Abstract
Counter immnuo-electrophoresis (CIEP) and Competitive ELISA (C-ELISA) tests were employed for seroprevalence of Peste des Petits Ruminants (PPR) infection in Sudan. The result of both tests showed high prevalence of PPRV antibodies in sheep and goats sera collected from six different regions of Sudan. Of the 519 serum samples examined for the presence of PPRV antibodies 307(59.15%) were positive by CIEP while 263(50.67%) were positive by C-ELISA. CIEP technique was shown to be more sensitive than C-ELISA technique for detection of PPRV antibodies (Kappa statistics 0.259). C-ELISA allowed rapid, simple, specific, sensitive and differential sero-diagnosis of PPRV and RPV in sheep, goats and cattle. CIEP is, unlike competitive ELISA, is group-specific test and can not differentiate between PPR and RP infections. Despite its low specificity CIEP can be a useful indicative screening test for PPRV antibodies in flocks that neither been vaccinated nor otherwise exposed to PPR or RP virus. Results obtained suggest that CIEP, like the HI test, could be a useful screening test where it is not possible to use C-ELISA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Peste des petits ruminants (PPR) is an acute and highly contagious viral disease of small ruminants with high rates of morbidity and sometimes high rates of mortality. Goats are usually more severely affected than sheep (Roeder et al. 1994; Diallo 2000). Economically, it has been the most important disease of these species in sub-Saharan Africa, the Middle East, and southwest Asia.
Severe outbreaks of PPR continued to occur in small ruminants in Sudan causing significant economic losses. In Sudan, detection of antibodies against PPRV has also been reported in several species including sheep, goats, cattle and camels (Anderson and McKay 1994; El Amin and Hassan 1999, Haroun et al. 2002). Rapid detection of infected animals is very important for PPR controls to be effective. Severe cases in which animals show clinical signs in the field can easily be detected through clinical surveillance and the detection of antigen in clinical samples, while the diagnosis of PPRV infection in subclinically infected animals can be achieved by serological surveillance.
In an attempt to investigate the prevalence of PPR in sheep and goats in Sudan, a study based on serosurveillance was conducted in different parts of Sudan. The aim of this study is to provide a simple, rapid and cheap serological test that can be used for routine diagnosis of PPR in Sudan.
Materials and methods
Test sera
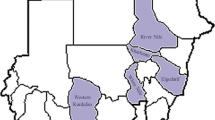
A total of 519 serum samples were collected from sheep and goats during the period 2001-2003 to study the seroprevalence of PPR in six different states of Sudan (Table 1). None of the animals was known to have been vaccinated against PPR before or at the time of sampling.
Reference PPR virus (Sinnar Strain)
The reference strain of PPRV was the Sudanese isolate SUD 72/1 PPRV Sinnar (BKC. P.4. LTC P.2) Nussieba et al. (2008). The reference virus was used as positive control antigen in CIEP.
Preparation of hyper-immune Serum
Hyper-immune antiserum to PPRV SUD 72/1 (Sinnar 105.4 TCID50/ml) was produced in goats (Nussieba et al. 2008).
Counter immnuo-electrophoresis (CIEP)
The gel used was agarose (Sigma, medium EEO, type II). The tank buffer was a barbitone acetate buffer pH 8.6, 0.1 M containing 2 g sodium azide per litre. A 1% (w/v) agar was prepared by boiling 1 g of agarose in 100 ml of barbitone acetate buffer. The CIEP protocol was essentially as described previously by Elhag Ali and Lees (1979), Majiyagbe et al. (1984) and Obi and Patrick (1984).
Competitive ELISA (C-ELISA)
All reagents were obtained in pre-titrated kit form prepared by BDSL, Flow Laboratories Ltd. & the Institute for Animal Health, Pirbright, Surrey, U.K. (BDSL 2000), in collaboration with the Animal Production Unit, Agriculture Laboratory, Agency's Laboratory Division, Seibersdrof, Austria. All procedures were carried out according to the instructions in the manual included with the kit.
Statistical analysis
The results of both tests were analyzed using Microsoft Office Excel and SPSS software. The statistical significance between groups of data was determined using the chi-square (δ2) test.
Results
Of the 519 serum samples examined for the presence of PPRV antibodies by CIEP, 307 serum samples (59.15%) were positive and 212 serum samples (40.85%) were negative (Table 2). Positive results were indicated by presence of 1-2 precipitin lines which formed between PPR antigen and suspected sera. The precipitation lines were seen after 70-90 minutes of connecting the electrophoresis apparatus to the electricity supply. The prevalence of PPRV antibodies in different States of Sudan detected by CIEP is shown in Table 3. The highest incidence of PPRV antibodies detected by CIEP was in River Nile State (69.81%) and the lowest incidence was in Kordofan State (42.50%).
Of the 519 serum samples examined for the presence of PPRV antibodies by C-ELISA, 263 serum samples (50.67%) were positive and 256 serum samples (49.33%) were negative (Table 2). The prevalence of PPRV antibodies in different States of Sudan detected by C-ELISA is shown in Table 3. The highest incidence of PPRV antibodies detected by C-ELISA was in Blue Nile State (60.49%) and the lowest incidence was in River Nile State (33.96%).
Out of the 519 sera examined for prevalence of PPRV antibodies by both CIEP and C-ELISA, 195 sera (37.57%) were positive and 133 sera (25.63%) were negative by both (Table 2). It was generally observed that more sera samples were positive with CIEP (59.15%) than with C-ELISA (50.67%). The results of both tests revealed that CIEP was more sensitive than C-ELISA for detection of PPRV antibodies (Kappa statistics 0.259).
Discussion
In this study, CIEP and C-ELISA were applied to the serosurveillance of PPR in sheep and goats in Sudan. The result of the CIEP could be obtained within 70-90 minutes of connecting the electrophoresis apparatus to the electricity supply. It was previously confirmed that the rapidity, simplicity and sensitivity of the CIEP made it a suitable technique in serological studies of PPR (Durojaiye and Taylor 1984; Majiyagbe et al. 1984). Elhag Ali and Lees (1979) indicated that CIEP was around 4-16 times more sensitive than the AGPT for detecting RP antigen and antibodies. However, Majiyagbe et al. (1984) indicated that it was 8-16 times more sensitive. In general most test samples gave a single line of precipitation in the CIEP assays while some samples gave two precipitation lines. For conducting the CIEP a very small volume of antigen and antiserum was required for performing the test and a large number of samples could be tested simultaneously. In this study, the test was performed at room temperature. When we used both PPR antigen and RP antigen in CIEP test the developed lines were clear with PPR antigen than with RP antigen. That is as confirmed by Majiyagbe et al. (1984) who observed that the number and intensity of the precipitin arcs were more for the homologous than the heterologous virus.
Monoclonal antibody-based C-ELISA was used for the specific detection of antibodies to PPRV in sera of sheep and goats. C-ELISA is simple, rapid, specific and sensitive and preferred over VNT for intensive surveillance (Singh et al. 2004). The test could clearly differentiate infected from uninfected population (Libeau et al. 1995; Singh et al., 2004). This test may be a useful tool for a standardized and accurate determination of the immune status of animals because of its superior sensitivity to conventional tests (Libeau et al. 1992).
Examination of 519 sera for PPRV antibodies from six different States of Sudan revealed 307(59.15%) positive and 212(40.85%) negative by CIEP and 263(50.67%) positive and 256(49.33%) negative by C-ELISA. In this study, it was clear that more positive serum samples were obtained by CIEP test than by C-ELISA when these tests were employed for detection of antibodies against PPRV (Kappa statistics 0.259). This result may be due to the fact that CIEP is group-specific test and can detect PPRV antibodies as well as some cross-reactive antibodies against RPV in sera of sheep and goats. This finding substantiated that of Obi and Patrick (1984) who reported that CIEP is group-specific test and may not distinguish between PPR and RP infections in small ruminants. Also it may be due to the different factors that affect the CIEP run compared with the optimized conditions of the C-ELISA. Despite its low specificity CIEP can be a useful indicative screening test for PPRV antibodies in flocks that neither been vaccinated nor otherwise exposed to PPR or RP virus. Results obtained suggest that CIEP, like the HI test, could be a useful screening test where it is not possible to use C-ELISA. One of the main advantages of the CIEP is its rapidity in producing results as precipitin lines were often visible after the test was run for 30-45 minutes. The CIEP, which can be performed under field conditions, is considered suitable for use for diagnosis and sero-epidemiological surveillance due to its simplicity and rapidity.
The detection of a high prevalence of antibodies against PPRV in sera collected from field samples of sheep and goats in this study indicated the exposure of these animals to the field virus. No vaccination with PPR vaccine was carried out previously. The prevalence of PPR antibodies in the six States under study indicated the wide spread of the disease in Northern, Southern, Western and Central Sudan. It was observed that the prevalence of PPR antibodies detected by both CIEP and C-ELISA was higher in States near the borders of the country. These would be attributed to animal movement between Sudan and neighbouring countries.
Abbreviations
- PPRV:
-
Peste des Petits Ruminants Virus
- CIEP:
-
Counter immnuo-electrophoresis
- C-ELISA:
-
Competitive ELISA
- RPV:
-
Rinderpest Virus
- HI:
-
Haemagglutination inhibition
- MAb:
-
Monoclonal antibodies
- SUD 72/1:
-
Sudan 72/1
- BKC. P.4:
-
Bovine kidney cells passage 4
- LTC. P.2:
-
Lamb testis cells passage 2
- TCID50 :
-
Tissue culture infective dose50
- AGPT:
-
Agar Gel Precipitation Test
- EEO:
-
Electroendosmosis
- PI:
-
Percentage inhibition
- VNT:
-
Virus neutralization test
References
Anderson, J. and McKay, J.A. (1994). The detection of antibodies against peste des petits ruminants virus in cattle, sheep and goats and the possible implication to rinderpest control programmes. Epidemiology and Infection, 112: 225-231.
Biological Diagnostic Supplies LTD (BDSL), Flow Laboratories and Institute for Animal Health, Pirbright, Surrey, England (2000). PPR competitive ELISA Kit. Competitive ELISA for detection of antibodies to P.P.R. virus. Developed in Collaboration with: The Animal Production and Health Section joint FAO/IAEA Division, IAEA, and the FAO/IAEA Central Laboratory for ELISA and molecular techniques in diagnosis of animal diseases, Vienna, Austria.
Diallo, A. (2000). Peste des petits ruminants. In: OIE Manual of Standards for Diagnostic Tests and Vaccines, 4th edition. Chapter 2.1.5. Office international des Epizooties, Paris, pp. 114-122.
Durojaiye, O.A. and Taylor, W.P. (1984). Application of countercurrent immuno-electro-osmo-phoresis to the serology of peste des petits ruminants. Revue d’Elevage et de Medecine Veterinaire des Pays Tropicaux, 37(3): 272-276.
El Amin, M.A.G. and Hassan, A.M. (1999). Rinderpest and PPR surveillance in Sudan (1996-1998). In: The Sermonitoring and Surveillance of RP Throughout Africa-phase III. Results for 1998. Proceedings of a research co-ordination meeting of the FAO/IAEA/OAU/IBAR/PARC co-ordinated research project organized by the joint FAO/IAEA division of nuclear techniques in food and agriculture, Machakos, Kenya, April 26-30.
Elhag Ali, B. and Lees, G.E. (1979). The application of immunoelectroprecipitation in the diagnosis of rinderpest. Bulletin of Animal Health and Production in Africa, 27: 1- 6.
Haroun, M., Hajer, I., Mukhtar, M. and Ali, B.E. (2002). Detection of antibodies against peste des petits ruminants virus in sera of cattle, camels, sheep and goats in Sudan. Veterinary Research Communications, 26(7): 537-541. doi:10.1023/A:1020239515020
Libeau, G., Diallo, A., Calvez, D. and Lefevre, P.C. (1992). A competitive ELISA using anti-N monoclonal antibodies for specific detection of rinderpest antibodies in cattle and small ruminants. Veterinary Microbiology, 31: 147-160. doi:10.1016/0378-1135(92)90073-3
Libeau, G., Prehaud, C., Lancelot, R., Colas, F., Guerre, L., Bishop, D.H.L. and Diallo, A. (1995). Development of a competitive ELISA for detecting antibodies to the peste des petits ruminants virus using a recombinant nucleoprotein. Research in Veterinary Science, 58: 50-55. doi:10.1016/0034-5288(95)90088-8
Majiyagbe, K.A., Nawathe, D.R. and Abegunde, A. (1984). Rapid diagnosis of peste des petits ruminants (PPR) infection, application of immunoelectroosmophoresis (IEOP) technique. Revue d’Elevage et de Medecine Veterinaire des Pays Tropicaux, 37(1): 11-15.
Nussieba, A.O., Mahasin, E.A., Ali, A.S. and Fadol, M.A. (2008). Rapid detection of Peste des Petits Ruminants (PPR) virus antigen in Sudan by Agar Gel Precipitation (AGPT) and Haemagglutination (HA) Tests. Tropical Animal Health and Production, 40(5): 363-368. doi:10.1007/s11250-007-9106-1
Obi, T.U. and Patrick, D. (1984). The detection of peste des petits ruminants (PPR) virus antigen by agar gel precipitation test and counter-immunoelectrophoresis. Journal of Hygiene, Cambridge, 93: 579-586.
Roeder, P.L., Abraham, G., Kenfe, G. and Barrett, T. (1994). Peste des petits ruminants in Ethiopian Goats. Tropical Animal Health and Production, 26: 69-73. doi:10.1007/BF02239901
Singh, R.P., Sreenivasa, B.P., Dhar, P., Shah, L.C. and Bandyopadhyay, S.K. (2004). Development of a monoclonal antibody based competitive-ELISA for detection and titration of antibodies to peste des petits ruminants (PPR) virus. Veterinary Microbiology, 98: 3-15. doi:10.1016/j.vetmic.2003.07.007
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Osman, N.A., Ali, A.S., A/Rahman, M.E. et al. Antibody seroprevalences against Peste des Petits Ruminants (PPR) virus in sheep and goats in Sudan. Trop Anim Health Prod 41, 1449–1453 (2009). https://doi.org/10.1007/s11250-009-9333-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-009-9333-8