Abstract
This study consists of the evaluation of the anticoccidial effect of Artemisia herba-alba Asso during experimental coccidial infection. Four groups of 30 broiler chickens were formed: the negative control (G1), the positive control (G2), the infected Monensin-treated group (G3), and the infected Artemisia-treated group (G4). Each infected bird received orally 105 sporulated oocysts of Eimeria tenella. No mortality was recorded in both G1 and G4. Haematocrit levels showed great variations from the 7th day post-infection, especially in G2 (20.87% ± 5.77). By day 10 P-I, haematocrit recovery was rapid particularly in G4 (28.07% ± 1.50). Haemoglobin concentration also decreased significantly (p < 0.05) in all infected groups by the 7th day P-I. The reduction was very marked, but not statistically significant, in G2 (6.47 g/dL ± 1.67) against (10.53 g/dL ± 0.25) in G1. It was less marked in G4 (8.05 g/dL ± 1.56). Results show the protective effect of A. herba-alba Asso by improving the lesion score and the haematological parameters affected during coccidian infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Because of their therapeutic properties, several species belonging to the genus Artemisia are increasingly attracting the attention of researchers. The genus Artemisia L. belonging to the Asteraceae family includes more than 200 species (Singh et al., 2012). Isolation and identification of the chemical structure in the early 1970s, for the first time, of artemisinin (Fig. 1) from Artemisia annua (Covello et al., 2007) have made these Asteraceae a valuable source of active ingredients with various biopharmaceutical properties (Tilaoui et al., 2011), especially antiprotozoal activity (Machín et al., 2020). In poultry farms, coccidiosis of broiler chicken is one of the main diseases to be controlled (Acharya and Acharya, 2017). Our knowledge of this protozoosis is quite considerable, but it still results in large losses worldwide, estimated by (Williams, 1999) at more than two billion dollars.
The sensitivity of different chicken strains to coccidia is different, but the development of poultry farming was possible only through the incorporation in feeding-stuff anticoccidial additives (ionophores or synthetic products). Since the 1950s, anticoccidial drugs have been used to control coccidiosis. These substances are currently subject to strict legislation which should lead to their prohibition in the coming years (Taljanski-Zygmunt et al., 1998). Furthermore, the appearance of resistant strains of coccidia, making most of the available substances ineffective.
Among the new strategies, the use of phytogenic feed additives is proposed (Habibi et al., 2016). They are products of plant origin used in animal feeding as non-nutrient substances to enhance their performance and health (Abbas, 2012). Recent studies have demonstrated the efficacy of natural products, which appear to affect the development of coccidia and may be promising as food additives (Yang et al., 2021). Allen et al. (1997), Arab et al. (2006), and Jiao et al. (2018) demonstrated the anticoccidial effect of two Artemisia species extracts, in experimentally infected chickens with different Eimeria species.
In the present study, A. herba-alba Asso, a species widespread in Algeria, is studied for its anticoccidial effect in chickens, experimentally infected with Eimeria tenella. The effects of the plant were compared with those of a coccidiostat (monensin sodium) widely used in poultry farming. The study focused on the main parasitological (Lesion score) and haematological parameters (haematocrit level, haemoglobin concentration) modified during cæcal coccidiosis infections. The study would also describe a simple method of using the plant for its exploitation by breeders who often use chemical anticoccidials without a veterinary prescription. To our knowledge, this is the first study conducted to examine comparatively the prophylactic effects of A. herba alba Asso leaves on experimentally challenged broilers in Algeria.
Material and methods
The experimental protocol followed in this study is consistent with the international guidelines of animal care and use in research and teaching (NRC, 2011). Our study was carried out within the Institute of Veterinary Sciences of Constantine, Algeria.
Plant material
The aerial parts of Artemisia herba alba Asso were harvested from the T’Kout region (Batna, Algeria). This region is located on a plateau at an altitude of 1.200 m. The climate is almost desert with a cold winter and a hot summer (Fig. 2). After drying in the shade, the aerial parts were finely cut before being mixed with the animal’s feed.
Parasite and inoculum preparation
A first inoculum, isolated from the litter of a broiler farm and kept in a 2.5% potassium dichromate solution in PADESCA laboratory, was multiplied to have a sufficient quantity of sporulated oocysts. Each millilitre of the solution contains an average of 24 × 103 sporulated oocysts. On the 8th day after inoculation, the animals were autopsied and the intestinal masses recovered. The analysis proceeds then as follows: caeca were ground in distilled water and filtered through cheesecloth. The filtrate was centrifuged (3200t/min for 15 min), and the pellets were re-suspended in saturated salt (NaCl) solution. After second centrifugation (3 min: the time required for oocyst flotation), the supernatants were re-suspended in distilled water to be washed and then centrifuged again. Finally, all the pellets were recovered and kept in a 2.5% potassium dichromate solution. In a water bath, oocysts collected were kept at 30 °C to undergo sporulation before they were infective. After 72 h, sporulated oocysts were kept at + 4 °C until the day of inoculation. On the 18th day of age (day 0 of infection), animals of the infected groups G2, G3, and G4 received individually by gavage, 1 ml of a solution containing 105 sporulated oocysts of Eimeria tenella (sporulation rate 93.15%).
Animals
Two hundred eighty Hubbard-ISA15 chicks of one day of age were bred under controlled hygiene conditions to prevent any contamination. The animals were raised in the pet store of PADESCA laboratory in the Institute of Veterinary Sciences. All breeding conditions were met (temperatures according to the age of the animals, light program, ventilation, ambient humidity, etc.). No adverse events were recorded. The animals were raised on a 10 cm new wood-shavings litter. Animals were vaccinated against Gumboro IBA-VAC® and Newcastle diseases BIO-VAC LA SOTA® on the 7th and the 14th day of age, respectively. At the age of 1 day, the animals were divided into 4 groups of 70 subjects (G1, G2, G3, G4). G3 animals received monensin, group 4 animals received Artemisia. Before infection, 30 subjects in each group were selected on a bodyweight basis to form homogeneous groups.
On the 17th day, four groups of 30 broiler chicken were formed (294.65 ± 20.58 g/subject): negative control uninfected untreated group (G1), positive control infected untreated group (G2), an infected group treated with Monensin (G3), and an infected group treated with A. herba-alba Asso (G4).
All feeds have been formulated to meet animal nutrient requirements (NRC, 1994). Standard ration (corn 62%, soybean cake 35%, mineral and vitamin supplement 1%, calcium carbonate 0.8%, bicalcic phosphate 1%, and sodium bicarbonate 0.02%) has been provided. Feed distributed to G1 and G2 was free of any anticoccidial supplementation, while feed distributed to groups G3 and G4 was supplemented with 100 ppm of monensin sodium (Elancoban®) until the 45th day of age and 5% dried leaves of A. herba alba Asso until the 30th day of age, respectively.
The mortality rate was determined up to the 9th day post-infection. Blood samples were delicately obtained from the ulnar (wing) vein before euthanasia. They were sent to the haematological laboratory (+ 4 °C). The haematological parameter analyses were carried out using an automaton, with kits (SPINREACT®). Haemoglobin concentration and haematocrit were measured for 06 subjects from each group of animals. The lesion score was evaluated for 6 subjects per infected group at the 7th, 10th, and 12th days post-infection according to the method described by Johnson and Reid (1970). Where animals are dead, immediately the samples of caeca were taken to evaluate the lesion score.
Statistical analysis
The statistical analysis was carried out by the Kruskal–Wallis test followed by the Mann–Whitney test of the XLSTAT 2010 software (Addinsoft SARL). The difference was considered significant when p < 0.05. Treatment groups were compared to the negative control uninfected untreated group (G1) and the positive control infected untreated group (G2) for statistical difference (p < 0.05).
Results
Mortality
Mortality rates are given in Table 1.
Lesion score
The results reported in Table 2 represent arithmetic means obtained from the numerical values (0, 1, 2, 3, or 4) attributed to the cæcal lesions.
Haematological parameters
Table 3 present the results of the haematocrit and haemoglobin analysis. Blood samples were taken from 6 subjects from each experimental group.
Discussion
During the post-infection period, clinical signs of cæcal coccidiosis were observed in all infected chickens; immobility, weakness, bloody diarrhoea, and recumbency. The latter is pathognomonic during coccidial infections due to E. tenella (Conway and McKenzie, 2008). Among the infected groups, the less severe clinical signs were observed in Artemisia-treated animals (G4), where it was observed only a slight weakness during 2 days. In the other groups, infected live birds appear thrifty with pale combs, ruffled feathers, and decreased activity. Diarrhoea and floated feathers were observed for 4 days after their disappearance in the animals of G3 and G3. Mortality rates were 10% and 3.33%, respectively, for the positive control (G2) and monensin-treated animals (G3), but no cases of mortality were recorded in the negative control (G1) and Artemisia-treated group. When added at 5% in the animal’s feed, A. herba alba Asso dried leaves protected infected chickens from mortality and pathological symptoms caused by E. Tenella. The severity of haemorrhagic diarrhoea has been considerably reduced in Artemisia-treated group.
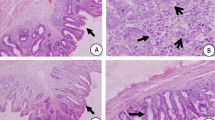
Lesion scoring is a technique developed to provide a numerical ranking of gross lesions caused by coccidian (Johnson and Reid, 1970). The cæcal lesions revealed at the autopsy were pathognomonic of a coccidial infection with Eimeria tenella: cæcums distended by bloody faeces and mucous debris, with haemorrhages on the mucosa. Such findings have been reported by several authors (Kadhim, 2014; Chen et al., 2020), in cases of cæcal coccidial infections. In our study, dried aerial parts of A. herba-alba Asso incorporated in the diet reduced the cæcal lesions in infected chicks. Nevertheless, although numerically lower, the lesion score recorded in the Artemisia-treated group was not statistically different (p < 0.05) from that observed in the positive control group. The lesion score is still a parameter that is not very precise to evaluate. It must be considered in conjunction with other parameters. The scale from 0 to 4 does not allow for a very accurate assessment. However, monensin has proven to be the most effective molecule in improving the lesion score, especially by the 12th day post-infection. This carboxylic polyether ionophore, by its coccidicide effect, limits parasitic development (Moraes et al., 2019), resulting in faster regeneration of cæcal lesions.
Our results are in agreement with those reported in multiple studies, which have shown the beneficial effect of certain plants and their extracts on the improvement of the lesion score during Eimeria tenella coccidial infections (Papazahariadou et al., 2010; Yang et al., 2021).
Previous studies indicated that many biological impacts have always been reported during chicken coccidiosis including haematological changes (Akhtar et al., 2015) and various plasma metabolites. Due to haemorrhagic lesions following parasitic development, disturbance of erythrocytic parameters has been studied in our work, including haematocrit and haemoglobin concentration. In all the infected groups, the haematocrit level varied strongly from the 7th day post-infection, especially in the positive control group (20.87 ± 5.77). Decreased haematocrit may reflect anaemia in most cases. It can have different causes: iron deficiency, inflammation, intestinal malabsorption, or excessive blood loss (Janssens, 2009).
Furthermore, the occurrence in infected groups of diarrhoea with malabsorption and the inflammatory and haemorrhagic nature of the cæcal lesions may explain the significant fall (p < 0.05) in haematocrit, especially on the 7th day post-infection. With Eimeria tenella, parasitic development leads to ulcerative lesions in the cæcums, and blood loss is almost constantly observed (Chandrakesan et al., 2009), hence the alteration of haematological parameters and the appearance of signs of anaemia. On the 10th day P-I, recovery of haematocrit was rapid in Artemisia-treated group but without significant difference from the positive control one.
As was observed for haematocrit, in all infected groups, the haemoglobin concentration decreased significantly from the 7th day post-infection. This reduction was very marked in the positive control group (6.47 ± 1.67) compared with the negative control group (10.53 ± 0.25). These results are in agreement with those reported recently by Aljedaie and Al-Malki (2020) who reported a significant decrease in haemoglobin concentration (Hb) in chicks infected with E. tenella oocysts. In our study, haemorrhagic diarrhoea detected from the 5th day post-infection, as well as the cæcal lesions revealed at autopsy, was accompanied by the appearance of anaemia in infected chickens. In Artemisia-treated animals, the haematocrit and haemoglobin concentrations were numerically better than those recorded in animals of the positive control group. This finding could be due to the protection conferred on animals by the bioactive constituents of the plant. Protection resulted in a reduction in the haemorrhagic nature of the infection, confirmed by an improvement in the lesion score.
Based on current knowledge, it could be assumed that the attenuation of the severity of cæcal lesions and the improvement of haematological parameters during E. tenella by phytogenics is attributed mainly to artemisinin (Dragan et al., 2010; Del Cacho et al., 2010), camphor and 1,8-cineole (Allen et al., 1997), tannins (Zaman et al., 2012), and antioxidant compounds (Almeida et al. 2012; Lu et al., 2019; Zhang et al., 2020). The content of A. herba-alba Asso in these compounds has been reported by several authors (Feuerstein et al., 1988; Messaï et al., 2008; Bezza et al., 2010) suggesting that the plant we studied contains one or more of these compounds, explaining the improvement of the lesion score in the Artemisia-treated group. However, among all these bioactive compounds, artemisinin is the most studied. Many reports suggested that the functional endoperoxide bridge of artemisinin induces oxidative stress by generating a cascade of free radicals. The key point in the antiprotozoal activities of artemisinin is the production of free radicals and subsequently alkylation of proteins and lipid peroxidation (Meshnick, 2002; Kaboutari et al., 2013; Pirali Kheirabadi et al., 2014). Furthermore, artemisinin can slow down the reproduction of E. tenella and subsequently decrease the sporulation and survival capability of the oocysts in the litter (Del Cacho et al. 2010). In addition to its direct action on Eimeria development, artemisinin may block proinflammatory factors activated by the parasite (Pirali Kheirabadi et al., 2014).
Through our study, the anticoccidial effect of Artemisia herba alba has not been completely understood. It seems that the plant may exert its effects via direct or indirect action of its active compounds on Eimeria tenella development.
Besides, in many studies on the anticoccidial effect of medicinal plants, authors have proven the effectiveness of certain plants in attenuation of the haemorrhage accompanying the development of Eimeria tenella, without identifying the active ingredient responsible for its effectiveness (Youn and Noh, 2001; Giannenas et al., 2003; Du and Hu, 2004). So, the beneficial effect of some of the unidentified compounds of the plant we have studied is also likely.
The prophylactic effects of A herba alba Asso have been examined in our study on Eimeria tenella-challenged broilers. Based on our results, it may be concluded that the addition of Artemisia herba-alba Asso at 5% to the broiler’s diet has a positive effect on lowering the bloody diarrhoea intensity. In the search for alternatives to conventional anticoccidial drugs, our findings are encouraging. The effect of A. herba alba Asso was comparable to that of traditional prophylactic anticoccidial drugs: reduction of mortality rate and prevention of collapse of haematological parameters. At this stage of our research, it is difficult to recommend the definitive replacement of monensin by 5% of Artemisia herba-alba Asso in animal’s diet, but the results are very promising for the plant to be the alternative in the future. Further studies with larger numbers of animals and assessing other biological parameters are needed. The studied plant deserves to be the subject of more in-depth studies for better exploitation of its properties. As a first step in applying our results, the plant may be indicated to be exploited by farmers to reduce the economic impact of coccidiosis, before use is standardized as regards the rate and duration of incorporation us a phytogenic feed additive.
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
Abbas, T.E., 2012. Phytogenic Feed Additives as a Coccidiostat in Poultry. Bulletin of Environment, Pharmacology, and Life Sciences, 7, 22–24.
Acharya, K.P. and Acharya, N., 2017. Alternatives To Fight Against Coccidiosis: A Review. Nepalese Veteinary Journal, 34, 152-167. https://doi.org/10.3126/nvj.v34i0.22918
Akhtar, M., Awais, M.M., Anwar, M.I., Ehtisham-ul, S., Nasir, A., Saleemi, M.K. and Ashraf, K., 2015. The effect of infection with mixed Eimeria species on hematology and immune responses following Newcastle disease and infectious bursal disease booster vaccination in broilers. Veterinary Quarterly, 35:1, 21-26. https://doi.org/10.1080/01652176.2014.991048
Aljedaie, M.M. and Al-Malki, E.S., 2020. Anticoccidial activities of Salvadora persica(arak), Zingiber officinale (ginger) and Curcuma longa (turmeric) extracts on the control of chicken coccidiosis. Journal of King Saud University - Science, 32:6, 2810–2817. https://doi.org/10.1016/j.jksus.2020.07.002
Allen, P.C., Lydon, J. and Danforth, H.D., 1997. Effects of Components of Artemisia annua on Coccidia Infections in Chickens. Poultry Science, 76:8, 1156–1163. https://doi.org/10.1093/ps/76.8.1156
Almeida, G.F.D., Horsted, K., Thamsborg, S.M., Kyvsgaard, N.C., Ferreira, J.F.S. and Hermansen, J.E., 2012. Use of Artemisia annua as a natural coccidiostat in free-range broilers and its effects on infection dynamics and performance. Veterinary Parasitology, 186:3-4, 178–187. https://doi.org/10.1016/j.vetpar.2011.11.058
Arab, H.A., Rahbari, S., Rassouli, A., Moslemi, M.H. and Khosravirad, F., 2006. Determination of artemisinin in Artemisia sieberi and anticoccidial effects of the plant extract in broiler chickens. Tropical Animal Health and Production, 38:6, 497–503. https://doi.org/10.1007/s11250-006-4390-8
Bezza, L., Mannarino, A., Fattarsi, K., Mikail, C., Abou, L., Hadji-Minaglou, F. and Kaloustian, J., 2010. Composition chimique de l’huile essentielle d’Artemisia herba-alba provenant de la région de Biskra (Algérie). Phytotherapie, 8 :5, 277–281. https://doi.org/10.1007/s10298-010-0576-3
Chandrakesan, P., Muralidharan, K., Kumar, V.D., Ponnudurai, G., Harikrishnan, T.J. and Rani, K.S.V.N., 2009. Efficacy of a herbal complex against caecal coccidiosis in broiler chickens. Veterinarski Arhiv, 79:2, 199–203.
Chen, H.L., Zhao, X.Y., Zhao, G.X., Huang, H.B., Li, H.R., Shi, C.W., Yang, W.T., Jiang, Y.L., Wang, J.Z., Ye, L.P., Zhao, Q., Wang, C.F. and Yang, G.L., 2020. Dissection of the cecal microbial community in chickens after Eimeria tenella infection. Parasites and Vectors, 13:1, 1–15. https://doi.org/10.1186/s13071-020-3897-6
Conway, D.P. and McKenzie, M.E., 2008. Poultry Coccidiosis: Diagnostic and Testing Procedures. Third Edition., Blackwel, Oxford OX4 2DQ, UK, pp. 168. https://doi.org/10.1002/9780470344620
Covello, P.S., Teoh, K.H., Polichuk, D.R., Reed, D.W. and Nowak, G., 2007. Functional genomics and the biosynthesis of artemisinin. Phytochemistry, 68:14, 1864–1871. https://doi.org/10.1016/j.phytochem.2007.02.016
Del Cacho, E., Gallego, M., Francesch, M., Quílez, J. and Sánchez-Acedo, C., 2010. Effect of artemisinin on oocyst wall formation and sporulation during Eimeria tenella infection. Parasitology International, 59:4, 506–511. https://doi.org/10.1016/j.parint.2010.04.001
Dragan, L., Titilincu, A., Dan, I., Dunca, I., Dragan, M. and Mircean, V., 2010. Effects of Artemisia annua and Pimpinella anisum on Eimeria tenella (Phylum Apicomplexa) low infection in chickens. Revista Scientia Parasitologica, 11:2, 77–82.
Du, A. and Hu, S., 2004. Effects of a herbal complex against Eimeria tenella infection in chickens. Journal of Veterinary Medicine Series B: Infectious Diseases and Veterinary Public Health, 51:4, 194–197. https://doi.org/10.1111/j.1439-0450.2004.00749.x
Feuerstein, I., Danin, A. and Segal, R., 1988. Constitution of the essential oil from an Artemisia herba-alba population of Spain. Phytochemistry, 27:2, 433–434. https://doi.org/10.1016/0031-9422(88)83114-5.
Giannenas, I., Florou-Paneri, P., Papazahariadou, M., Christaki, E., Botsoglou, N.A. and Spais, A.B., 2003. Effect of dietary supplementation with oregano essential oil on performance of broilers after experimental infection with Eimeria tenella. Archives of Animal Nutrition, 57:2, 99–106. https://doi.org/10.1080/0003942031000107299
Habibi, H., Firouzi, S., Nili, H., Razavi, M., Asadi, S.L. and Daneshi, S., 2016. Anticoccidial effects of herbal extracts on Eimeria tenella infection in broiler chickens: in vitro and in vivo study. Journal of Parasitic Diseases, 40:2, 401–407. https://doi.org/10.1007/s12639-014-0517-4
Janssens, G., 2009. Répertoire d’analyses de biologie clinique. Institut de biologie clinique. Université libre de Bruxelles. 257p. http://www.ulb-ibc.be/Repertoire_IBC_2009_2.pdf.
Jiao, J., Yang, Y., Liu, M., Li, J., Cui, Y., Yin, S. and Tao, J., 2018. Artemisinin and Artemisia annua leaves alleviate Eimeria tenella infection by facilitating apoptosis of host cells and suppressing inflammatory response. Veterinary Parasitology, 254, 172–177. https://doi.org/10.1016/j.vetpar.2018.03.017
Johnson, J. and Reid, W.M., 1970. Anticoccidial drugs: Lesion scoring techniques in battery and floor-pen experiments with chickens. Experimental Parasitology, 28:1, 30–36. https://doi.org/10.1016/0014-4894(70)90063-9
Kaboutari, J., Arab, H.A., Ebrahimi, K. and Rahbari, S., 2013. Prophylactic and therapeutic effects of a novel granulated formulation of Artemisia extract on broiler coccidiosis. Tropical Animal Health and Production, 46:1, 43–48. https://doi.org/10.1007/s11250-013-0444-x
Kadhim, L.I., 2014. Histopathological Changes of Broilers Immunized With Sonicated Oocysts Against Eimeria Tenella. International Journal of Advanced Biological Research, 4:1, 31–35.
Lu, F., He, X.L., Richard, C. and Cao, J., 2019. A brief history of artemisinin: Modes of action and mechanisms of resistance. Chinese Journal of Natural Medicines, 17:5, 331–336. https://doi.org/10.1016/s1875-5364(19)30038-x
Machín, L., Nápoles, R., Gille, L. and Monzote, L., 2021. Leishmania amazonensis response to artemisinin and derivatives. Parasitology International, 80, 102218. https://doi.org/10.1016/j.parint.2020.102218
Meshnick, S.R., 2002. Artemisinin: mechanisms of action, resistance and toxicity. International Journal for Parasitology, 32, 1655–1660. https://doi.org/10.1016/S0020-7519(02)00194-7
Messaï, L., Hegazy, M.E.F., Ahmed, A.A., Ali, K., Belkacemi, D. and Ohta, S., 2008. Sesquiterpene lactones from Algerian Artemisia herba-alba. Phytochemistry Letters, 1:2, 85–88. https://doi.org/10.1016/j.phytol.2008.04.002
Moraes, P.O., Cardinal, K.M., Gouvêa, F.L., Schroeder, B., Ceron, M.S., Lunedo, R., Frazzon, A.P.G., Frazzon, J. and Ribeiro, A.M.L., 2019. Comparison between a commercial blend of functional oils and monensin on the performance and microbiota of coccidiosis-challenged broilers. Poultry Science, 98:11, 5456–5464. https://doi.org/10.3382/ps/pez345
NRC, 1994. National Research Council, Nutrient Requirements of Poultry. 9th Ed., National Academy Press, Washington DC, USA.
NRC, 2011. National Research Council, Guide for the Care and Use of Laboratory Animals: Eighth Edition. Washington, DC: The National Academies Press.
Papazahariadou, M., Papadopoulos, E., Christaki, E., Georgopoulou, I., Florou-Paneri, P., Tserveni-Goussi, A., Yannakopoulos, A., 2010. Use of Fraxinus ornus as an alternative. Revue de Médecine Vétérinaire, 161:7, 326-331.
Pirali Kheirabadi, K., Kaboutari Katadj, J., Bahadoran, S., Teixeira da Silva, J.A., Dehghani Samani, A. and Cheraghchi Bashi, M., 2014. Comparison of the anticoccidial effect of granulated extract of Artemisia sieberi with monensin in experimental coccidiosis in broiler chickens. Experimental Parasitology, 141, 129–133. https://doi.org/10.1016/j.exppara.2014.03.022
Singh, R., Verma, P. and Singh, G., 2012. Total phenolic, flavonoids and tannin contents in different extracts of Artemisia absinthium. Journal of Intercultural Ethnopharmacology, 1:2, 101-104. https://doi.org/10.5455/jice.20120525014326
Taljanski-Zygmunt, W., Grzesiuk, E., Zabielski, R. and Pierzynowski, S.G., 1998. Is the use of antimicrobial drugs in agriculture risky for human health? Journal of Animal and Feed Sciences, 7, 289–295.
Tilaoui, M., Mouse, H.A., Jaafari, A., Aboufatima, R., Chait, A. and Zyad, A., 2011. Chemical composition and antiproliferative activity of essential oil from aerial parts of a medicinal herb Artemisia herba-alba. Brazilian Journal of Pharmacognosy, 21:4, 781–785. https://doi.org/10.1590/S0102-695X2011005000114
Williams, R.B., 1999. A compartmentalised model for the estimation of the cost of coccidiosis to the world’s chicken production industry. International Journal for Parasitology, 29:8, 1209–1229. https://doi.org/10.1016/S0020-7519(99)00086-7
Yang, Y., Memon, F.M., Hao, K., Jiang, M., Guo, L., Liu, T., Lv, F., Zhang, W., Zhang, Y., Si, H., 2021. The combined use of Bacillus subtilis-based probiotic and anticoccidial herb had a better anti-Eimeria tenella efficiency. Journal of Applied Poultry Research, https://doi.org/10.1016/j.japr.2021.100181
Youn, H.J. and Noh, J.W., 2001. Screening of the anticoccidial effects of herb extracts against Eimeria tenella. Veterinary Parasitology, 96:4, 257–263. https://doi.org/10.1016/S0304-4017(01)00385-5
Zaman, M.A., Iqbal, Z., Abbas, R.Z. and Khan, M.N., 2012. Anticoccidial activity of herbal complex in broiler chickens challenged with Eimeria tenella. Parasitology, 139:2, 237–243. https://doi.org/10.1017/S003118201100182X
Zhang, W., Heng, J., Kim, S.W., Chen, F., Deng, Z., Zhang, S. and Guan, W., 2020. Dietary enzymatically-treated Artemisia annua L. supplementation could alleviate oxidative injury and improve reproductive performance of sows reared under high ambient temperature. Journal of Thermal Biology, 94, 102751. https://doi.org/10.1016/j.jtherbio.2020.102751
Acknowledgements
We thank all the members of the PADESCA Research Laboratory (Institute of Veterinary Sciences of Constantine, Algeria) for their warm welcome.
Funding
This study was partially funded by the DGRSDT and the Ministry of Higher Education and Scientific Research of Algeria for PRFU Project (D04N01UN070120190002).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Writing the original draft and revising methodology were performed by Messaï Ahmed. Reviewing the paper and analysing data were performed by Redouane-Salah Sara. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study followed the international guidelines of animal care and use in research and teaching (NRC, 2011). All procedures performed in this research were approved by the ethics committee on the use of animals from the Institute of Veterinary Sciences-University of Constantine 1.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Messaï, A., Redouane-Salah, S. Dietary use of Artemisia herba alba Asso as a potential coccidiostat against cæcal coccidiosis: haematological parameter variations. Trop Anim Health Prod 54, 28 (2022). https://doi.org/10.1007/s11250-021-03038-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11250-021-03038-x