Abstract
During 2015 and 2016, from five different States of the Sudan, a total of 1000 cattle sera were purposively collected from many herds of apparently healthy cattle regardless of their age, sex, and breed. Assessment of the sero-prevalence of PPRV antibodies using competitive ELISA (C-ELISA) yielded a higher overall sero-prevalence of 42.0% (420/1000) among cattle populations in the Sudan which is higher than previously reported. Within Sudanese States under study, the highest sero-prevalence of 53.5% (107/200 sera) was demonstrated in Khartoum State while the least sero-prevalence of 31.5% (63/200 sera) was demonstrated in White Nile State. The higher PPRV sero-prevalence values detected in cattle suggested the potential exposure of cattle to PPRV via contact with infected small ruminants and thus might be an indicator of infection of small ruminants. There is a need to include serological surveillance of PPR in cattle within the sero-monitoring program of PPR to give a better indication of the national herd immunity and to assess in the ongoing eradication program.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Peste des petits ruminants (PPR) is a fatal viral disease characterized by high morbidity (100%) and mortality (50–90%) rates (Lefevre and Diallo 1990; OIE 2013). Peste des petits ruminants virus (PPRV), the causative agent, belongs to the Morbillivirus genus of the Paramyxoviridae family (OIE 2013). Nowadays the disease is endemic in Africa and Asia, the Middle East and Turkey, with recent outbreaks in Georgia and Mongolia (Banyard et al. 2010; OIE 2013; Baron et al. 2017).
Sheep and goats are the main natural hosts of PPR which can be acutely infected (Lefevere and Diallo 1990; Parida et al. 2015). Cattle are also considered susceptible to subclinical infection by PPR either by direct contact with infected sheep and goats or via direct inoculation with PPRV (Anderson and Mckay 1994; Lembo et al. 2013; Sen et al. 2014). However, it is not excreting virus and does not pose any potential role in the transmission of PPRV (Parida et al. 2015). A seroconversion and a humoral antibody response against PPRV was demonstrated following PPRV infection of cattle (Dardiri et al. 1977; Anderson and Mckay 1994; Sen et al. 2014).
PPR was known in the Sudan since 1970th (Elhag Ali 1973) as a Rinderpest-like disease infecting mainly sheep and goats, while in contact cattle remains apparently healthy. Many years later, the disease was diagnosed as PPR (Elhag Ali and Taylor 1984). Since then, outbreaks of the disease in sheep and goats were reported regularly. For control of PPR in small ruminants in the field, the live-attenuated cell culture PPRV Nigeria 75/1 vaccine has been used in the Sudan since 1989 (Fadol and El Hussein 2004).
Outbreaks of PPR in sheep and goats in the Sudan, in the past, occurred in Gezira, White Nile, Blue Nile, Khartoum, River Nile, Elgedarif, Kassala, Red Sea, and Kurdufan States (Osman et al. 2008; Saeed et al. 2010; Ali et al. 2014; Saeed et al. 2017). Due to the continual reported incidence of PPR in sheep and goats in the Sudan and the documented cases of PPR in camels which highlighted the possibility of existence of other susceptible hosts of the disease, the present study aimed to perform serological investigations to assess the sero-prevalence of PPRV antibodies among cattle populations in the Sudan.
Materials and methods
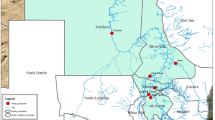
Between 2015 and 2016, during a surveillance program, a total of 1000 cattle sera were purposively collected from many herds form five different States of the Sudan. A total of 200 cattle sera were collected from each State, of which 20 sera were from each locality within the State and around 5–10 sera were collected from each herd. Sera were collected from River Nile (Northern Sudan), White Nile (middle Sudan); Elgedarif (Eastern Sudan) States during December 2015 and from Khartoum (middle Sudan); and Western Kurdufan (South-Western Sudan) States during January 2016 (Fig. 1; Table 1). Sera were collected from many herds of apparently healthy cattle regardless of their age, sex and breed.
A competitive-enzyme linked immunosorbent assay (C-ELISA) was performed, for detection of PPRV antibodies in the suspected cattle sera, using a competitive screening ELISA kit “ID Screen® PPR Competition kit” (IDVet Innovative Diagnostics, France) following the manufacturer’s instructions.
Results and discussion
The present study was performed during 2015–2016 for serological investigations of PPR among cattle populations in areas located in five different States of the Sudan considering the fact that cattle in the Sudan had never been vaccinated against PPR. Indeed, there was also no history of previous vaccination of cattle herds against Rinderpest since it was terminated in 2002 in the Sudan during the global Rinderpest eradication program which accomplished successfully in 2011 (OIE 2016).
Screening of 1000 cattle sera for detection of PPRV antibodies by C-ELISA indicated that only 420/1000 sera were found positive while all the remainder were negative. In this study, serological surveillance for screening of PPRV antibodies in cattle sera, as indicated by C-ELISA, revealed a higher sero-prevalence of PPRV antibodies of 42.0% among cattle populations in the country. In contrast, lower antibody sero-prevalence values of 25.8% (Saeed et al. 2017) and 11.4% (Haroun et al. 2002) were demonstrated previously among cattle population in the Sudan. The findings revealed a higher sero-prevalence of PPRV antibodies in cattle than previously reported in the country.
Similarly, a higher sero-prevalence of PPRV antibodies in cattle sera of 41.86% was documented in cattle in Pakistan (Khan et al. 2008) in contrary to a much lower value of only 8% (Rashid et al. 2008) and 10% reported recently (Abubakar et al. 2017). Moreover, several sero-surveillance studies indicated the presence of PPR antibodies in cattle sera in different countries with variable sero-prevalence values, for instance 0.9% and 18% in Turkey (Özkul et al. 2002; Albayrak and Gür 2010), 9% in Ethiopia (Abraham et al. 2005), 4.5% in Cameron (Ngangnou et al. 1996), 1.78% in Mali (Tounkara et al. 1996), 26.7% and 5.9% in Tanzania (Lembo et al. 2013), and finally 4.58% and 11.07% in India (Balamurugan et al. 2012; Balamurugan et al. 2014).
In fact, cattle which were vaccinated against RP could not produce detectable antibodies against PPRV due to the immunological interaction and cross-reactivity between the two viruses (Anderson and Mckay 1994). Detection of PPRV antibodies in cattle sera, in absence of a vaccination history, indicated the potential exposure of these animals to PPRV, via contact with infected small ruminants, which probably leads to development of a subclinical infection. Antibody sero-prevalences detected in cattle suggested the potential exposure of cattle to PPRV via contact with infected small ruminants and indicted the natural transmission of PPRV from small ruminants to cattle under field conditions which is as stated previously by Khan et al. (2008). Thus, the higher sero-prevalence of PPRV demonstrated among cattle populations in the Sudan might be an indicator of infection of small ruminants and might be attributed to the mixed free grazing rangelands for all animal species, especially small ruminants, cattle and camel, which facilitate the exposure of cattle to the virus. This could be explained better by the frequently occurred outbreaks of PPR in small ruminants, in the five States under study in the Sudan, as officially reported to the OIE (OIE-WAHIS 2018). PPR outbreaks were reported in River Nile, White Nile, Elgedarif, and Khartoum States during 2013; River Nile and Western Kurdufan States during 2014; River Nil, Khartoum and Kurdufan States during 2015; River Nile, Elgedarif and Kurdufan States during 2016; White Nile, Elgedarif, Khartoum and Kurdufan States during 2017 as annually reported to the OIE (OIE-WAHIS 2018).
According to the geographic distribution of the cattle herds in the Sudanese States under study, results yielded the highest sero-prevalence of 53.5% (107/200 sera) in Khartoum State (middle Sudan) followed by 45.5% (91/200 sera) in River Nile State (Northern Sudan), then 40.0% (80/200 sera) in Western Kurdufan State (South-Western Sudan), 39.5% (79/200 sera) in Elgedarif State (Eastern Sudan) and finally the least sero-prevalence of 31.5% (63/200 sera) was demonstrated in White Nile State (middle Sudan) (Table 1; Fig. 1). The result of the present study demonstrated the prevalence of PPRV antibodies in cattle sera as documented previously (El Amin and Hassan 1999; Haroun et al. 2002; Saeed et al. 2017) and reflects the wide distribution of PPRV among cattle populations in the country keeping in mind that PPR is endemic in small ruminants throughout Sudanese States under study.
It is important to include serological surveillance of PPR in cattle within the sero-monitoring program of PPR to give a better indication of the national herd immunity and to assess in the ongoing eradication program. The findings of the present study suggested cattle are subclinically infected hosts of PPR in the Sudan, however, there is no evidence if it can transmit the disease to closely in contact susceptible animal species thus there potential contribution (or not) in the epidemiology of the disease remains questionable. Additional studies to investigate the possible role that cattle might play (or not) in PPRV transmission and thus infection to other animals and also in the epidemiology of PPR are needed.
References
Abraham, G., Sintayehu, A., Libeau, G., Albina, E., Roger, F., Laekemariam, Y., Abayneh, D., Awoke, K.M., 2005. Antibody seroprevalences against peste des petits ruminants (PPR) virus in camels, cattle, goats and sheep in Ethiopia, Preventive Veterinary Medicine, 70(1–2), 51–57.
Abubakar, M., Mahapatra, M., Muniraju, M., Arshed, M.J., Khan, E.H., Banyard, A.C., Ali, Q., Parida, S., 2017. Serological detection of antibodies to peste des petits ruminants virus in large ruminants, Transboundary and Emerging Diseases, 64(2), 513–519.
Albayrak, H., Gür, S., 2010. A serologic investigation for Peste des petits ruminants infection in sheep, cattle and camels (Camelus dromedarius) in Aydın province, West Anatolia, Tropical Animal Health and Production, 42, 151–153.
Ali, Y.H., Intisar, K.S., Khalafalla, A.I., 2014. Outbreaks of Peste des petits ruminants in two different localities in Sudan, Journal of Veterinary Medicine and Animal Health, 6(6), 174–177.
Anderson, J., McKay, J.A., 1994. The detection of antibodies against peste des petits ruminants virus in cattle, sheep and goats and the possible implications to rinderpest control programmes, Epidemiology and Infection, 112, 225–231.
Balamurugan, V., Krishnamoorthy, P., Veeregowda, B.M., Sen, A., Rajak, K.K., Bhanuprakash, V., Gajendragad, M.R., Prabhudas, K., 2012. Seroprevalence of peste des petits ruminants in cattle and buffaloes from Southern Peninsular India, Tropical Animal Health and Production, 44(2), 301–306.
Balamurugan, V., Krishnamoorthy, P., Raju, D.S., Rajak, K.K., Bhanuprakash, V., Pandey, A.B., Gajendragad, M.R., Prabhudas, K., Rahman, H., 2014. Prevalence of Peste-des-petits-ruminant virus antibodies in cattle, buffaloes, sheep and goats in India, Virusdisease, 25, 85–90.
Banyard, A.C., Parida, S., Batten, C., Oura, C., Kwiatek, O., Libeau, G., 2010. Global distribution of peste des petits ruminants virus and prospects for improved diagnosis and control, Journal of General Virology, 91(12), 2885–2897.
Baron, M.D., Diop, B., Njeumi, F., Willett, B.J., Bailey, D., 2017. Future research to underpin successful peste des petits ruminants virus (PPRV) eradication, Journal of General Virology, 98, 2635–2644.
Dardiri, A.H., DeBoer, C.J., Hamdy, F.M., 1977. Response of American Goats and cattle to peste des petits ruminants virus, In: Proceedings of the 19th Annual Meeting of the American Association of Veterinary Laboratory Diagnosticians, Florida, p. 337–44.
El Amin, M.A.G., Hassan, A.M., 1999. Rinderpest and PPR surveillance in Sudan (1996–1998), In: The seromonitoring and surveillance of rinderpest throughout Africa - Phase III, Results for 1998. Proceedings of a Research Co-ordination Meeting of the FAO/IAEA/OAU/IBAR/PARC Co-ordinated Research Project Organized by the joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture, Machakos, Kenya, 26–30 April 1999.
Elhag Ali, B., 1973. A natural outbreak of rinderpest involving sheep, goats and cattle in Sudan, Bulletin of Epizootic Diseases of Africa, 21, 421–428.
Elhag Ali, B., Taylor, W.P., 1984. Isolation of peste des petits ruminants virus from the Sudan, Research in Veterinary Science, 36, 1–4.
Fadol, M.A., El Hussein, A.M., 2004. Production of PPR vaccine in the Sudan, Journal of Science and Technology, 5(1), 81–86.
Haroun, M., Hajer, I., Mukhtar, M., Ali, B.E., 2002. Detection of antibodies against peste des petits ruminants virus in sera of cattle, camels, sheep and goats in Sudan, Veterinary Research Communication, 26(7), 537–541.
Khan, H.A., Siddique, M., Rahman, S., Abubakar, M., Ashraf, M., 2008. The detection of antibody against peste des petits ruminants virus in Sheep, Goats, Cattle and Buffaloes, Tropical Animal Health and Production, 40, 521–527
Lefevre, P.C., Diallo, A., 1990. Peste des petits ruminants, Revue Scientifique et Technique de l' Office International des Epizooties, 9(4), 951–965.
Lembo, T., Oura, C., Parida, S., Hoare, R., Frost, L., Fyumagwa, R., Kivaria, F., Chubwa, C., Kock, R., Cleaveland, S., Batten, C., 2013. Peste des petits ruminants infection among cattle and wildlife in Northern Tanzania, Emerging Infectious Diseases, 19, 2037–2040.
Ngangnou, A., Zoyem, N., Hamet, M. and Abdoulkadiri, S., 1996. Evaluation de la protection vaccinale contre la peste bovine au Cameroun III, Evaluation globale, Revue d’élevage et de Médecine Vétérinaire des Pays Tropicaux, 49(1), 18–22.
OIE, 2013. Peste des petits ruminants, In: Manual of diagnostic tests and vaccines for terrestrial animals, 7th Edition, Chapter 2.7.11., Office international des Epizooties (OIE), Paris.
OIE, 2016. Rinderpest, In: Manual of diagnostic tests and vaccines for terrestrial animals, 7th Edition, Chapter 2.1.19., Office international des Epizooties (OIE), Paris.
OIE-WAHIS, 2018. Annual Animal Health Report, World Animal Health Information Database (WAHIS Interface) – Version 1, World Organisation for Animal Health (OIE). (Retrieved from: https://www.oie.int/wahis_2/public/wahid.php/Reviewreport/).
Osman, N.A., A/Rahman, M.E., Ali, A.S., Fadol, M.A., 2008. Rapid detection of peste des petits ruminants (PPR) virus antigen in Sudan by agar gel precipitation (AGPT) and haemagglutination (HA) Tests, Tropical Animal Health and Production, 40(5), 363–368.
Özkul, A., Akça, Y., Alkan, F., Barrett, T., Karaoğlu, M.T., Dağalp, S.B., Anderson, J., Yeşilbağ, K., Çokçalışkan, C., Gencay, A., Burgu, I., 2002. Prevalance, distribution and host range of peste des petits ruminants virus, Turkey, Emerging Infectious Diseases, 8, 708–712
Parida, S., Muniraju, M., Mahapatra, M., Muthuchelvan, D., Buczkowski, H., Banyard, A.C., 2015. Peste des petits ruminants, Veterinary Microbiology, 181(1–2), 90–106.
Rashid, A., Asim, M., Hussain, A., 2008. Seroprevalence of peste des petits ruminants (PPR) virus in goats, sheep and cattle at Livestock Production Research Institute Bahadurnagar Okara, Journal of Animal and Plant Sciences, 18(4), 114–116.
Saeed, I.K., Ali, Y.H., Khalafalla, A.I., A/Rahman, M.E., 2010. Current situation of peste des petits ruminants (PPR) in the Sudan, Tropical Animal Health and Production, 42(1), 89–93.
Saeed, I.K., Ali, Y.H., Haj, M.A., Sahar, M.A.T., Shaza, M.M., Baraa, A.M., Ishag, O.M., Nouri, Y.M., Taha, K.M., Nada, E.M., Ahmed, A.M., Khalafalla, A.I., Libeau, G., Diallo, A., 2017. Peste des petits ruminants infection in domestic ruminants in Sudan, Tropical Animal Health and Production, 49(4), 747–754.
Sen, A., Saravanan, P., Balamurugan, V., Bhanuprakash, V., Venkatesan, G., Sarkar, J., Rajak, K.K., Ahuja, A., Yadav, V., Sudhakar, S.B., Parida, S., Singh, R.K., 2014. Detection of subclinical peste des petits ruminants virus infection in experimental cattle, VirusDisease, 25(3), 408–411.
Tounkara, K., Traore, A., Traore, A. P., Sidibe, S., Samake, Diallo, B.O. & Diallo, A., 1996. Epidémiologie de la peste des petits ruminants (PPR) et de la peste bovine au Mali: enquètes sérologiques, Revue d’élevage et de Médecine Vétérinaire des Pays Tropicaux, 49 (4), 273–277.
Author information
Authors and Affiliations
Contributions
Wegdan H. Ali: designed the work. Nussieba A. Osman: performed data analysis, results interpretation, prepared and finalized the manuscript. Rayan M. Asil, Baraa A. Mohamed, Salma O. Abdelgadir, Shaza M. Mutwakil and Nafeesa E.B. Mohamed: performed the laboratory work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
This article belongs to the Topical Collection: The Gordon Scott Collection - articles on peste des petits ruminants and other infectious diseases of tropical livestock
Rights and permissions
About this article
Cite this article
Ali, W.H., Osman, N.A., Asil, R.M. et al. Serological investigations of peste des petits ruminants among cattle in the Sudan. Trop Anim Health Prod 51, 655–659 (2019). https://doi.org/10.1007/s11250-018-1740-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-018-1740-2