Abstract
Bovine viral diarrhoea virus (BVDV) and infectious bovine rhinotracheitis virus (IBRV) are important viral diseases around the world. The objective of this study was to estimate the incidence of seroconversion to BVDV and IBRV and to identify associated risk factors in dairy herds of Michoacan, Mexico. The longitudinal study included 62 herds and ran from December 2001 to November 2002. The total number of animals enrolled and completing the study were 392 and 342 animals for BVDV and 925 and 899 animals for IBRV. Animals were tested monthly for 12 months, for the presence of antibodies. Risk factors were: herd size (2–9, 10–25 and 26–55 animals), herd serostatus (seropositive or seronegative, only for IBRV), age group of the animal (6 to 12, 13 to 24, 25 to 48 and > 48 months) and animal origin (born in farm, purchased). The cumulative incidences for BVDV and IBRV were 16.4% and 3.4%, respectively; whereas, the incidence density rates for BVDV and IBRV were 15.9 and 2.9 per 1000 animal-months at risk, respectively. Seroconversion curves were statistically different for age group for BVDV and IBRV and for herd status for IBR. The relatively high incidence of seroconversion for BVDV suggests that a successful control programme should be oriented towards the identification and elimination of the PI animals and towards avoiding the introduction of PI cattle to the farm. The scenario of IBRV is favourable to implement a programme directed to reduce the number of new seropositive herds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In Mexico, the diseases that cause abortions in cattle produce economical losses of about $937 to $1100 per aborted cow (Córdova et al. 2003). Abortions have been associated with bacterial, viral or fungal infections. Bovine viral diarrhoea and infectious bovine rhinotracheitis are among the viral diseases present in Mexico. These diseases may cause embryonic death, mummification, abortion, infertility and dead or weak calves that die within the first days of life (Fray et al. 2000). These viruses are also implicated in the bovine respiratory disease complex, which is considered one of the major causes of economic losses in the cattle industry (Houe 2003, Muylkens et al. 2007). A previous seroprevalence study in Michoacan, Mexico reported herd-level and animal-level seroprevalences of 98.4% and 63.2% for bovine viral diarrhoea virus (BVDV), and 38.7% and 10.1% for infectious bovine rhinotracheitis virus (IBRV) (Solorio 2004). However, to the authors´ knowledge there are no studies about the incidences of these diseases in Mexico.
Knowledge of the incidence of cattle being infected with BVDV or IBRV could provide information to control and prevent transmission of those diseases, minimizing their adverse effects on herd health. The objective of this study was to estimate the incidences of seroconversion to BVDV and IBRV and to determine the seroconversion curves of some descriptive factors of dairy herds in Michoacan, Mexico.
Materials and methods
Localization and climate
The study was carried out in the localities of Cotzio and Tejaro, Michoacan, Mexico located between 19° 45′ and 19° 54′ north and 101° 03′ and 101° 11′ west, at 1860 meters above sea level (INEGI 2004). Cotzio and Tejaro are important places for cow-milk production within the Morelia-Querendaro Valley. Cow milk production is practiced by rural families of low socio-economic status and occupies the second most economically important activity in the region, with an approximate inventory of 154 herds and 3180 cows. Herds are small (2–55 animals) and contain mainly cattle of the Holstein breed. Cows are kept in stationary enclosures built of brick or concrete with natural or concrete floor. Cows are milked by hand twice a day with average daily milk production of 7.3 litres /cow. Rations are based on fresh Lucerne and corn straw (April-May) when pasture is scarce. Animals were reproduced mainly by natural mating. Vaccination is not a common practice in the region; however, when done, it is mainly against rabies, pasteurellosis and black leg.
Study design and population
A prospective longitudinal study was conducted. Sixty-two herds previously chosen at random for a seroprevalence study conducted in November 2000 to September 2001 (Solorio 2004) were used. The numbers of herds to sample was calculated considering a population of 154 herds, 50% herd seroprevalence, 95% confidence level and 10% precision, using the formula for a random simple survey (Segura and Honhold 2000). All seronegative animals of the 62 herds were involved in a follow-up study from December, 2001 to November, 2002. All the seronegative animals (>6 month old), from each herd, were monthly tested for 12 months for the presence of antibodies to BVDV and IBRV. An animal that seroconverted for the first time during the period of study was considered to be a case and it was further sampled until seroconversion occurred for the two infections or until the end of the study. Animals were identified individually by numbered ear tags. The serological status of animals at risk was considered an indicator of infection, because vaccination against those diseases was not practiced in the region. Information about herd size, cattle introduced to the farm and herd serostatus was obtained from a previous cross-sectional study (Solorio 2004); whereas, age of the animal at the start of the study was determined at the start of the study and from the monthly visits for the newly enrolled animals. The populations at risk at the beginning of the study were all the seronegative animals in the 62 herds (349 animals for BVDV and 820 animals for IBRV). The number of herds and animals for each stratum of the descriptive factors are shown in Tables 1 and 2. Herd size was defined as the number of animals in the herd (positive or negative). Herd serostatus (only for IBRV, because all herds were positive for BVDV) was defined as negative (herds with zero or one seropositive animal) or seropositive (herds with at least 2 seropositive animals, due to false positive test results, because specificity of the ELISA test used was not 100%).
Sampling and laboratory analysis
Blood samples (10 ml) were collected from the coccygeal vein of each animal, using disposable needles (21 × 1 1/2 mm) and vacutainer tubes, and transported on ice to the laboratory. The samples were centrifuged at 2000 × g for 10 min to obtain the serum. Sera were stored in identified vials at –20 C until testing. Serum samples were tested (all at one time) for antibodies against BVDV with an indirect enzyme-linked immunosorbent assay kit (ELISA for BVDV-Ab, Svanova Biotech AB: 10-2200-10, SE-751 83 Uppsala, Sweden) with sensitivity (Se) and specificity (Sp) of 97.8% and 94.6%, respectively (Provided by the manufacturer). The results were read by a microplate photometer, where the optical density (OD) was measured at 450 nm and using air as blank.
For the diagnosis of seropositivity to IBRV an indirect enzyme-linked immunosorbent assay kit (ELISA for IBR-Ab, Svanova Biotech AB: 10-2100-10, SE-751 83 Uppsala, Sweden) with sensitivity and specificity of 95.8% and 94.4%, respectively was used (Provided by the manufacturer). Sera with corrected OD ≥ 0.25 were considered positives and all assays were performed at one time.
Data from all seronegative animals at enrolment and that completed at least one monthly follow-up visit (of 12) were included in the statistical analysis. The events of interest in this study were BVDV and IBRV seroconversion.
Statistical analyses
Data on time to seroconversion until the end of the observation period, or time of sale or death during the course of the study were right-censored at the month the study was terminated or the month the animal left the herd. Time to seroconversion was the accumulated number of cattle-months until seroconversion. The average incidence rates (IR) for each of the infections were estimated as the number of animals that seroconverted divided between the number of animal-months at risk during the period of study. In this analysis zero was defined as the month of enrolment. To facilitate interpretation of the survival curves, herd sizes were grouped to make categorical variable with three levels: from 2 to 9, from 10 to 25 and from 26 to 55. Age group of cattle at the start of the study was categorized as: animals from 6 to 12, 13 to 24 and >24 months old. To test the equality of the seroconversion curves between strata for each descriptive factor, the long rank test of the life-table option was used (SAS 2000).
Results
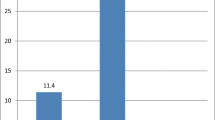
Twenty-eight out of the 62 herds had at least one animal that seroconverted (median 1; range 1–7) for BVDV. Of the 392 animals studied, 50 seroconverted and 342 were censored (3313 animal-months at risk). The estimated overall cumulative incidence during the 12 months of study was 16.6% and the average IR was 15.1 per 1000 animal-months at risk. The first case of seroconversion was observed after 3 months of exposure. The incidence rates by strata for each of the descriptive factors as well as the significances of the survival analysis are shown in Table 1. The life-table method only showed significant (P < 0.05) differences between seroconversion curves of the age group factor (Fig. 1).
Fifteen out of the 62 herds had at least one animal that seroconverted (median 2; range 1–3) for IBRV. Of the 925 animals studied, 26 seroconverted and 899 were censored (8978.5 animal-months at risk). The estimated overall cumulative incidence during the 12 months of study was 3.4% and the average IR was 2.9 per 1000 animal-months at risk. The first case of seroconversion was observed after 2 months of exposure (Fig. 2). The incidence by strata for each of the risk factors is shown in Table 2. The survival analysis showed differences between strata for herd seropositivity (P < 0.01) and age group of the animal (P < 0.06).
Discussion
The high herd-level (98.4%) and animal-level (63.2%) seroprevalences for BVDV found in a previous cross-sectional study (Solorio 2004) indicates that the disease is endemic in the region. Also, the presence of new seropositive animals to BVDV (16.4% cumulative incidence, during the 12 month period of study) suggest that animals were exposed to BVDV, and the existence of PI-animals in those herds. The IR (15.1 per animal-month at risk) found in our study is lower than that reported by Rush et al. (2001) in calves born without evidence of BVDV infection in two herd of California USA, under more intensive management conditions. The fact that no all herds experienced seroconversion during the 12 month study period could be partially explained by differences in management practices and biosecurity measures carried out in each herd. However, as pointed out by Rush et al. (2001) even herds with similar management could have different IR, because of differences in animal contact patterns within herds, sources of PI animals, differential susceptibility, cattle density and virulence of the BVDV strains in the herd. Some studies show that culling of PI animal and good biosecurity measures might decrease the prevalence (Self-clearance) of BVDV (Kampa et al. 2004, Lindberg and Houe 2005, Stahl et al. 2008). The principal factors for introduction or reintroduction of an infection into a herd are purchase of PI animals, dams carrying PI foetus or contact with PI cattle from an infected herd (Bitsch et al. 2000). Therefore, management procedures need to be reviewed to ensure that adequate biosecurity measures are provided and that any reintroduction is detected and dealt with rapidly (Valle et al. 2001).
Herd health programmes should be oriented to maintain the subclinical status of the disease, but trying to reduce the incidence to achieve a phase of herds free of infection and to include measurements to avoid the introduction of the agent to the susceptible herds (Gunn 2002). In unvaccinated dairy herds (like in this study) serological testing of bulk milk is a convenient method for BVDV prevalence screening (Stahl et al. 2002, Stahl et al. 2008). Bulk milk testing for detection of antibodies is a fast, non-invasive and cost-effective method invaluable in the control programmes for BVDV and bovine herpesvirus type 1 in some countries (Lindberg and Alenius 1999, Nylin et al. 2000).
The highest seroconversion rate for the young animals (Table 1) may suggest that young cattle are more susceptible than old animals to BVDV infection. However, it is also probable that low seroconversion in old animals are the result of culling cows with low performance as a result of subclinical infection with BVDV.
The low cumulative incidence and IR of IBRV explain the low herd-prevalence and animal-prevalence found by Solorio (2004) in a previous study. Hage et al. (1996) mentioned that after the introduction of the IBRV in a susceptible herd the infection is distributed rapidly resulting in clinical or subclinical manifestations with seroconversion. IBRV seropositive animals persist for long periods of time, where antibodies could be detected up to three years after infection (Kaashoek et al. 1996, Nardelli et al 2008). Bulk milk tests for antibody detection might be a good alternative to control the incidence of IBRV. To minimise the risk of introducing the virus into BHV-1-free herds, the recognition and removal of animals that are latent carriers is important in control programmes (Muylkens et al. 2007).
Herd serostatus was a significant risk factor for IBRV seroconversion. The incidence of seroconversion was higher for an animal in a seropositive herd (5.7%) than one in a seronegative herd (0.8%). This is logical due to that the presence of infected animals increase the risk of infection for the susceptible ones. Similar results were obtained by Nardelli et al (2008) in dairy cattle in Italy.
The patterns of IBVR seroconversion by age groups were similar to those for BVDV. Animals lower than 24 months of age had higher incidences than those >24 months of age; which could be explained by a higher susceptibility of young animals or to a process of culling of unproductive adult cows, some of them probably due to IBRV infection.
Even though introducing cattle to the farm was not associated with the incidence rate of seroconversion (P > 0.10), cross-sectional studies with beef (Solis-Calderon et al., 2003) and dairy cattle (Córdova et al. 2003) in Mexico and other countries (Muylkens et al. 2007) indicate that the introduction of the animals is potentially associated with prevalent BVDV or IBRV infection. Therefore biosecurity measures should be encouraged in the target population.
In conclusion, the high herd-level and animal-level seroprevalences found in a previous study and the relatively high incidence of seroconversion for BVDV suggest that a successful BVDV control should be oriented towards the identification and elimination of the PI animals and towards avoiding the introduction of PI cattle to the farm. The scenario of IBRV is favourable to implement a programme direct to reduce the number of new seropositive herds. The low cumulative incidence (here obtained) and low herd-level and animal-level seroprevalences found in the same herds might let us think in the implementation of a culling programme.
References
Bitsch, V., Hansen, K.E., Rønsholt, L., 2000. Experiences from the Danish programme for eradication of bovine virus diarrhoea (BVD) 1994-1998 with special reference to legislation and causes of infection. Veterinary Microbiology, 77,137–143.
Córdova, L.D., Urritia, R.M., Zacarias, M.L., Rosado, M.I, Guzmán, C.C., López, M.J., Moles, L.P., Mojarro, F.J., García, V.Z., Zermeño, P.A., Aguilar, R.F., Hernández, A.L., Patiño, A.P., 2003. Epidemiología y factores de riesgo asociados a algunas enfermedades causantes de aborto bovino (leptospirosis, diarrea viral, rinotraqueitis infecciosa, leucosis bovina, neosporosis y hemofilosis) en el Estado de Guanajuato, México. Memorias III Congreso Internacional de Epidemiología Veterinaria, A.C. Oaxaca, Oaxaca, México. 16 al 18 de Octubre de 2003. pp. 219–228.
Fray, M.D., Paton, D.J., Alenius, S., 2000. The effects of bovine viral diarrhoea virus on cattle reproduction in relation to disease control. Animal Reproduction Science, 60–61,615–627.
Gunn, G.J., 2002. Bovine virus diarrhoea virus-which way to turn? The Veterinary Journal, 163,226–227.
Hage, J.J., Schukken, Y.H., Barkema, H.W., Benedictus, G., Rijsewijk, F.A., Wentink, G.H., 1996. Population dynamics of bovine herpesvirus-I infection in a dairy herd. Veterinary Microbiology, 53, 169–180.
Houe, H., 2003. Economic impact of BVDV infection in dairies. Biologicals, 31, 137–143.
INEGI, 2004. Anuario Estadístico de Yucatán. Instituto Nacional de Estadística, Geografía e Informática. Aguascalientes, México.
Kaashoek, M.J., Rijsewijk, F.A.M., van Oirschot, J.T., 1996. Persistence of antibodies against bovine herpesvirus 1 and virus reactivation two to three years after infection. Veterinary Microbiology, 53,103–110.
Kampa, J., Stahl, K., Moreno-López,J., Chanlun, A., Aiumlamai, S., Alenius, S., 2004. BVDV and BHV-1 infections in dairy herds in northern and northeastern Thailand. Acta Veterinaria Scandinavica, 45,181–192.
Lindberg, A.L.E., Alenius, S. 1999. Principles for eradication of bovine viral diarrhoea virus (BVDV) infection in cattle populations. Veterinary Microbiology, 64, 197–222.
Lindberg, A., Houe, H., 2005. Characteristics in the epidemiology of bovine viral diarrhea virus (BVDV) of relevance to control. Preventive Veterinary Medicine, 72, 55–73.
Muylkens, B., Thiry, J., Kirten, P., Schynts, F., Thiry, E., 2007. Bovine herpesvirus 1 infection and infectious bovine rhinotrachaeitis. Veterinary Research, 38, 181–209.
Nardelli S., Farina G., Lucchini R., Valorz C., Moresco A., Dal-Zooto R., Constanzi C., 2008. Dynamics of infection and immunity in a dairy cattle population undergoing and eradication programme for Infectious Bovine Rhinotracheitis (IBR). Preventive Veterinary Medicine, 85, 68–80.
Nylin, B., Strøger, U., Rønsholt, L., 2000. A retrospective evaluation of a bovine herpesvirus-1 (BHV-1) antibody ELISA on bulk-tank milk samples for classification of the BHV-1 status of Danish dairy herds. Preventive Veterinary Medicine, 47, 91–105.
Rush, M.D., Thurmond, M.C., Muñoz-Sanzi, C.A., Hietala, S.K. 2001. Descriptive epidemiology of potsnatal bovine viral diarrhea virus infection in intensively managed dairy herd heifers. JAVMA, 219:1426–1431.
SAS, 2000. SAS/STAT User’s Guide, Version 8.1, SAS Institute Inc., Cary, NC.
Segura, J.C., Honhold, N., 2000. Métodos de Muestreo para la Producción y Salud Animal. Universidad Autónoma de Yucatán. Mérida, Yucatán, México.
Solis-Calderon, .J.J., Segura-Correa, V.M., Segura-Correa, .J.C., Alvarado-Islas, A., 2003. Seroprevalence of and risk factors for infectious bovine rhinotracheitis in beef cattle herds of Yucatan, Mexico. Preventive Veterinary Medicine, 57, 199–208.
Solorio J.L. 2004. Análisis de riesgo de enfermedades abortivas en el sistema de lechería familiar en la región centro del estado de Michoacán. Doctoral Thesis, Universidad Autónoma de Yucatán. Mérida, Yucatán, México.
Stahl, K., Rivera, H., Vagsholm, I., Moreno-López, J., 2002. Bulk milk testing for antibody seroprevalences to BVDV and BHV-1 in a rural region of Peru. Preventive Veterinary Medicine, 56,193–202.
Stahl, K., Lindberg, A., Rivera, H., Ortiz, C., Moreno-López, J., 2008. Self-clearance from BVDV infections- A frequent finding in dairy herds in an endemically infected region in Peru. Preventive Veterinary Medicine, 83, 285–296.
Valle, P.S., Martin, S.W., Skjerve, E., 2001. Time to first calving and calving interval in bovine virus diarrhoea virus (BVDV) seroconverted dairy herds in Norway. Preventive Veterinary Medicine, 51, 17–36.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Segura-Correa, J.C., Solorio-Rivera, J.L. & Sánchez-Gil, L.G. Seroconversion to bovine viral diarrhoea virus and infectious bovine rhinotracheitis virus in dairy herds of Michoacan, Mexico. Trop Anim Health Prod 42, 233–238 (2010). https://doi.org/10.1007/s11250-009-9411-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-009-9411-y