to be similar in all outlines, with concrete floor having wood shavings as bedding at a depth of 5–10 cm, and built in accordance with the international and institutional research ethics and welfare requirements for experimental animals. Each pen was set with separate feeding and watering troughs, and an electrically controlled warming 200 W bulb lamp hung from the top of each pen. Indoor heating temperature was controlled using wall-fixed digital thermometers and maintained at 30 ± 1.0 °C across the starter periods of the experiment. All experimental chickens were procedurally vaccinated against infectious bursal (Gumboro) disease, Newcastle disease and fowl typhoid as per the manufacturer’s recommended vaccination protocols for day-old chickens.
Determination of growth performance indicators
Performance indicating factors were assessed for chickens in each experimental unit. Following fasting for 12 h, chickens were weighed on weekly basis. The average weekly feed intake (g/wk), body weight gain (g/wk) and feed conversion ratio (FCR, g/g) were evaluated at each sampling time point following standard procedures. Mortality cases were checked and recorded from every replicate of each treatment group (N = 80) on a daily basis and correction factors were made for all dependent parameters across the study period. Mortality was calculated as follows:
where, N stands for the number of dead chicken in every replicate of each treatment group; 80 is the total number of chickens per group.
Determination of apparent nutrient digestibility
Marker-feed preparation and feeding
Starting from day 22 onwards, titanium (IV) oxide (VWR Chemicals®, Belgium, PN84853; > 99.0% purity) was supplemented into each experimental diet of grower chickens at 0.4% addition. To assure homogenous mixing of the marker, premixing was applied on a small amount of diet (1% of the total diet). Feeding with the marker diet started on the first day of the growth phase (day 22) and continued till day 42 of the experimental period.
Excreta collection and processing
Prior to sampling, all wood shavings were removed and replaced with plastic mats for easier collection of excreta. Excreta sampling from each experimental unit was carried out for six consecutive days of the last week of the experimental period, days 37–42. Excreta were collected using a blunt-flat scraper from the floor quantitatively for 24 h at 2 × /day (06:00 and 18:00), pooled and weighed daily and kept frozen (− 20 °C) during the sampling days. Finally, samples were pooled per pen, homogenized with a mixer (1–2 min), and sample of known weight (about 300 g/pen) was taken in plastic bags from each experimental unit. Excreta samples were then freeze-dried to stable weight and kept at − 20 °C till analysed (Schaafstra et al. 2017). Feed and water were given ad libitum across the collection period.
Measurement of apparent ileal digestibility coefficients
Freeze-dried excreta samples and the experimental diets were all analysed for their individual nutrients. The DM, CP and EE contents were evaluated according to AOAC (1990). The CP contents were evaluated using the Kjeldahl method as described by AOAC (2001) and the TiO2 contents as per the procedures stated by Myers et al. (2004). All samples were evaluated in duplicates at Ghent University, Belgium. Obtained mean values were used for the calculation of apparent digestibility coefficients of individual nutrients using the biomarker and the following formula:
Evaluation of chicken blood biochemical profiles
Blood sample collection and processing
Blood samples (5 mL/chicken) were drawn from wing veins of three randomly selected chickens from each experimental unit at the end of the starter (day 22) and grower phases (day 43). Blood samples were allowed to coagulate at room temperature for 15 min, then centrifuged (1500 × g for 10 min) and serum were collected in Eppendorf tubes and stored at − 20 °C until analyses. The same chickens were traced and sampled for their serum in round two blood sampling.
Measurement of biogenic amines and oxidative stress biomarkers
Serum samples (100 µL) were evaluated for histamine concentration. Following sample preparation and acylation, histamine quantification was performed using enzyme-linked immunosorbent assay (ELISA) following the manufacturer’s kit protocol (Ridascreen® Histamine (enzymatic), Art No. R1605) at Ghent University, Belgium. Serum (150 µL) malondialdehyde concentrations were analysed as thiobarbituric acid reactive substances (TBARS) according to the method of Grotto et al. (2007) at Lanupro, Ghent University.
Acylcarnitine profiling
For the evaluation of shifts in nutrient metabolism, profiling of acylcarnitine concentrations was carried out. A drop (50 µL) of blood was taken from each of the whole blood samples collected and placed on circular specimen collection papers (Whatman protein saver cards, 903™, UK). Each specimen kit was allowed to dry, and later sent to Ghent University Hospital (Laboratory for Clinical Chemistry) for their biochemical assay.
Measurement of intestinal histomorphometry
Intestinal sample collection
At the end of the experimental feeding period, following 12 h fasting, the traced 3 chickens from each experimental unit were sampled for their intestines on day 42. Chickens were weighed and then euthanized humanely using sodium pentobarbital (IP, 28 mg/kg BW) to death. The entire small intestine segment was exposed under aseptic post-mortem examination.
A fixative solution (mixture of glutaraldehyde [3%] and paraformaldehyde [4%] fixative solution in 0.1 mol/L cacodylate buffer [pH 7.4]) was prepared and injected into the intestinal lumen of the middle part of each intestinal segment. The entire small intestine spanning from the gizzard to pancreatic and bile duct (duodenum), from bile duct to Meckel’s diverticulum (jejunum) and from diverticulum to the ileo-caecal-colonic junction (ileum) was then excised and placed into separate bottles containing the same fixative solution. Similarly, the cecum part were also separately removed and placed in the same fixative solution until morphologically evaluated.
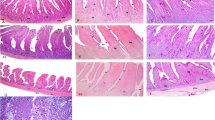
Histomorphometry examination
Gut tissue samples (2 cm long) were collected from the middle part of each intestinal segment, dehydrated under graded alcohols and xylene and then embedded in paraffin. Serial paraffin Sects. (5 μm) were made from samples and kept at 37 °C for 12 h or more. Then after, sample sections were prepared in slides and stained with haematoxylin–eosin stain following a routine protocol. Finally, the mucosal structures were observed under a Nikon phase-contrast microscope and examined using MicroComp integrated digital image-analysis system with the help of microscope camera. The villous height and crypt depth of 10 well-oriented villi were measured per section. The average value for each tissue was obtained from 3 sections per tissue sample. That is, 10 villi/crypt per section and 3 sections per sample were measured and the average value used for statistical analysis (Fan et al. 1997). Furthermore, the villus height to crypt depth ratio was also calculated for each value obtained.
Everted sac test (ex vivo)
A 20 cm segment of the hind intestine (ileum part) was cut and washed in phosphate buffer saline (PBS) solution along the hollow, weighed and then kept in an ice water mounted Petri dish. The hollow segment was then inverted using a small crochet needle inserted into the lumen. One part of the lumen was ligated while filled with the PBS solution from an elevated container through the other end of the sac and then ligated on either side. The sacs were fully immersed and incubated in a glass beaker filled with PBS solution containing a cobalt marker with a constant oxygenation through aqua-aerators. The glass beakers were kept in an open water bath set up at 39.3 ± 0.8 °C for an hour. Then after, sacs were taken out, rinsed with water, and 10 ml of the intestinal fluid contents was collected with syringe into a tube and stored at until analysed. Similar 10 mL sample was collected from the ringers’ solution with markers at the start of the experiment for conjoint evaluation. Spectrophotometric reading of cobalt concentration was done at 640 nm (Hamilton and Butt 2013).
Statistical analysis
Every replicate was considered the experimental unit for all statistical analyses. Treatment groups were compared for growth performance, apparent ileal digestibility, histomorphometry, histamine and malondialdehyde. Data were assessed for the assumptions of normality of distribution and equality of variance across treatment groups using graphical inspection of Q-Q plots, and Shapiro–Wilk and Leven’s test statistics. Since our variables deviated from these assumptions, we applied the distribution-free Kruskal–Wallis one-way ANOVA for overall group comparison. In the presence of significant overall group difference, pair-wise comparison of the treatment groups was performed using the Dunn’s test. Data were presented using medians (P25, P75) and Box-Whisker plots. Statistical analysis was performed using SPSS statistics for windows, version 27.0 (IBM® Corp., Armonk, NY, USA), and a P < 0.05 was considered statistically significant.
Results
Growth performance indicators
Mortality throughout the experimental period was low: three dead chickens in the control group and one dead in the 10% garra meal group, all in the first week, with no apparent relationship to the treatments.
There was a significant increase in feed intake in both the starter and grower phases of broilers receiving garra meal compared with the control group (P < 0.05). Similarly, increased weight gains were recorded at the grower and overall growth phases of broilers with garra meal addition (P < 0.05; Table 5).
Broiler chickens fed with 30% garra meal were more efficient in converting feed to body weight (1.8 g feed/g gain; P < 0.05) across the feeding experiment. Likewise, the highest carcass weight (1050 g; P < 0.05) was recorded at the highest garra meal addition (30%). However, there was no difference in carcass dressing percentages among treatment groups (P > 0.05; Table 5).
Apparent ileal nutrient digestibility
Apparent ileal crude protein digestibility was higher with the 20% garra meal receiving broilers (76.2%; P < 0.05). Similarly, crude fat ileal digestibility was higher with the 20 and 30% garra meal additions, 92.1 and 92.6%, respectively (P < 0.05). No difference in dry matter ileal digestibility was noticed among the treatment groups (P > 0.05; Table 6).
Acylcarnitine profiling
Higher blood spot concentrations of the short-chain acylcarnitine moieties — free carnitine (C0), propionylcarnitine (C3), butyrylcarnitine (C4), isovalerylcarnitine (C5) and hexanoylcarnitine (C6) — were observed in the garra meal addition than the control groups (P < 0.05; Fig. 1) with the highest concentration observed at the 30% garra meal addition groups. Also, the sum of all acylcarnitines (Sumcarn) was higher in the garra meal receiving groups than the control group (P < 0.05). However, the ratio of bound to free carnitine (acyl:free) decreased along the treatment groups with the highest and least being for the control and 30% garra meal addition, respectively (P < 0.05). The concentration of acetylcarnitine (C2) did not vary across the treatment groups (P > 0.05).
Box and whisker plots of acylcarnitine concentrations in dried blood spots of broiler chickens fed with different garra meal additions. a C0, free carnitine; b C2, acetylcarnitine; c C3, propionylcarnitine; d C4, butyrylcarnitine; e C5, isovalerylcarnitine; f C6, hexanoylcarnitine; g Acyl:free, ratio of acylated against free carnitine; h Sumcarn, sum of all carnitine esters. Box plots show the medians (solid bar), interquartile ranges (IQRs) (box) and 90th and 10th percentiles (whiskers) based on 3 birds per replicate and 5 replicates per treatment (N = 60). P-values report the overall group comparison performed using Kruskal–Wallis one-way ANOVA. Pair-wise comparisons of the treatment groups were performed using the Dunn’s test with different superscript letters indicating significant differences
The ratio of propionylcarnitine to acetylcarnitine (C3:C2) was higher with garra meal addition than the control groups (P < 0.05: Fig. 2). The ratio of 3-hydroxybutyrylcarnitine to acetylcarnitine (3OHC4:C2) was higher for the 10 and 20% garra meal receiving groups (P < 0.05), while there was no difference in the malonylcarnitine to acetylcarnitine ratio (C3DC:C2) across the treatment groups (P > 0.05).
Box and whisker plots of concentration ratios of targeted acylcarnitines from dried blood spots of broiler chickens fed diets with graded additions of garra meal. a C3:C2, propionylcarnitine to acetylcarnitine ratio; b 3OHC4:C2, 3-hydroxybutyrylcarnitine to acetylcarnitine ratio; c C3DC:C2, malonylcarnitine to acetylcarnitine ratio. Box plots show the medians (solid bar), interquartile ranges (IQRs) (box) and 90th and 10th percentiles (whiskers) based on 3 birds per replicate and 5 replicates per treatment (N = 60). P-values report the overall group comparison performed using Kruskal–Wallis one-way ANOVA. Pair-wise comparisons of the treatment groups were performed using the Dunn’s test with different superscript letters indicating significant differences
Dried blood spot concentrations of the free amino acids leucine, methionine and citrulline increased with garra meal addition than the control groups (P < 0.05; Fig. 3). On the other hand, phenylalanine and tyrosine concentrations were lower in the garra meal addition than the control groups (P < 0.05) and no difference in ornithine concentrations was noticed across the treatment groups (P < 0.05).
Box and whisker plots of free amino acid concentrations in dried blood spots of broiler chickens fed diets with graded additions of garra meal. a Leu, leucine; b Met, methionine; c Fen, phenylalanine; d Tyr, tyrosine; e Orn, ornithine; f Cit, citrulline. Box plots show the medians (solid bar), interquartile ranges (IQRs) (box) and 90th and 10th percentiles (whiskers) based on 3 birds per replicate and 5 replicates per treatment (N = 60). P-values report the overall group comparison performed using Kruskal–Wallis one-way ANOVA. Pair-wise comparisons of the treatment groups were performed using the Dunn’s test with different superscript letters indicating significant differences
Serum histamine and malondialdehyde levels
Chicken serum samples from all treatment groups contained histamine levels far below the detection limit, 5 mg/mL, and no difference was observed among treatment groups in both days 21 and 42 (P > 0.05). Likewise, there was no difference in serum malondialdehyde concentration among treatment groups at days 21 and 42 (P > 0.05) although all values recorded were above the detection limit set, 2.5 nmol/mL (Table 7).
Intestinal histomorphometry
A 20% garra meal addition elevated the villus height in the duodenum and jejunum segments compared with the control group (P < 0.05) while no difference was seen between 10% addition and the control group (P > 0.05). However, a decreased villus height of ileum was noted with the 20 and 30% garra meal addition than the control and 10% addition groups (P < 0.05).
The crypts of the duodenum and jejunum in the 20% garra meal group were significantly deeper than the rest treatment groups (P < 0.05). The crypt depth of ileum was deeper in the control than the garra meal receiving groups (P < 0.05), while there was no difference among the 10, 20 and 30% garra meal additions (P > 0.05).
The villus:crypt ratio of the duodenum and jejunum of broilers was higher in the 20 and 30% garra meal addition than the control group (P < 0.05). The ileum of 20% garra meal receiving broilers showed the lowest villus:crypt ratio compared to the other treatment groups (P < 0.05).
The cecal internal endothelial folding/crust heights were higher for the garra meal addition groups than that of the control group (P < 0.05), while no difference was noticed among the different garra meal receiving groups (P > 0.05; Table 8).
Everted sac test
Cobalt (Co) absorption across the ileal lumen was higher in the 30% garra meal addition than the other groups (P < 0.05; Fig. 4), while there was no difference noticed among the control, 10 and 20% garra meal receiving groups (P > 0.05).
Ex vivo mean cobalt ions absorbed across the intestinal lumen using the everted sac test from chickens fed diets with graded additions of garra meal. Absorbance differences of cobalt concentrations were measured at 640 nm spectrophotometry. Box plots show the medians (solid bar), interquartile ranges (IQRs) (box) and 90th and 10th percentiles (whiskers) based on 3 birds per replicate and 5 replicates per treatment (N = 60). P-values report the overall group comparison performed using Kruskal–Wallis one-way ANOVA. Pair-wise comparisons of the treatment groups were performed using the Dunn’s test with different superscript letters indicating significant differences
Discussion
The increase in performance of the broiler chickens with addition of garra meal was according to expectations, given the high protein and energy content of this fish meal made of garra. Adding garra meal on top of an assumedly balanced diet still induced a far better growth performance. Garra meal indeed appears as a balanced diet ingredient with highly digestible nutrients and high palatability.
Less obvious than the increased growth was the observed increase in feed intake with increasing garra meal, as in most situations, chickens will reduce feed intake with increasing energy density (Mikulec et al. 2004; Karimi 2006; Incharoen et al. 2010). Yet, other factors than just energy density are regulating feed intake. This was not the focus of our study, but we refer to, for instance, the minimal total discomfort theory (Forbes 2003) that showed that any nutrient or other requirement can affect voluntary feed intake. The increased intake can explain the higher growth rate with increasing garra meal, but the associated higher feed utilisation efficiency emphasizes the balanced supply of digestible nutrients from garra meal.
Data on whole-body composition of garra were recently published by Bayissa et al. (2021a) but we added here the fatty acid profile of garra, demonstrating its interestingly high content of long-chain n-3 fatty acids. These fatty acids have well-known health effects in chickens (Swanson et al. 2012; Al-Zuhairy and Jameel 2014; Ibrahim et al. 2018) and the accumulation of these fatty acids in chicken tissues can affect human health as well (Baiao and Lara 2005; Al-Zuhairy and Jameel 2014). As tackled further in this discussion, garra meal is also rich in specific functional nutrients such as carnitine and citrulline that may support the animal’s metabolism.
Clearly, the maximum for protein deposition was not yet reached with the basal diet without garra meal, because the birds not only grew faster with garra meal addition, but still had the same dressing percentage (and even numerically higher). Also, the unaltered malonylcarnitine to acetylcarnitine ratio in the dried bloodspots confirms that fat synthesis remained independent of garra meal addition. Malonyl coenzyme A is indeed a known marker for lipogenesis (Schooneman et al. 2013) and the higher production of malonylcarnitine is a step forward in the synthesis of fats in the citric acid cycle (Yang et al. 2019). It also matches the lower feed conversion ratio due to garra meal addition, which implies that the nutrients and energy from garra meal were more efficiently used than that of the basal diet. An important factor to explain this effect is the combination of highly digestible ingredients with a low fibre content. More garra meal addition led to higher lipid digestibility, even up to the 30% addition, but protein digestibility seemed to reach an optimum at 20% addition, likely because the capacity for protein digestion was reached at that point. A concomitant concern of suboptimal protein digestion is the consequences for gut health, because providing more protein as substrate to the cecal microbiome may lead to damaging fermentation end-products such as biogenic amines (Apajalahti and Vienola 2016; Mejrhit et al. 2018). In the present study, the serum concentrations of histamine, a major biogenic amine, and malondialdehyde, an oxidative stress indicator, could however not show such a negative effect of high garra meal addition. The only measurement that pointed to a possible optimum for garra meal addition was the increased gut permeability at 30% garra meal addition. Increased gut permeability, as proven ex vivo in the present study, could evoke increase in intestinal mucosal paracellular permeability enhancing entry of pathogenic bacteria and/or their toxins into the systemic circulations that could trigger diseases (Chelakkot et al. 2018; Hollander and Kaunitz 2020).
Despite this impact on gut permeability at the higher garra meal addition level, the intestinal development was prominently affected: gradual increases in villus height, crypt depth and their ratio in the proximal parts of the intestine (duodenum and jejunum) typically reflect a higher digestive and absorptive capacity when adding more garra meal. Irrefutably, Incharoen et al. (2010) have found that the villus heights in duodenum and jejunum were lower in chickens fed low-crude protein diets with a lower mitotic number in crypt cells than those on normal dietary crude protein. In fact, the intestinal endothelium, with its villi, is a dynamic environment and often changes to adjust itself in response to intestinal contents, including dietary compositions (Yamauchi et al. 2002; Prakatur et al. 2019). Hence, longer intestinal villi are associated with increased surface areas and capacity for absorption of nutrients (Izadi et al. 2013) and deeper crypts reflect active endothelial cell mitosis beneath each villus for the regeneration of new villi in the lumen (Hamedi et al. 2011). On the other side, lower villus:crypt ratios are associated with fewer absorptive cells and numerous epithelial secretory cells. The latter are involved in excess mucus section that can reduce nutrient absorption and lead to increased intestinal coping functions (Hamedi et al. 2011). In the more distal part (ileum), the opposite was observed, likely referring to the fact that higher proximal digestibility led to less digesta in the distal part of the chickens’ digestive tract. Ileum is indeed a site where water and minerals are absorbed while the absorption of proteins, carbohydrates and fat mainly takes place in the jejunum (Svihus et al. 2010; Ariyadi et al. 2019).
The avian caecum is particularly important to process uric acid from protein breakdown (Clench 1999), hence the increase in cecal crust height with more garra meal addition. The larger and numerous cecal folds can further be related to its involvement in the digestion of small protein loads being pushed against the cecal sphincter from the intestinal loads and/or their absorption (Clench 1999; Majeed et al. 2009).
As a result of increased absorptive capacity and increased feed intake, garra meal additions have indeed induced a higher supply of nutrients in metabolism. This requires a metabolic “machinery” that can handle the elevated demand for nutrient processing. Our group has been using carnitine esters in diverse species to reflect changes in the corresponding coenzyme A moieties in nutrient metabolism (e.g. frogs: Brenes-Soto et al. 2019; cows: Bedada et al. 2021; fish: Bayissa et al. 2021b) but on itself, the carnitine status of an animal can affect metabolic efficiency (Schooneman et al. 2013; Gucciardi et al. 2015; Yang et al. 2019). In the present study, the main acylcarnitines and their sum positively responded to increased garra meal addition, demonstrating the increased metabolic activity due to enhanced nutrient absorption and concomitant growth. The increase of the propionylcarnitine to acetylcarnitine ratio shows a relatively better supply of the often limiting glucogenic substrates to the citric acid cycle (Bayissa et al. 2021b). It is not sure if this was instrumental for improving feed utilisation efficiency because no significant effect was observed on the production of ketone bodies deviating from the citric acid cycle, as measured through the 3-hydroxybutyrylcarnitine to acetylcarnitine ratio. 3-Hydroxybutyrylcarnitine is an indicatory moiety of ketogenesis in the body (Xu et al. 2016). Yet, it likely shows that part of the higher amino acid uptake due to garra meal was used as glucogenic substrate. Methionine is an example of a glucoplastic amino acid that can enter the citric acid cycle through propionyl coenzyme A converting into succinyl coenzyme A (Krajcovicova-Kudlackova et al. 2000), and its free concentration in the dried bloodspots was clearly rising with garra meal addition. Yet, even the concentrations of the pure lipogenic amino acid leucine (Schooneman et al. 2013) went up with garra addition, whereas other free amino acids such as tyrosine and its precursor phenylalanine decreased when more garra meal was added. This suggests that these free amino acid profiles are not just representing metabolic requirements but also simply an influence of the dietary amino acid composition.
At least for citrulline and carnitine, two amino-acid-like substances, their dose-responsive increase in concentration could have been due to their supply through garra meal. Carnitine is not present in plant material, whereas most animals including fish are rich sources of carnitine (Li et al. 2020). There was a marked increase in the free as well as bound carnitine in our study. The reduction in the bound to free carnitine ratio that was seen with higher garra meal addition could be interpreted as a relative drop in metabolic rate, but in this case, this was clearly due to the much higher absorption of carnitine from the garra meal that may even have supported metabolic efficiency. We acknowledge that we cannot claim this effect since other factors may have caused improved efficiency independent of carnitine supply, but at least studies have demonstrated beneficial effects on carnitine supplementation to plant-based diets for broiler chickens (Janssens et al. 2000; Karadeniz et al. 2008; Hrnčár et al. 2015).
Citrulline cannot be synthesized in birds because they lack the urea cycle — in contrast with mammals — that can generate citrulline from ornithine (Tsuji and Kanazawa 1987). Citrulline can be an interesting functional nutrient given its antioxidant action (Coles 2007), especially in hot environments: heat stress is a strong inducer of oxidative stress (Akbarian et al. 2016). This effect was not studied here but we hypothesize that the better broiler performance in this study when feeding more garra meal was due to nutritional effects (improved supply of digestible nutrients) as well as metabolic modulator effects (e.g. carnitine, citrulline, n-3 fatty acids). The decrease in ornithine with garra meal additions however could possibly reflect dietary dilution effect of garra meal in the experimental diets.
In conclusion, garra meal improved broiler performance when added to a plant-based diet. Only few parameters warranted for caution when using up to 30% garra meal addition, but apart from digestibility and absorptive capacity, also nutrient metabolization efficiency seemed increased. The latter may have been caused by a number of functional components in the garra meal that may warrant further investigation together with meat quality characteristics of chickens.
Data availability
Any further data related to the manuscript will be available upon request.
References
Akbarian, A., Michiels, J., Degroote, J., Majdeddin, M., Golian, A., De Smet, S., 2016. Association between heat stress and oxidative stress in poultry; mitochondrial dysfunction and dietary interventions with phytochemicals. Journal of Animal Science and Biotechnology, 7, 1–14.
Alagawany, M., Elnesr, S.S., Farag, M.R., Abd El-Hack, M.E., Khafaga, A.F., Taha, A.E., Tiwari, R., Yatoo, M.I., Bhatt, P., Khurana, S.K., Dhama, K., 2019. Omega-3 and Omega-6 fatty acids in poultry nutrition: Effect on production performance and health. Animals, 9, 573. https://doi.org/10.3390/ani9080573
Al-Zuhairy, M.A., Jameel, Y.J., 2014. Effect of ND vaccine, multivitamins AD3E, and omega-3 on performance and immune response of broilers. Mirror of Research in Veterinary Sciences and Animals, 3, 42–50.
Amaral, A.B., da Silva, M.V., Lannes, S.C.D., 2018. Lipid oxidation in meat: mechanisms and protective factors–a review. Food Science and Technology, 38, 1–15.
AOAC, 1990. Official Methods of Analysis of the Association of Official Analytical Chemists (15th ed), (Arlington, USA).
AOAC, 2001. Official Methods of Analysis of the Association of Official Analytical Chemists (17th ed.), (Gaithersburg publishing, Washington, USA).
Apajalahti, J., Vienola, K., 2016. Interaction between chicken intestinal microbiota and protein digestion. Animal Feed Science and Technology, 221, 323–330.
Ariyadi, B., Sudaryati, S., Harimurti, S., Sasongko, H., Habibi, M.F., Rahayu, D., 2019. Effects of feed form on small intestine histomorphology of broilers. In: IOP Conference Series: Earth and Environmental Science. IOP Publishing, Vol. 387, No. 1, p. 012047.
Auerswald, L., Morren, C., Lopata, A.L., 2006. Histamine levels in seventeen species of fresh and processed South African seafood. Food Chemistry, 98, 231–239.
Baiao, N.C., Lara, L.J.C., 2005. Oil and fat in broiler nutrition. Brazilian Journal of Poultry Science, 7, 129–141. https://doi.org/10.1590/S1516-635X2005000300001.
Bayissa, T.N., Geerardyn, M., Gobena, S., Vanhauteghem, D., Du Laing, G., Wakijra Kabeta, M., Janssens, G.P.J., 2021a. Impact of species and their edible parts on the macronutrient and mineral composition of fish from the same aquatic environment, the Gilgel Gibe Reservoir, Ethiopia. Journal of Animal Physiology and Animal Nutrition, 00, 1-9.
Bayissa, T.N., Geerardyn, M., Vanhauteghem, D., Wakjira, M., Janssens, G.P.J., 2021b. Nutrient-related metabolite profiles explain differences in body composition and size in Nile tilapia from different lakes. Scientific Reports, 11, 1–8.
Bedada, K.W., Kechero, Y., Janssens, G.P.J., 2021. Seasonal and agro-ecological associations with feed resource use and milk production of ranging dairy cows in the Southern Ethiopian Rift Valley. Tropical animal health and production, 53, 1–8.
Blair, R., 2018. Nutrition and Feeding of Organic Poultry, 2nd ed., (CAB International, Boston, MA).
Brenes-Soto, A., Dierenfeld, E.S., Muñoz-Saravia, A., Janssens, G.P., 2019. No longer a leap in the dark: the importance of protein as an energy source in amphibians. Wildlife Biology, 1, 1–9.
Chelakkot, C., Ghim, J., Ryu, S.H., 2018. Mechanisms regulating intestinal barrier integrity and its pathological implications. Experimental & molecular medicine, 50, 1–9.
Clench, M.H., 1999. The avian cecum: update and motility review. Journal of experimental Zoology, 283, 441–447.
Coles, K.E., 2007. Investigation into the antioxidant capacity of L-arginine and L-citrulline in relation to their vascular protective properties, (unpublished Ph.D. thesis, Cardiff University). https://orca.cardiff.ac.uk/id/eprint/55641
Dejenie, T., Asmelash, T., De Meester, L., Mulugeta, A., Gebrekidan, A., Risch, S., Pals, A., Van der Gucht, K., Vyverman, W., Nyssen, J., Deckers, J., 2008. Limnological and ecological characteristics of tropical highland reservoirs in Tigray, Northern Ethiopia. Hydrobiologia, 610, 193–209. https://doi.org/10.1007/s10750-008-9435-8
Ebsa, Y.A., Harpal, S., Negia, G.G., 2019. Challenges and chicken production status of poultry producers in Bishoftu, Ethiopia. Poultry Science, 98, 5452–5455.
Eliaszewicz, M., 2004. BSE overview: consumer – risk assessment. Strategies and methods to detect and quantify mammalian tissues in feedingstuffs. In: International Symposium on “Food and feed safety in the context of prion diseases”, in the framework of the European project n°G6RD-2000-CT-00414, Namur, Belgium. https://stratfeed.cra.wallonie.be/page/Symposium_results.php
Fan, Y.K., Croom, J., Christensen, V.L., Black, B.L., Bird, A.R., Daniel, L.R., McBride, B.W., Eisen, E.J., 1997. Jejunal glucose uptake and oxygen consumption in turkey poults selected for rapid growth. Poultry Science, 76, 1738–1745.
Feng, C., Teuber, S., Gershwin, M.E., 2016. Histamine (scombroid) fish poisoning: a comprehensive review. Clinical Reviews in Allergy & Immunology, 50, 64–69.
Fickler, J., 2002. Fish meal: high protein does not stand for high quality. Feed International, 23, 13–16.
Forbes, J.M., 2003. The multifactorial nature of food intake control. Journal of Animal Science, 81, 139–144.
Getahun, A., 2000. Systematic Studies of the African species of the Genus Garra (Pisces: Cyprinidae), Identifier Id: OCLC: 82162791, (unpublished Ph.D. thesis, City University of New York).
Getahun, A. Stiassny, M.L.J., 1998. The freshwater biodiversity crisis: the case of the Ethiopian fish fauna. SINET: Ethiopian Journal of Sciences, 21, 207–230.
Gorsuch, T.T., 1970. The destruction of organic matter (1st ed.), (Braunschweig: Pergamon Press Ltd, Oxford, New York, Toronto, Sydney).
Grotto, D., Santa Maria, L.D., Boeira, S., Valentini, J., Charão, M.F., Moro, A.M., Nascimento, P.C., Pomblum, V.J., Garcia, S.C., 2007. Rapid quantification of malondialdehyde in plasma by high performance liquid chromatography–visible detection. Journal of pharmaceutical and biomedical analysis, 43, 619–624.
Gucciardi, A., Zaramella, P., Costa, I., Pirillo, P., Nardo, D., Naturale, M., Chiandetti, L., Giordano, G., 2015. Analysis and interpretation of acylcarnitine profiles in dried blood spot and plasma of preterm and full-term newborns. Pediatric Research, 77, 36–47. https://doi.org/10.1038/pr.2014.142
Hamedi, S., Rezaian, M., Shomali, T., 2011. Histological changes of small intestinal mucosa of cocks due to sunflower meal single feeding. American Journal of Animal and Veterinary Sciences, 6, 171–175.
Hamilton, K.L., Butt, A.G., 2013. Glucose transport into everted sacs of the small intestine of mice. Advances in Physiology Education, 37, 415–426.
Holecek, M., 2020. Histidine in health and disease: Metabolism, physiological importance, and use as a supplement. Nutrients, 12, 848.
Hollander, D., Kaunitz, J.D., 2020. The “Leaky Gut”: Tight Junctions but Loose Associations?. Digestive Diseases and Sciences, 65, 1277–1287. https://doi.org/10.1007/s10620-019-05777-2
Hrnčár, C., Verguliaková, S., Svorad, P., Weis, J., Aprášová, H., Mindek, S., Fik, M., Bujko, J., 2015. Effect of L-carnitine supplementation on fattening and carcass parameters of broiler chickens. Acta fytotechnica et zootechnica, 18, 15–19.
Ibrahim, D., El-Sayed, R., Khater, S.I., Said, E.N., El-Mandrawy, S.A.M., 2018. Changing dietary n-6: n-3 ratio using different oil sources affects performance, behavior, cytokines mRNA expression and meat fatty acid profile of broiler chickens. Animal Nutrition, 4, 44–51.
Incharoen, T., Yamauchi, K.E., Erikawa, T., Gotoh, H., 2010. Histology of intestinal villi and epithelial cells in chickens fed low-crude protein or low-crude fat diets. Italian Journal of Animal Science, 9, e82.
ISO, 1998. Meat and Meat Products - Determination of total ash, Switzerland: ISO/936:1998. https://www.iso.org/standard/24783.html
Izadi, H., Arshami, J., Golian, A., Reza Raji, M., 2013. Effects of chicory root powder on growth performance and histomorphometry of jejunum in broiler chicks. Veterinary Research Forum, 4, 169–174.
Janssens, G.P.J., Mast, J., Goddeeris, B.M., Cox, E., Hesta, M., De Wilde, R.O.M., 2000. Enhanced specific antibody response to bovine serum albumin in pigeons due to L-carnitine supplementation. British Poultry Science, 41, 448–453.
Karadeniz, A., Simsek, N., Cakir, S., 2008. Haematological effects of dietary L-carnitine supplementation in broiler chickens. Revue de Médecine Vétérinaire, 159, 437–444.
Karimi, A., 2006. The effects of varying fishmeal addition levels (%) on performance of broiler chicks. International Journal of Poultry Science, 5, 255–258.
Krajcovicova-Kudlackova, M., Simoncic, R., Bederova, A., Babinska, K., Beder, I., 2000. Correlation of carnitine levels to methionine and lysine intake. Physiological Research, 49, 399–402.
Li, L.Y., Lu, D.L., Jiang, Z.Y., Limbu, S.M., Qiao, F., Chen, L.Q., Zhang, M.L., Du, Z.Y., 2020. Dietary L-carnitine improves glycogen and protein accumulation in Nile tilapia via increasing lipid-sourced energy supply: an isotope-based metabolic tracking. Aquaculture Reports, 17, p.100302.
Majeed, M.F., Al-Asadi, F.S., Nassir, A.A., Rahi, E.H., 2009. The morphological and histological study of the caecum in broiler chicken. Basrah Journal of Veterinary Research, 8, 19–25.
Mejrhit, N., Azdad, Y., Azdad, O., Aarab, L., 2018. Determination of histamine levels in commonly consumed fish in the region of Fez: The effect of heating and enzymatic treatments. British Food Journal, 120, 2388–2394. https://doi.org/10.1108/BFJ-12-2017-0670
Mekonnen, A., Jones, N., Tefera, B., 2005. Tackling Child Malnutrition in Ethiopia: Do the Sustainable Development Poverty Reduction Programme’s Underlying Policy Assumptions Reflect Local Realities? Young Lives, Save the Children UK, Working Paper No.19. https://idl-bnc-idrc.dspacedirect.org/bitstream/handle/10625/46813/133263.pdf?sequence=1
Mikulec, Ž., Mas, N., Mašek, T., Strmotić, A., 2004. Soybean meal and sunflower meal as a substitute for fish meal in broiler diet. Veterinarski arhiv, 74, 271–279.
Myers, W.D., Ludden, P.A., Nayigihugu, V., Hess, B.W., 2004. Technical Note: A procedure for the preparation and quantitative analysis of samples for titanium dioxide, Journal of Animal Science, 82, 179–183.
Prakatur, I., Miskulin, M., Pavic, M., Marjanovic, K., Blazicevic, V., Miskulin, I., Domacinovic, M., 2019. Intestinal morphology in broiler chickens supplemented with propolis and bee pollen. Animals, 9, 301. https://doi.org/10.3390/ani9060301
Schaafstra, F.J.W.C., van Doorn, D.A., Schonewille, J.T., van Riet, M.M.J., Visser, P., Blok, M.C. and Hendriks, W.H., 2017. Evaluation of methodological aspects of digestibility measurements in ponies fed different haylage to concentrate ratios. Animal, 11, 1922–1929.
Schooneman, M.G., Vaz, F.M., Houten, S.M., Soeters, M.R., 2013. Acylcarnitines: reflecting or inflicting insulin resistance?. Diabetes, 62, 1–8.
Svihus, B., Hetland, H., Choct, M., Sundby, F., 2010. British Poultry Science, 43, 662–668.
Swanson, D., Block, R., Mousa, S.A. 2012. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Advances in Nutrition, 3, 1–7. https://doi.org/10.3945/an.111.000893.
Thiry, E., Saegerman, C., Xambeu, L., Penders, J., 2004. Current status of transmissible spongiform encephalopathies in ruminants. Biotechnology, Agronomy and Society and Environment, 8, 221–228.
Tsuji, S., Kanazawa, S., 1987. Chicken ornithine transacarbamylase: Its unexpected expression. Biochemical genetics, 25, 259–266.
Xu, G., Hansen, J.S., Zhao, X.J., Chen, S., Hoene, M., Wang, X.L., Clemmesen, J.O., Secher, N.H., Haring, H.U., Pedersen, B.K., Lehmann, R., Weigert, C., Plomgaard. P., 2016. Liver and muscle contribute differently to the plasma acylcarnitine pool during fasting and exercise in humans. The Journal of Clinical Endocrinology and Metabolism, 101, 5044–5052.
Yamauchi, T., Kamon, J., Minokoshi, Y.A., Ito, Y., Waki, H., Uchida, S., Yamashita, S., Noda, M., Kita, S., Ueki, K., Eto, K., 2002. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nature medicine, 8, 1288–1295.
Yang, Y., Sadri, H., Prehn, C., Adamski, J., Rehage, J., Dänicke, S., Saremi, B., Sauerwein, H., 2019. Acylcarnitine profiles in serum and muscle of dairy cows receiving conjugated linoleic acids or a control fat supplement during early lactation. Journal of dairy science, 102, 754–767. https://doi.org/10.3168/jds.2018-14685
Acknowledgements
The authors do further acknowledge Donna Vanhauteghem for histamine analysis, Herman De Rycke for proximate analysis, Biruk Mekonnen and Belay Gebreyohannes for blood and garra sampling assistance.
Funding
The project was financially supported by the VLIR-UOS TEAM project under the title “Win–win protein: reconciling fish protein generation and water quality management” [grant number ET2018TEA462A101].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was carried out following the guidelines and approval of the research policy of the Animal Ethics Committee, Mekelle University, Ethiopia. The care of experimental chickens was performed in accordance with the international ethical standards declaration.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mebratu, A.T., Asfaw, Y.T. & Janssens, G.P.J. Exploring the functional and metabolic effects of adding garra fish meal to a plant-based broiler chicken diet. Trop Anim Health Prod 54, 196 (2022). https://doi.org/10.1007/s11250-022-03200-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11250-022-03200-z